The Temporal Order of Mixed Viral Infections Matters: Common Events That Are Neglected in Plant Viral Diseases
Abstract
:1. Introduction
2. The Insect-Vector Role in Mixed Infections of Plant Viruses
3. Impact of Temporal Order of Infection on Plant Viral Disease: A Case Study of Two Aphid-Transmitted Viruses
4. Consequences of Infection Order on Population Dynamics, Virus Transmission, and Agroecosystems
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abedon, S.T. Bacteriophage secondary infection. Virol. Sin. 2015, 30, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W.; Hughes, K.A.; Skillman, L.C.; Tait, K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 2004, 232, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hillman, B.I.; Annisa, A.; Suzuki, N. Viruses of Plant-Interacting Fungi. Adv. Virus Res. 2018, 100, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Tugume, A.K.; Mukasa, S.B.; Valkonen, J.P.T. Mixed Infections of Four Viruses, the Incidence and Phylogenetic Relationships of Sweet Potato Chlorotic Fleck Virus (Betaflexiviridae) Isolates in Wild Species and Sweetpotatoes in Uganda and Evidence of Distinct Isolates in East Africa. PLoS ONE 2016, 11, e0167769. [Google Scholar] [CrossRef]
- Tollenaere, C.; Susi, H.; Laine, A.L. Evolutionary and Epidemiological Implications of Multiple Infection in Plants. Trends Plant Sci. 2016, 21, 80–90. [Google Scholar] [CrossRef]
- Susi, H.; Sallinen, S.; Laine, A. Coinfection with a virus constrains within-host infection load but increases transmission potential of a highly virulent fungal plant pathogen. Ecol. Evol. 2022, 12, e8673. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-infection of swine with porcine circovirus type 2 and other swine viruses. Viruses 2019, 11, 16–20. [Google Scholar] [CrossRef]
- Musa, W.I.; Sa’idu, L.; Bello, M.; Abdu, P.A. Co-inections of domestic and wild birds with avian influenza and Newcastle disease viruses: Implications for control and genetic mutations. Vet. Res. Commun. 2020, 44, 159–166. [Google Scholar] [CrossRef]
- Antalis, E.; Oikonomopoulou, Z.; Kottaridi, C.; Kossyvakis, A.; Spathis, A.; Magkana, M.; Katsouli, A.; Tsagris, V.; Papaevangelou, V.; Mentis, A.; et al. Mixed viral infections of the respiratory tract; an epidemiological study during consecutive winter seasons. J. Med. Virol. 2018, 90, 663–670. [Google Scholar] [CrossRef]
- Howell, A.K.; McCann, C.M.; Wickstead, F.; Williams, D.J.L. Co-infection of cattle with Fasciola hepatica or F. gigantica and Mycobacterium bovis: A systematic review. PLoS ONE 2019, 14, e0226300. [Google Scholar] [CrossRef]
- Bursakov, S.A.; Kovalchuk, S.N. Co-infection with tick-borne disease agents in cattle in Russia. Ticks Tick. Borne. Dis. 2019, 10, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The impact of co-infections on fish: A review. Vet. Res. 2016, 47, 98. [Google Scholar] [CrossRef] [PubMed]
- Cawcutt, K.; Kalil, A.C. Pneumonia with bacterial and viral coinfection. Curr. Opin. Crit. Care 2017, 23, 385–390. [Google Scholar] [CrossRef]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.H.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respi. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef]
- Szymański, K.; Cieślak, K.; Kowalczyk, D.; Brydak, L.B. Co-infection with influenza viruses and influenza-like virus during the 2015/2016 epidemic season. Adv. Exp. Med. Biol. 2017, 968, 7–12. [Google Scholar] [CrossRef]
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, a017871. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Jones, R.A.C. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Moreno, A.B.; López-Moya, J.J. When Viruses Play Team Sports: Mixed Infections in Plants. Phytopathology 2020, 110, 29–48. [Google Scholar] [CrossRef]
- de Moya-Ruiz, C.; Juárez, M.; Gómez, P. Revealing hidden viruses inducing similar yellowing symptoms or remaining asymptomatic in cucurbit crops. Plant Pathol. 2025, 74, 270–282. [Google Scholar] [CrossRef]
- Gómez-Aix, C.; Alcaide, C.; Agüero, J.; Faize, M.; Juárez, M.; Díaz-Marrero, C.J.; Botella-Guillén, M.; Espino, A.I.; Aranda, M.A.; Gómez, P. Genetic diversity and population structure of Pepino mosaic virus in tomato crops of Spain and Morocco. Ann. Appl. Biol. 2019, 174, 284–292. [Google Scholar] [CrossRef]
- Alcaide, C.; Rabadán, M.P.; Juárez, M.; Gómez, P. Long-Term Cocirculation of Two Strains of Pepino Mosaic Virus in Tomato Crops and Its Effect on Population Genetic Variability. Phytopathology 2020, 110, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rabadán, M.P.; Juárez, M.; De Moya-Ruiz, C.; Gómez, P. Aphid-borne viruses infecting cultivated watermelon and squash in Spain: Characterization of a variant of cucurbit aphid-borne yellows virus (CABYV). Plant Pathol. 2021, 70, 1476–1485. [Google Scholar] [CrossRef]
- Kassem, M.A.; Juárez, M.; Gómez, P.; Mengual, C.M.; Sempere, R.N.; Plaza, M.; Elena, S.F.; Moreno, A.; Fereres, A.; Aranda, M.A. Genetic Diversity and Potential Vectors and Reservoirs of Cucurbit aphid-borne yellows virus in Southeastern Spain. Phytopathology 2013, 103, 1188–1197. [Google Scholar] [CrossRef]
- Syller, J. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol. Plant Pathol. 2011, 13, 204–216. [Google Scholar] [CrossRef]
- Rubio, L.; Guerri, J.; Moreno, P. Genetic variability and evolutionary dynamics of viruses of the family Closteroviridae. Front. Microbiol. 2013, 4, 151. [Google Scholar] [CrossRef]
- Singhal, P.; Nabi, S.U.; Yadav, M.K.; Dubey, A. Mixed infection of plant viruses: Diagnostics, interactions and impact on host. J. Plant Dis. Prot. 2021, 128, 353–368. [Google Scholar] [CrossRef]
- Alcaide, C.; Rabadán, M.P.; Moreno-Pérez, M.G.; Gómez, P. Implications of mixed viral infections on plant disease ecology and evolution. Adv. Virus Res. 2020, 106, 145–169. [Google Scholar] [CrossRef]
- Syller, J. Biological and molecular events associated with simultaneous transmission of plant viruses by invertebrate and fungal vectors. Mol. Plant Pathol. 2014, 15, 417–426. [Google Scholar] [CrossRef]
- Syller, J.; Grupa, A. Antagonistic within-host interactions between plant viruses: Molecular basis and impact on viral and host fitness. Mol. Plant Pathol. 2016, 17, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Mascia, T.; Gallitelli, D. Synergies and antagonisms in virus interactions. Plant Sci. 2016, 252, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.; Sempere, R.N.; Elena, S.F.; Aranda, M.A. Mixed Infections of Pepino Mosaic Virus Strains Modulate the Evolutionary Dynamics of this Emergent Virus. J. Virol. 2009, 83, 12378–12387. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.K.; Falk, B.W. Virus-Vector Interactions Mediating Nonpersistent and Semipersistent Transmission of Plant Viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Peters, D.; Matsumura, E.E.; van Vredendaal, P.; van der Vlugt, R.A.A. The plant virus transmissions database. J. Gen. Virol. 2024, 105, 001957. [Google Scholar] [CrossRef]
- Fereres, A.; Raccah, B. Plant Virus Transmission by Insects; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–12. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus-insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef]
- Gallet, R.; Michalakis, Y.; Blanc, S. Vector-transmission of plant viruses and constraints imposed by virus–vector interactions. Curr. Opin. Virol. 2018, 33, 144–150. [Google Scholar] [CrossRef]
- Zhou, J.S.; Drucker, M.; Ng, J.C. Direct and indirect influences of virus–insect vector–plant interactions on non-circulative, semi-persistent virus transmission. Curr. Opin. Virol. 2018, 33, 129–136. [Google Scholar] [CrossRef]
- Moury, B.; Fabre, F.; Senoussi, R. Estimation of the number of virus particles transmitted by an insect vector. Proc. Natl. Acad. Sci. USA 2007, 104, 17891–17896. [Google Scholar] [CrossRef] [PubMed]
- McLeish, M.J.; Fraile, A.; García-Arenal, F. Ecological Complexity in Plant Virus Host Range Evolution. Adv. Virus Res. 2018, 101, 293–339. [Google Scholar] [PubMed]
- Carrillo-Hernández, M.Y.; Ruiz-Saenz, J.; Villamizar, L.J.; Gómez-Rangel, S.Y.; Martínez-Gutierrez, M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect. Dis. 2018, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Kazazian, L.; Lima Neto, A.S.; Sousa, G.S.; Do Nascimento, O.J.; Castro, M.C. Spatiotemporal transmission dynamics of co-circulating dengue, zika, and chikungunya viruses in Fortaleza, Brazil: 2011–2017. PLoS Negl. Trop. Dis. 2020, 14, e0008760. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Rückert, C.; Cavany, S.M.; Perkins, T.A.; Ebel, G.D.; Grubaugh, N.D. Arbovirus coinfection and co-transmission: A neglected public health concern? PLoS Biol. 2019, 17, e3000130. [Google Scholar] [CrossRef]
- Juárez, M.; Legua, P.; Mengual, C.M.; Kassem, M.A.; Sempere, R.N.; Gómez, P.; Truniger, V.; Aranda, M.A. Relative incidence, spatial distribution and genetic diversity of cucurbit viruses in eastern Spain. Ann. Appl. Biol. 2013, 162, 362–370. [Google Scholar] [CrossRef]
- Kassem, M.A.; Sempere, R.N.; Juárez, M.; Aranda, M.A.; Truniger, V. Cucurbit aphid-borne yellows virus Is Prevalent in Field-Grown Cucurbit Crops of Southeastern Spain. Plant Dis. 2007, 91, 232–238. [Google Scholar] [CrossRef]
- De Moya-Ruiz, C.; Rabadán, P.; Juárez, M.; Gómez, P. Assessment of the Current Status of Potyviruses in Watermelon and Pumpkin Crops in Spain: Epidemiological Impact of Cultivated Plants and Mixed Infections. Plants 2021, 10, 138. [Google Scholar] [CrossRef]
- Tamborindeguy, C.; Hata, F.T.; Molina, R.d.O.; Nunes, W.M.d.C. A New Perspective on the Co-Transmission of Plant Pathogens by Hemipterans. Microorganisms 2023, 11, 156. [Google Scholar] [CrossRef]
- Allen, L.J.S.; Bokil, V.A.; Cunniffe, N.J.; Hamelin, F.M.; Hilker, F.M.; Jeger, M.J. Modelling vector transmission and epidemiology of co-infecting plant viruses. Viruses 2019, 11, 1153. [Google Scholar] [CrossRef]
- Srinivasan, R.; Hall, D.G.; Cervantes, F.A.; Alvarez, J.M.; Whitworth, J.L. Strain specificity and simultaneous transmission of closely related strains of a potyvirus by Myzus persicae. J. Econ. Entomol. 2012, 105, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Gray, S.M. Sequential acquisition of Potato virus Y strains by Myzus persicae favors the transmission of the emerging recombinant strains. Virus Res. 2017, 241, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Lin, Y.H.; Carroll, J.E.; Wenninger, E.J.; Bosque-Pérez, N.A.; Whitworth, J.L.; Hutchinson, P.; Eigenbrode, S.; Gray, S.M. Potato virus y transmission efficiency from potato infected with single or multiple virus strains. Phytopathology 2017, 107, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, M.J.; Hong, J.S.; Choi, J.K.; Ryu, K.H. Patterns in disease progress and the influence of single and multiple viral infections on pepper (Capsicum annuum L.) growth. Eur. J. Plant Pathol. 2010, 127, 53–61. [Google Scholar] [CrossRef]
- Mascia, T.; Cillo, F.; Fanelli, V.; Finetti-Sialer, M.M.; De Stradis, A.; Palukaitis, P.; Gallitelli, D. Characterization of the Interactions Between Cucumber mosaic virus and Potato virus Y in Mixed Infections in Tomato. Mol. Plant-Microbe Interact. 2010, 23, 1514–1524. [Google Scholar] [CrossRef]
- Borgolte, S.; Varrelmann, M.; Hossain, R. Time point of virus yellows infection is crucial for yield losses in sugar beet, and co-infection with beet mosaic virus is negligible under field conditions. Plant Pathol. 2024, 73, 2056–2070. [Google Scholar] [CrossRef]
- Wintermantel, W.M. Co-infection of Beet mosaic virus with Beet Yellowing Viruses Leads to Increased Symptom Expression on Sugar Beet. Plant Dis. 2005, 89, 325–331. [Google Scholar] [CrossRef]
- Anderson, E.J.; Kline, A.S.; Morelock, T.E.; McNew, R.W. Tolerance to blackeye cowpea mosaic potyvirus not correlated with decreased virus accumulation or protection from cowpea stunt disease. Plant Dis. 1996, 80, 847–852. [Google Scholar] [CrossRef]
- Ribeiro, G.P. Cowpea Stunt: A Disease Caused by a Synergistic Interaction of Two Viruses. Phytopathology 1978, 68, 1260. [Google Scholar] [CrossRef]
- Ryang, B.S.; Kobori, T.; Matsumoto, T.; Kosaka, Y.; Ohki, S.T. Cucumber mosaic virus 2b protein compensates for restricted systemic spread of Potato virus Y in doubly infected tobacco. J. Gen. Virol. 2004, 85, 3405–3414. [Google Scholar] [CrossRef]
- Takeshita, M.; Koizumi, E.; Noguchi, M.; Sueda, K.; Shimura, H.; Ishikawa, N.; Matsuura, H.; Ohshima, K.; Natsuaki, T.; Kuwata, S.; et al. Infection dynamics in viral spread and interference under the synergism between Cucumber mosaic virus and Turnip mosaic virus. Mol. Plant-Microbe Interact. 2012, 25, 18–27. [Google Scholar] [CrossRef]
- Murphy, J.F.; Bowen, K.L. Synergistic disease in pepper caused by the mixed infection of Cucumber mosaic virus and Pepper mottle virus. Phytopathology 2006, 96, 240–247. [Google Scholar] [CrossRef]
- Wang, Y.; Gaba, V.; Yang, J.; Palukaitis, P.; Gal-On, A. Characterization of synergy between Cucumber mosaic virus and potyviruses in cucurbit hosts. Phytopathology 2002, 92, 51–58. [Google Scholar] [CrossRef]
- Zeng, R.; Liao, Q.; Feng, J.; Li, D.; Chen, J. Synergy between cucumber mosaic virus and zucchini yellow mosaic virus on cucurbitaceae hosts tested by real-time reverse transcription-polymerase chain reaction. Acta Biochim. Biophys. Sin. 2007, 39, 431–437. [Google Scholar] [CrossRef]
- Choi, S.K.; Yoon, J.Y.; Ryu, K.H.; Choi, J.K.; Palukaitis, P.; Park, W.M. Systemic movement of a movement-deficient strain of Cucumber mosaic virus in zucchini squash is facilitated by a cucurbit-infecting potyvirus. J. Gen. Virol. 2002, 83, 3173–3178. [Google Scholar] [CrossRef]
- Jiménez, J.; Sadras, V.O.; Espaillat, N.; Moreno, A.; Fereres, A. Interplay between drought and plant viruses co-infecting melon plants. Sci. Rep. 2024, 14, 15833. [Google Scholar] [CrossRef]
- Martín, S.; Elena, S.F. Application of game theory to the interaction between plant viruses during mixed infections. J. Gen. Virol. 2009, 90, 2815–2820. [Google Scholar] [CrossRef]
- Pruss, G.; Ge, X.; Shi, X.M.; Carrington, J.C.; Vance, V.B. Plant viral synergism: The potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 1997, 9, 859–868. [Google Scholar] [CrossRef]
- Srinivasan, R.; Alvarez, J.M. Effect of mixed viral infections (potato virus Y-potato leafroll virus) on biology and preference of vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 646–655. [Google Scholar] [CrossRef]
- Barker, H. Specificity of the effect of sap-transmissible viruses in increasing the accumulation of luteoviruses in co-infected plants. Ann. Appl. Biol. 1989, 115, 71–78. [Google Scholar] [CrossRef]
- Erickson, A.; Falk, B.W. Dissecting dynamic plant virus synergism in mixed infections of poleroviruses, umbraviruses, and tombusvirus-like associated RNAs. Front. Microbiol. 2023, 14, 1223265. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Huang, X.; Zhou, G. Impact of two reoviruses and their coinfection on the rice RNAi system and vsiRNA production. Viruses 2018, 10, 594. [Google Scholar] [CrossRef]
- Anjos, J.R.; Jarlfors, U.; Ghabrial, S.A. Soybean Mosaic Potyvirus Enhances the Titer of Two Comoviruses in Dually Infected Soybean Plants. Phytopathology 1992, 82, 1022. [Google Scholar] [CrossRef]
- García-Cano, E.; Resende, R.O.; Fernández-Muñoz, R.; Moriones, E. Synergistic interaction between Tomato chlorosis virus and Tomato spotted wilt virus results in breakdown of resistance in tomato. Phytopathology 2006, 96, 1263–1269. [Google Scholar] [CrossRef]
- Ajjikuttira, P.; Loh, C.-S.; Wong, S.-M. Reciprocal function of movement proteins and complementation of long-distance movement of Cymbidium mosaic virus RNA by Odontoglossum ringspot virus coat protein. J. Gen. Virol. 2005, 86, 1543–1553. [Google Scholar] [CrossRef]
- Chávez-Calvillo, G.; Contreras-Paredes, C.A.; Mora-Macias, J.; Noa-Carrazana, J.C.; Serrano-Rubio, A.A.; Dinkova, T.D.; Carrillo-Tripp, M.; Silva-Rosales, L. Antagonism or synergism between papaya ringspot virus and papaya mosaic virus in Carica papaya is determined by their order of infection. Virology 2016, 489, 179–191. [Google Scholar] [CrossRef]
- Aguilar, I.; Sánchez, F.; Ponz, F. Different forms of interference between two tobamoviruses in two different hosts. Plant Pathol. 2000, 49, 659–665. [Google Scholar] [CrossRef]
- Alcaide, C.; Sardanyés, J.; Elena, S.F.; Gómez, P. Increasing temperature alters the within-host competition of viral strains and influences virus genetic variability. Virus Evol. 2021, 7, veab017. [Google Scholar] [CrossRef]
- Vargas-Mejía, P.; Vega-Arreguín, J.; Chávez-Calvillo, G.; Ibarra-Laclette, E.; Silva-Rosales, L. Differential Accumulation of Innate- and Adaptive-Immune-Response-Derived Transcripts during Antagonism between Papaya Ringspot Virus and Papaya Mosaic Virus. Viruses 2020, 12, 230. [Google Scholar] [CrossRef]
- De Moya-Ruiz, C.; Gómez, P.; Juárez, M. Occurrence, Distribution, and Management of Aphid-Transmitted Viruses in Cucurbits in Spain. Pathogens 2023, 12, 422. [Google Scholar] [CrossRef]
- Agüero, J.; Gómez-Aix, C.; Sempere, R.N.; García-Villalba, J.; García-Núñez, J.; Hernando, Y.; Aranda, M.A. Stable and broad spectrum cross-protection against pepino mosaic virus attained by mixed infection. Front. Plant Sci. 2018, 9, 1810. [Google Scholar] [CrossRef]
- Wang, H.L. Effectiveness of Cross Protection by a Mild Strain of Zucchini Yellow Mosaic Virus in Cucumber, Melon, and Squash. Plant Dis. 1991, 75, 203. [Google Scholar] [CrossRef]
- Ziebell, H.; Carr, J.P. Cross-Protection. In Natural and Engineered Resistance to Plant Viruses, Part B; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 76, pp. 211–264. [Google Scholar]
- Gal-On, A.; Shiboleth, Y.M. Cross-Protection. In Natural Resistance Mechanisms of Plants to Viruses; Loebenstein, G., Carr, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 261–288. ISBN 978-1-4020-3780-1. [Google Scholar]
- Lecoq, H. Control of Zucchini Yellow Mosaic Virus in Squash by Cross Protection. Plant Dis. 1991, 75, 208. [Google Scholar] [CrossRef]
- Khanal, V.; Wells, H.; Ali, A. High Prevalence of Three Potyviruses Infecting Cucurbits in Oklahoma and Phylogenetic Analysis of Cucurbit Aphid-Borne Yellows Virus Isolated from Pumpkins. Pathogens 2021, 10, 53. [Google Scholar] [CrossRef]
- Francis, F.; Chen, J.; Yong, L.; Bosquee, E. Aphid Feeding on Plant Lectins Falling Virus Transmission Rates: A Multicase Study. J. Econ. Entomol. 2020, 113, 1635–1639. [Google Scholar] [CrossRef]
- Medina-Ramos, G.; De La Torre-Almaráz, R.; Bujanos-Muñiz, R.; Guevara-González, R.G.; Tienanegra-García, N.; Guevara-Olvera, L.; González Chavira, M.M.; Torres-Pacheco, I. Co-transmission of Pepper huasteco yellow vein virus and Pepper golden mosaic virus in chili pepper by Bemisia tabaci (Genn.). J. Entomol. 2008, 5, 176–184. [Google Scholar] [CrossRef]
- Ohnishi, J.; Kitamura, T.; Terami, F.; Honda, K. Co-transmission of Tomato yellow leaf curl virus (TYLCV)-Mld and TYLCV-IL by the whitefly Bemisia tabaci. J. Gen. Plant Pathol. 2011, 77, 54–59. [Google Scholar] [CrossRef]
- Domingo-Calap, M.L.; Moreno, A.B.; Pendón, J.A.D.; Moreno, A.; Fereres, A.; López-Moya, J.J. Assessing the impact on virus transmission and insect vector behavior of a viral mixed infection in melon. Phytopathology 2020, 110, 174–186. [Google Scholar] [CrossRef]
- McLaughlin, A.A.; Hanley-Bowdoin, L.; Kennedy, G.G.; Jacobson, A.L. Vector acquisition and co-inoculation of two plant viruses influences transmission, infection, and replication in new hosts. Sci. Rep. 2022, 12, 20355. [Google Scholar] [CrossRef]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Virus–virus interactions in a plant host and in a hemipteran vector: Implications for vector fitness and virus epidemics. Virus Res. 2020, 286, 198069. [Google Scholar] [CrossRef]
- Hossain, R.; Willems, G.; Wynant, N.; Borgolte, S.; Govaerts, K.; Varrelmann, M. Aphid-mediated beet yellows virus transmission initiates proviral gene deregulation in sugar beet at early stages of infection. PLoS ONE 2024, 19, e0311368. [Google Scholar] [CrossRef]
- Bello, V.H.; Ghosh, S.; Krause-Sakate, R.; Ghanim, M. Competitive Interactions Between Whitefly and Aphid Transmitted Poleroviruses within the Plant Host and the Insect Vectors. Phytopathology® 2021, 111, 1042–1050. [Google Scholar] [CrossRef]
- Cunniffe, N.J.; Taylor, N.P.; Hamelin, F.M.; Jeger, M.J. Epidemiological and ecological consequences of virus manipulation of host and vector in plant virus transmission. PLoS Comput. Biol. 2021, 17, e1009759. [Google Scholar] [CrossRef]
- Kazinczi, G.; Horváth, J.; Takács, A.P.; Gáborjányi, R.; Béres, I. Susceptibility of some weed species to Pepino mosaic virus (PepMV). Commun. Agric. Appl. Biol. Sci. 2005, 69, 53–60. [Google Scholar]
- Chen, G.; Pan, H.; Xie, W.; Wang, S.; Wu, Q.; Fang, Y.; Shi, X.; Zhang, Y. Virus infection of a weed increases vector attraction to and vector fitness on the weed. Sci. Rep. 2013, 3, 3–8. [Google Scholar] [CrossRef]
- Szabó, A.K.; Várallyay, É.; Demian, E.; Hegyi, A.; Galbács, Z.N.; Kiss, J.; Bálint, J.; Loxdale, H.D.; Balog, A. Local Aphid Species Infestation on Invasive Weeds Affects Virus Infection of Nearest Crops Under Different Management Systems—A Preliminary Study. Front. Plant Sci. 2020, 11, 684. [Google Scholar] [CrossRef]
- Srinivasan, R.; Riley, D.; Diffie, S.; Shrestha, A.; Culbreath, A. Winter weeds as inoculum sources of tomato spotted wilt virus and as reservoirs for its vector, frankliniella fusca (Thysanoptera: Thripidae) in farmscapes of Georgia. Environ. Entomol. 2014, 43, 410–420. [Google Scholar] [CrossRef]
- Prendeville, H.R.; Ye, X.; Jack Morris, T.; Pilson, D. Virus infections in wild plant populations are both frequent and often unapparent. Am. J. Bot. 2012, 99, 1033–1042. [Google Scholar] [CrossRef]
- Malmstrom, C.M.; Melcher, U.; Bosque-Perez, N.A.; Bosque-Pérez, N.A. The expanding field of plant virus ecology: Historical foundations, knowledge gaps, and research directions. Virus Res. 2011, 159, 84–94. [Google Scholar] [CrossRef]
- Wyant, P.S.; Strohmeier, S.; Schäfer, B.; Krenz, B.; Assunção, I.P.; Lima, G.S.d.A.; Jeske, H. Circular DNA genomics (circomics) exemplified for geminiviruses in bean crops and weeds of northeastern Brazil. Virology 2012, 427, 151–157. [Google Scholar] [CrossRef]
- Vincent, S.J.; Coutts, B.A.; Jones, R.A.C. Effects of introduced and indigenous viruses on native plants: Exploring their disease causing potential at the agro-ecological interface. PLoS ONE 2014, 9, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.; Rabadán, M.P.; Martínez, L.D.; Tayahi, M.; Grande-Pérez, A.; Gómez, P. Natural Hosts and Genetic Diversity of the Emerging Tomato Leaf Curl New Delhi Virus in Spain. Front. Microbiol. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
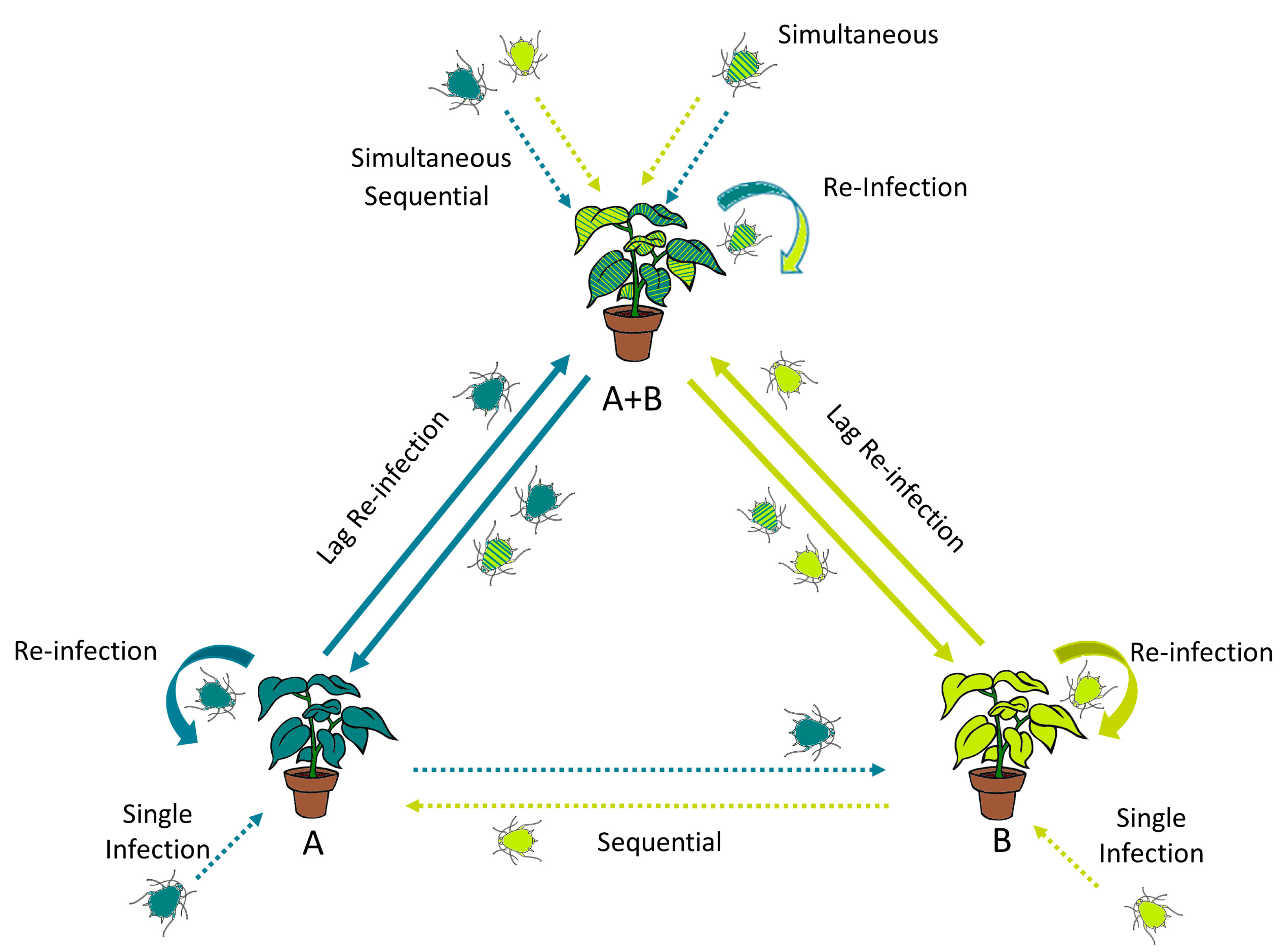
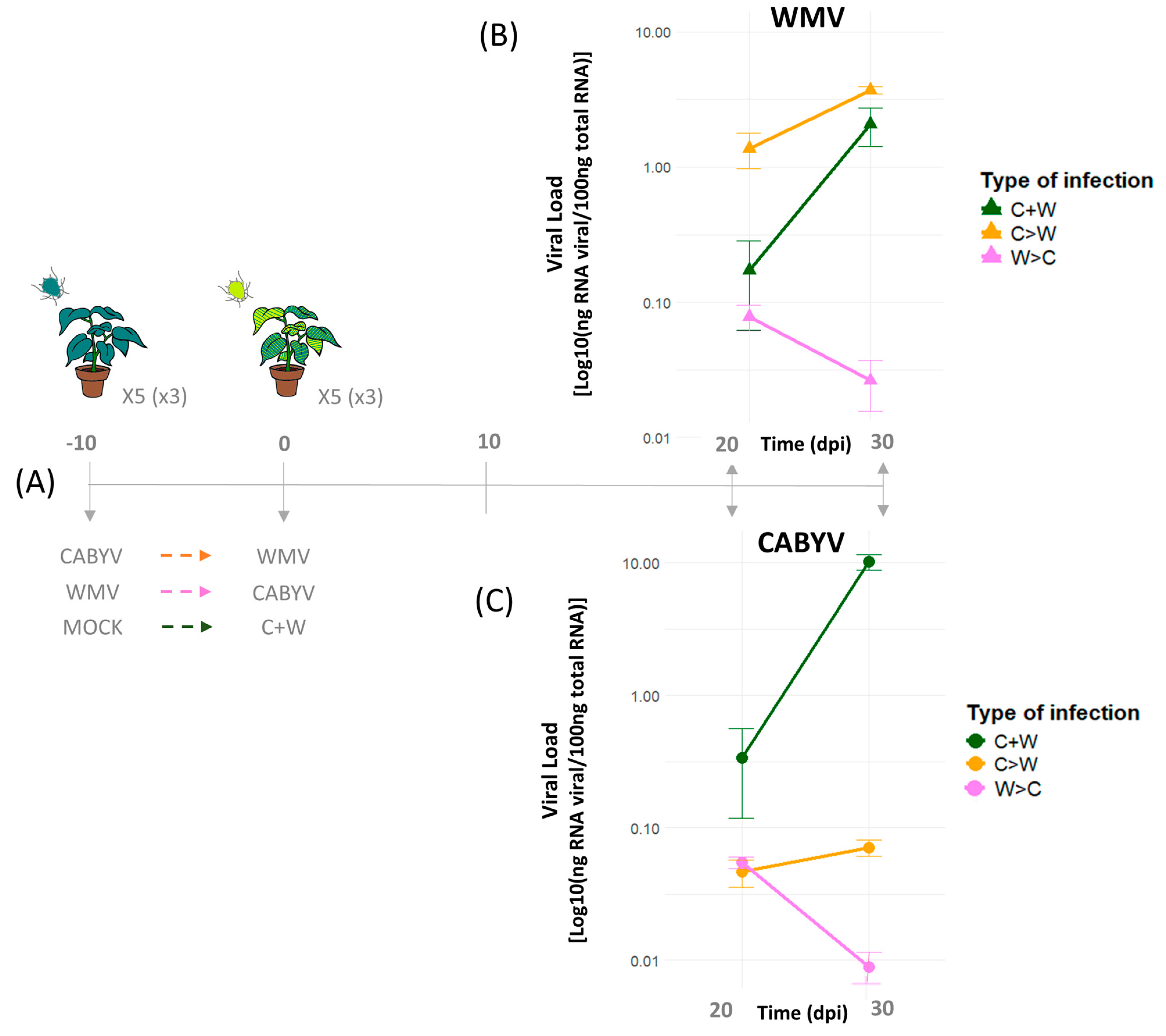
| Infection Type | Genus | Virus | Host | Interaction | Viral Trait | Cite |
|---|---|---|---|---|---|---|
| Sequential | Potyvirus */Cucumovirus * | PVY/CMV | Tomato | Synergism | Viral titer and symptoms | [56] |
| Potyvirus */Polerovirus ▫ | BTMV/BChV | Sugar beet | Neutral | Symptoms | [57] | |
| Potyvirus */Polerovirus ▫ | BTMV/BMYV | Sugar beet | Synergism | Symptoms | [57] | |
| Sequential and simultaneous | Potyvirus */Polerovirus ▫ | WMV/CABYV | Melon | Synergism | Viral titer | This study |
| Potyvirus */Closterovirus † | BTMV/BYV | Sugar beet | Neutral/Synergism | Symptoms and viral titer | [57,58] | |
| Cucumovirus */Potyvirus * | CMV/PepMoV | Pepper | Synergism | Viral titer and symptoms | [55] | |
| Simultaneous | Cucumovirus */Potyvirus * | CMV/BICMV 1 | Cowpea | Synergism | Viral titer and symptoms | [59,60] |
| Cucumovirus */Potyvirus * | CMV/PVY | Tobacco | Synergism | Viral titer and symptoms | [61] | |
| Cucumovirus */Potyvirus * | CMV/TuMV 2 | Nicotiana benthamiana | Synergism | Symptoms | [62] | |
| Cucumovirus */Potyvirus * | CMV/PepMoV | Pepper | Synergism | Viral titer and symptoms | [63] | |
| Cucumovirus */Potyvirus * | CMV/WMV | Zucchini squash and melon | Synergism | Viral titer and symptoms | [64] | |
| Cucumovirus */Potyvirus * | CMV/ZYMV | Bottle gourd, Zucchini squash and melon | Synergism | Viral titer and symptoms | [64,65,66] | |
| Cucumovirus */Polerovirus ▫ | CMV/CABYV | Melon | Synergism | Symptoms | [67] | |
| Potyvirus/Caulimovirus † | TuMV/CaMV 3 | Arabidopsis thaliana | Neutral | Viral titer and symptoms | [68] | |
| Potyvirus */Cucumovirus * | TEV 4/CMV | N. benthamiana | Synergism | Viral titer and symptoms | [69] | |
| Potyvirus */Polerovirus ▫ | PVY/PLRV 5 | Potato | Synergism | Viral titer and symptoms | [70] | |
| Potyvirus */Polerovirus ▫ | BTMV/BWYV 6 | Sugar beet | Synergism | Symptoms and viral titer | [58] | |
| Potyvirus */Polerovirus ▫ | PVY/PLRV | N. clevelandii | Synergism | Viral titer | [71] | |
| Polerovirus */Umbravirus ▫ | TuYV 7/CMoV 8 | N. benthamiana | Synergism | Viral titer | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya-Ruiz, C.d.; Ferriol, I.; Gómez, P. The Temporal Order of Mixed Viral Infections Matters: Common Events That Are Neglected in Plant Viral Diseases. Viruses 2024, 16, 1954. https://doi.org/10.3390/v16121954
Moya-Ruiz Cd, Ferriol I, Gómez P. The Temporal Order of Mixed Viral Infections Matters: Common Events That Are Neglected in Plant Viral Diseases. Viruses. 2024; 16(12):1954. https://doi.org/10.3390/v16121954
Chicago/Turabian StyleMoya-Ruiz, Celia de, Inmaculada Ferriol, and Pedro Gómez. 2024. "The Temporal Order of Mixed Viral Infections Matters: Common Events That Are Neglected in Plant Viral Diseases" Viruses 16, no. 12: 1954. https://doi.org/10.3390/v16121954
APA StyleMoya-Ruiz, C. d., Ferriol, I., & Gómez, P. (2024). The Temporal Order of Mixed Viral Infections Matters: Common Events That Are Neglected in Plant Viral Diseases. Viruses, 16(12), 1954. https://doi.org/10.3390/v16121954







