Quantitation of Enterovirus A71 Empty and Full Particles by Sedimentation Velocity Analytical Ultracentrifugation
Abstract
:1. Introduction
2. Materials and Methods
2.1. EV71 EP and FP Production and Purification
2.2. Purification of EV71 Vaccine Bulks
2.3. Characterization of EV71 EPs and FPs
2.4. Sample Preparation and SV-AUC Data Acquisition
2.5. SV-AUC Data Analysis
2.6. Statistical Analysis
3. Results
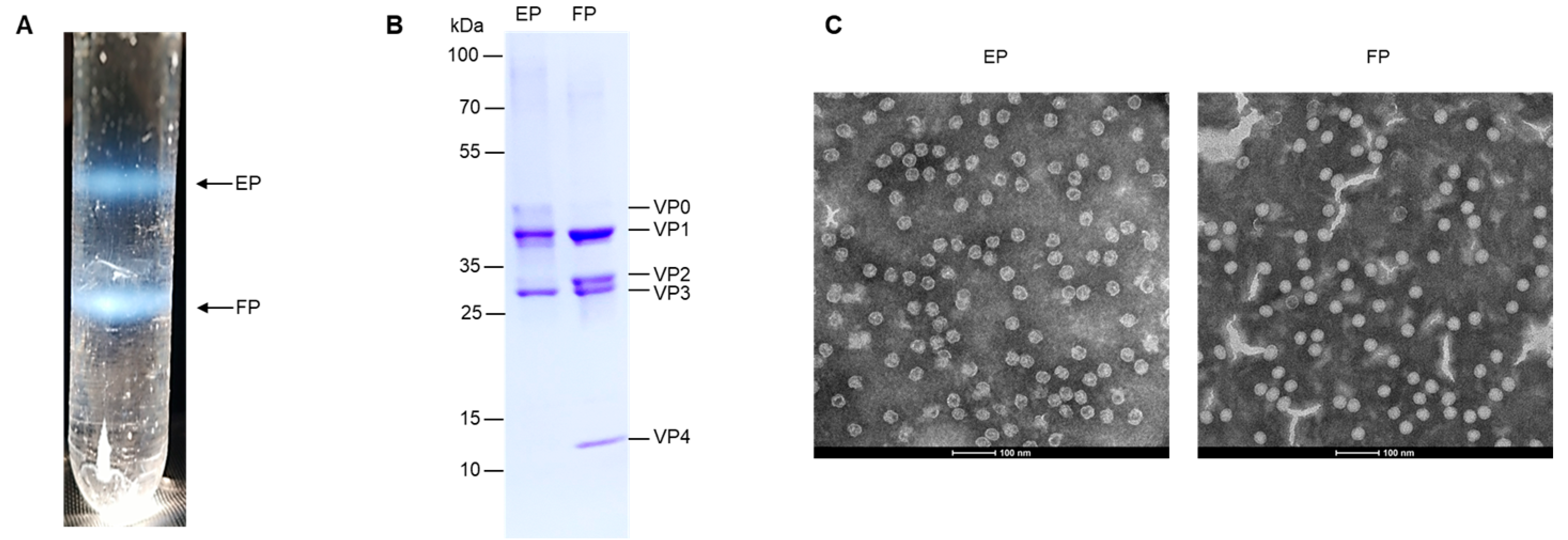
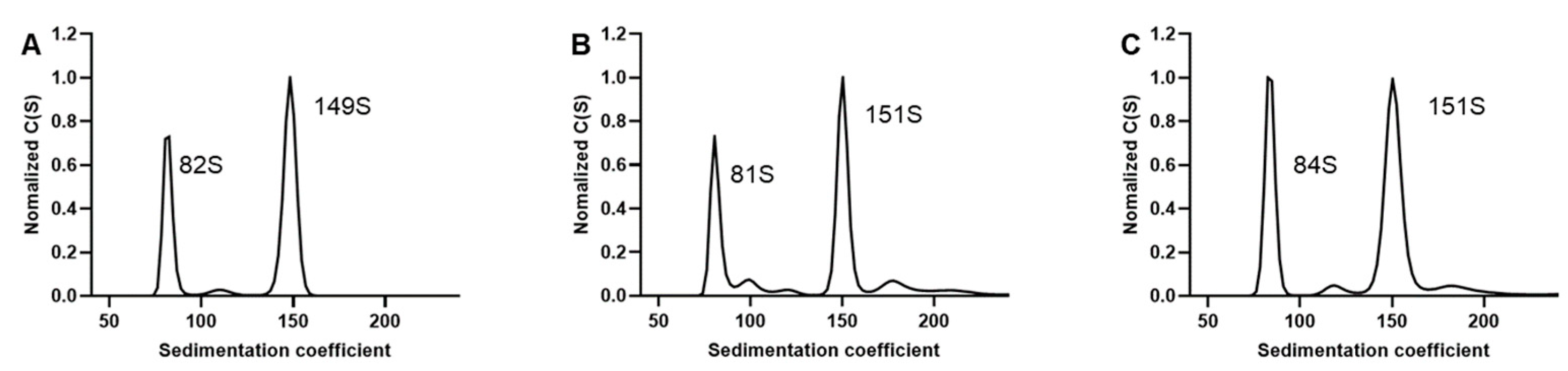
3.1. Preparation and Characterization of EV71 EPs and FPs
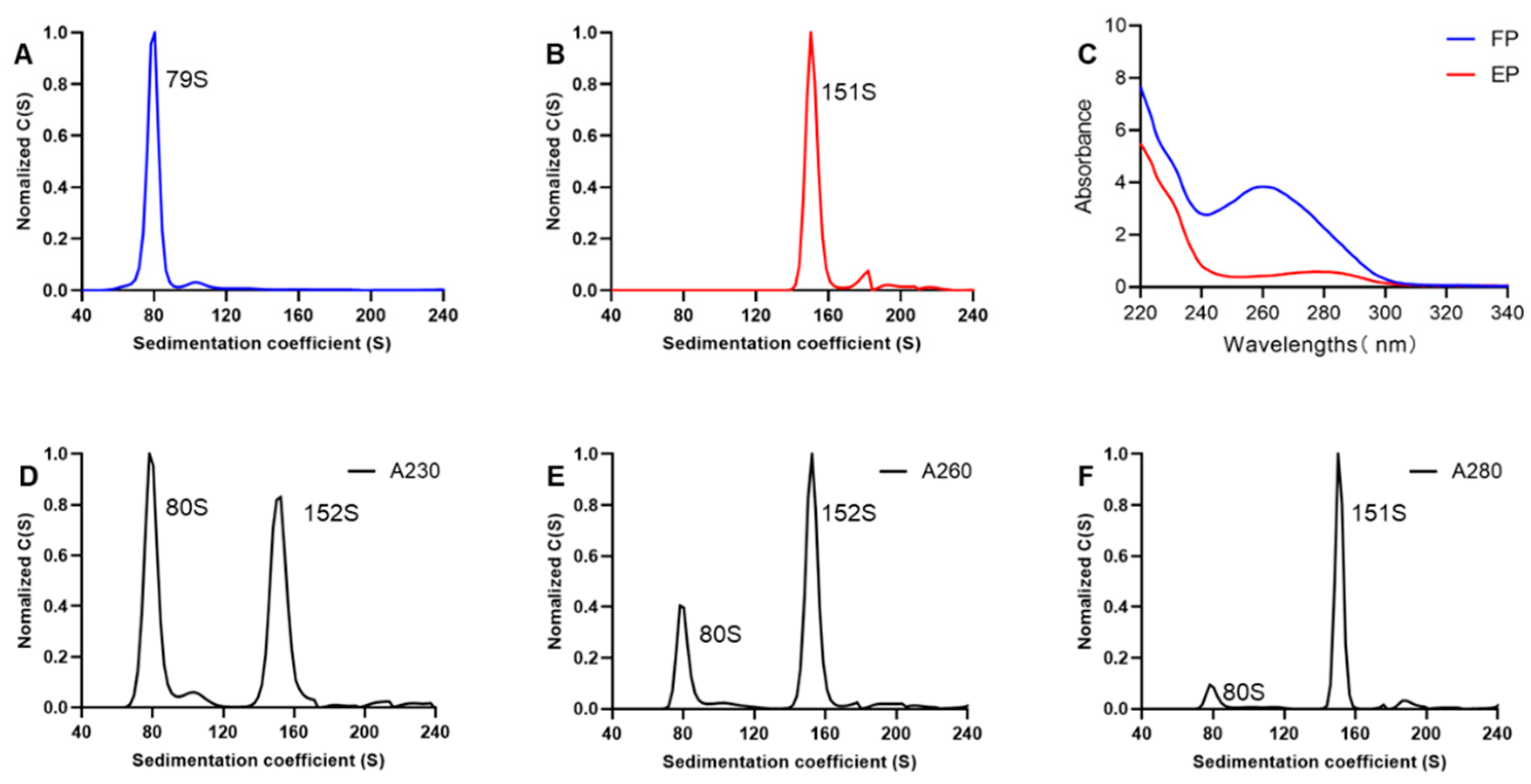
3.2. SV-AUC for EV71 EPs and FPs
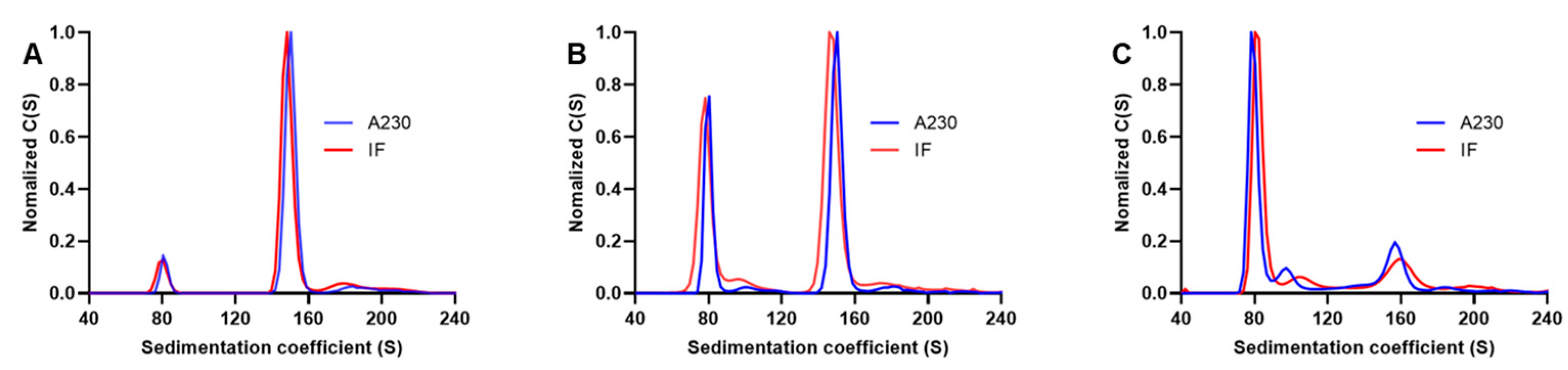
3.3. Comparison of Interference and Ultraviolet Detection
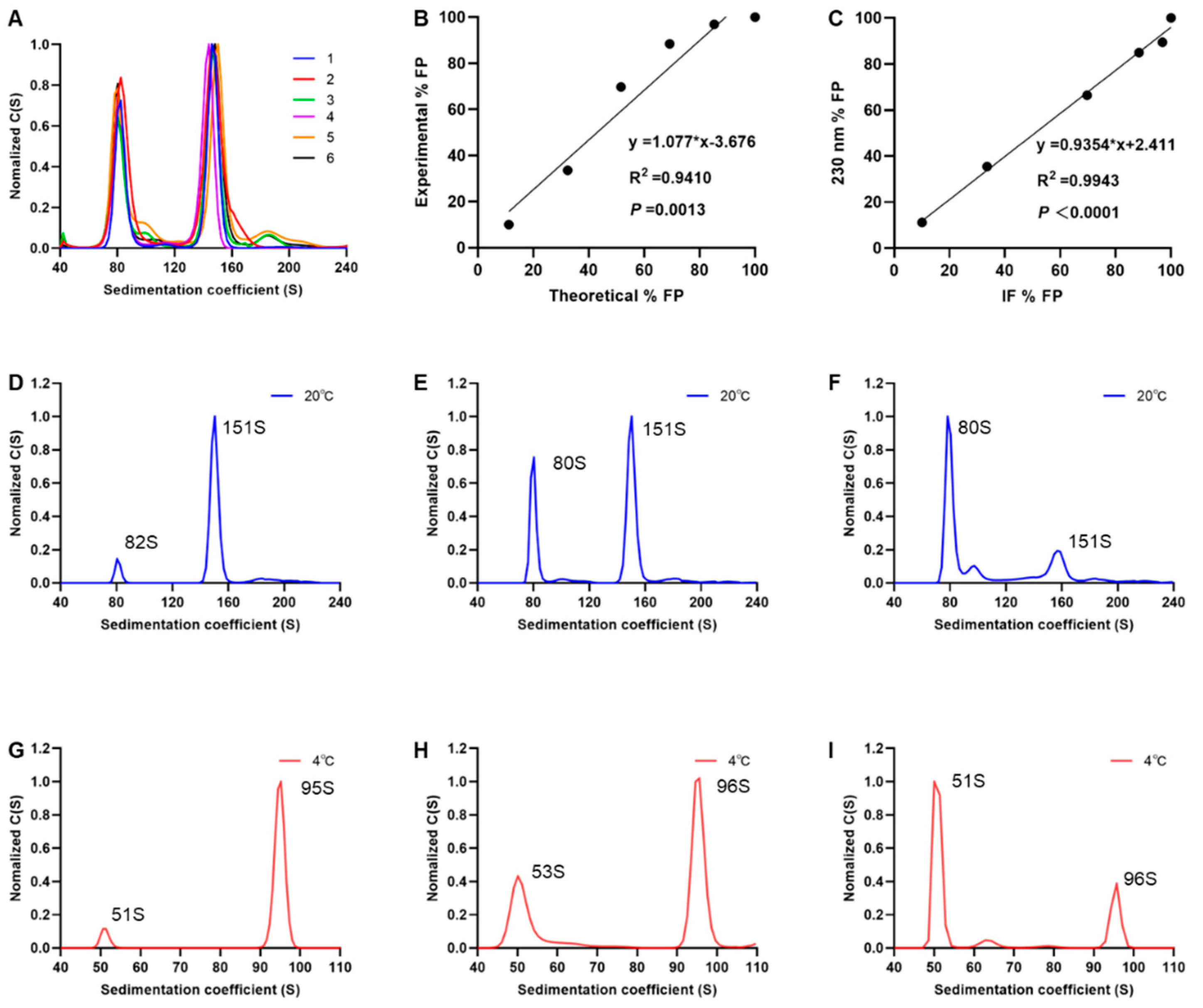
3.4. Performance of SV-AUC for Quantifying EV71 EPs and FPs
3.5. SV-AUC Analysis of Multiple Batches of EV71 Vaccine Bulks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, C.Y.; Liu, C.C. Novel strategies for the development of hand, foot, and mouth disease vaccines and antiviral therapies. Expert Opin. Drug Discov. 2022, 17, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Min, N.; Ong, Y.H.B.; Han, A.X.; Ho, S.X.; Yen, E.W.P.; Ban, K.H.K.; Maurer-Stroh, S.; Chong, C.Y.; Chu, J.J.H. An epidemiological surveillance of hand foot and mouth disease in paediatric patients and in community: A Singapore retrospective cohort study, 2013–2018. PLoS Negl. Trop. Dis. 2021, 15, e0008885. [Google Scholar] [CrossRef]
- Wu, Y.; Yeo, A.; Phoon, M.C.; Tan, E.L.; Poh, C.L.; Quak, S.H.; Chow, V.T. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: The role of enterovirus 71 and coxsackievirus A strains. Int. J. Infect. Dis. 2010, 14, e1076–e1081. [Google Scholar] [CrossRef]
- Alexander, J.P., Jr.; Baden, L.; Pallansch, M.A.; Anderson, L.J. Enterovirus 71 infections and neurologic disease—United States, 1977–1991. J. Infect. Dis. 1994, 169, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.P.; Goh, K.T.; Chong, C.Y.; Teo, E.S.; Lau, G.; Ling, A.E. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg. Infect. Dis. 2003, 9, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Chang, H.L.; Wang, S.T.; Cheng, Y.T.; Yang, J.Y. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics 2007, 120, e244–e252. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.J.; Lennette, E.H.; Ho, H.H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974, 129, 304–309. [Google Scholar] [CrossRef]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Pu, D.; Mo, X.; Zhu, C.; Gong, S.; Xu, Y.; Lin, G.; Wu, B.; He, S.; Jiao, X.; et al. Children of rural-to-urban migrant workers in China are at a higher risk of contracting severe hand, foot and mouth disease and EV71 infection: A hospital-based study. Emerg. Microbes Infect. 2013, 2, e72. [Google Scholar] [CrossRef]
- Li, J.X.; Song, Y.F.; Wang, L.; Zhang, X.F.; Hu, Y.S.; Hu, Y.M.; Xia, J.L.; Li, J.; Zhu, F.C. Two-year efficacy and immunogenicity of Sinovac Enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev. Vaccines 2016, 15, 129–137. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Mo, Z.; Wang, X.; Xia, J.; Liang, Z.; Zhang, Y.; Li, Y.; Mao, Q.; Wang, J.; et al. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014, 370, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Y.; Wang, Y.; Bian, L.; Xu, M.; Liang, Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev. Vaccines 2016, 15, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Guo, X.; Zhang, M.; Chen, Y.; Zhang, Y.; Yu, X.; Luo, L.; Chen, H.; Xu, W.; Liu, H.; et al. Safety and Immunogenicity of Enterovirus 71 Vaccine (Vero Cell) Administered Simultaneously with Trivalent Split-Virion Influenza Vaccine in Infants Aged 6-7 Months: A Phase 4, Randomized, Controlled Trial. Vaccines 2023, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.C.; Meng, F.Y.; Li, J.X.; Li, X.L.; Mao, Q.Y.; Tao, H.; Zhang, Y.T.; Yao, X.; Chu, K.; Chen, Q.H.; et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013, 381, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Pallansch, M.A. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 1995, 39, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, B. Universal PCR Primers Are Critical for Direct Sequencing-Based Enterovirus Genotyping. J. Clin. Microbiol. 2017, 55, 339–340. [Google Scholar] [CrossRef]
- Liu, B.M.; Mulkey, S.B.; Campos, J.M.; DeBiasi, R.L. Laboratory diagnosis of CNS infections in children due to emerging and re-emerging neurotropic viruses. Pediatr. Res. 2024, 95, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Shingler, K.L.; Yoder, J.L.; Carnegie, M.S.; Ashley, R.E.; Makhov, A.M.; Conway, J.F.; Hafenstein, S. The enterovirus 71 A-particle forms a gateway to allow genome release: A cryoEM study of picornavirus uncoating. PLoS Pathog. 2013, 9, e1003240. [Google Scholar] [CrossRef]
- Wang, X.; Peng, W.; Ren, J.; Hu, Z.; Xu, J.; Lou, Z.; Li, X.; Yin, W.; Shen, X.; Porta, C.; et al. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat. Struct. Mol. Biol. 2012, 19, 424–429. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, Q.; Li, S.; He, M.; Wu, Y.; Li, Y.; Zhu, R.; Yu, H.; Hong, Q.; Jiang, J.; et al. Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody. Nat. Commun. 2017, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, H.; Qu, M.; Hou, A.; Zhou, Y.; Sun, B.; Cai, L.; Gao, F.; Su, W.; Jiang, C. Determination of the cleavage site of enterovirus 71 VP0 and the effect of this cleavage on viral infectivity and assembly. Microb. Pathog. 2019, 134, 103568. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yu, S.L.; Chen, Y.T.; Chen, Y.H.; Hsiao, P.W.; Chow, Y.H.; Chen, J.R. The mature EV71 virion induced a broadly cross-neutralizing VP1 antibody against subtypes of the EV71 virus. PLoS ONE 2019, 14, e0210553. [Google Scholar] [CrossRef] [PubMed]
- Tedcastle, A.; Mao, Q.; Hockley, J.; Pegg, E.; EV71 Study Group; Gao, F.; Liang, Z.; Matejtschuk, P.; Duru, C.; Minor, P.; et al. Report on the WHO Collaborative Study to Establish the 1st International Standard for Enterovirus A71 Inactivated Vaccine; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Sawyer, L.A.; McInnis, J.; Albrecht, P. Quantitation of D antigen content in inactivated poliovirus vaccine derived from wild-type or sabin strains. Biologicals 1993, 21, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kouiavskaia, D.; Puligedda, R.D.; Dessain, S.K.; Chumakov, K. Universal ELISA for quantification of D-antigen in inactivated poliovirus vaccines. J. Virol. Methods 2020, 276, 113785. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.M.; Smith, P.H.; Parthasarathy, S.; Isaacs, J.; Vijay, S.; Kieran, J.; Powell, S.K.; McClelland, A.; Wright, J.F. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 2003, 7, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lock, M.; Alvira, M.R.; Wilson, J.M. Analysis of particle content of recombinant adeno-associated virus serotype 8 vectors by ion-exchange chromatography. Hum. Gene Ther. Methods 2012, 23, 56–64. [Google Scholar] [CrossRef]
- Burnham, B.; Nass, S.; Kong, E.; Mattingly, M.; Woodcock, D.; Song, A.; Wadsworth, S.; Cheng, S.H.; Scaria, A.; O’Riordan, C.R. Analytical Ultracentrifugation as an Approach to Characterize Recombinant Adeno-Associated Viral Vectors. Hum. Gene Ther. Methods 2015, 26, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Pierson, E.E.; Keifer, D.Z.; Asokan, A.; Jarrold, M.F. Resolving Adeno-Associated Viral Particle Diversity with Charge Detection Mass Spectrometry. Anal. Chem. 2016, 88, 6718–6725. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Maurer, A.C.; Bator, C.M.; Makhov, A.M.; Conway, J.F.; Turner, K.B.; Marden, J.H.; Vandenberghe, L.H.; Hafenstein, S.L. Filling Adeno-Associated Virus Capsids: Estimating Success by Cryo-Electron Microscopy. Hum. Gene Ther. 2019, 30, 1449–1460. [Google Scholar] [CrossRef]
- McIntosh, N.L.; Berguig, G.Y.; Karim, O.A.; Cortesio, C.L.; De Angelis, R.; Khan, A.A.; Gold, D.; Maga, J.A.; Bhat, V.S. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021, 11, 3012. [Google Scholar] [CrossRef]
- Khasa, H.; Kilby, G.; Chen, X.; Wang, C. Analytical band centrifugation for the separation and quantification of empty and full AAV particles. Mol. Ther. Methods Clin. Dev. 2021, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Werle, A.K.; Powers, T.W.; Zobel, J.F.; Wappelhorst, C.N.; Jarrold, M.F.; Lyktey, N.A.; Sloan, C.D.K.; Wolf, A.J.; Adams-Hall, S.; Baldus, P.; et al. Comparison of analytical techniques to quantitate the capsid content of adeno-associated viral vectors. Mol. Ther. Methods Clin. Dev. 2021, 23, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Yarawsky, A.E.; Zai-Rose, V.; Cunningham, H.M.; Burgner, J.W., 2nd; DeLion, M.T.; Paul, L.N. AAV analysis by sedimentation velocity analytical ultracentrifugation: Beyond empty and full capsids. Eur. Biophys. J. 2023, 52, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Philo, J.S. Monitoring the homogeneity of adenovirus preparations (a gene therapy delivery system) using analytical ultracentrifugation. Anal. Biochem. 2007, 362, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Agarwala, S.; Ravindran, S.; Vellekamp, G. Determination of particle heterogeneity and stability of recombinant adenovirus by analytical ultracentrifugation in CsCl gradients. J. Pharm. Sci. 2008, 97, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P. Sedimentation analysis of noninteracting and self-associating solutes using numerical solutions to the Lamm equation. Biophys. J. 1998, 75, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Dam, J.; Schuck, P. Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Methods Enzymol. 2004, 384, 185–212. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, B.; Sun, S.; Fu, B.; Liu, C.; Liu, D.; Chu, Y.; Ma, Y.; Bai, L.; Wu, Y.; et al. Characterization of human enterovirus71 virus-like particles used for vaccine antigens. PLoS ONE 2017, 12, e0181182. [Google Scholar] [CrossRef]
- Lien, S.C.; Lu, C.C.; Shen, Y.S.; Yang, Y.T.; Wu, S.R.; Fang, C.Y.; Chow, Y.H.; Liao, C.L.; Chiang, J.R.; Liu, C.C. Separation and purification of highly infectious enterovirus A71 particles using a strong anion-exchange column. J. Chromatogr. A 2022, 1680, 463427. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Forman, M.; Valsamakis, A. Optimization and evaluation of a novel real-time RT-PCR test for detection of parechovirus in cerebrospinal fluid. J. Virol. Methods 2019, 272, 113690. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, A.; Luo, Y.; Yang, J.; Xie, T.; Wang, W.; Wan, X.; Wang, K.; Pang, D.; Yang, D.; Dai, H.; et al. Quantitation of Enterovirus A71 Empty and Full Particles by Sedimentation Velocity Analytical Ultracentrifugation. Viruses 2024, 16, 573. https://doi.org/10.3390/v16040573
Yang A, Luo Y, Yang J, Xie T, Wang W, Wan X, Wang K, Pang D, Yang D, Dai H, et al. Quantitation of Enterovirus A71 Empty and Full Particles by Sedimentation Velocity Analytical Ultracentrifugation. Viruses. 2024; 16(4):573. https://doi.org/10.3390/v16040573
Chicago/Turabian StyleYang, Anna, Yun Luo, Jie Yang, Tingbo Xie, Wenhui Wang, Xin Wan, Kaiwen Wang, Deqin Pang, Dongsheng Yang, Hanyu Dai, and et al. 2024. "Quantitation of Enterovirus A71 Empty and Full Particles by Sedimentation Velocity Analytical Ultracentrifugation" Viruses 16, no. 4: 573. https://doi.org/10.3390/v16040573
APA StyleYang, A., Luo, Y., Yang, J., Xie, T., Wang, W., Wan, X., Wang, K., Pang, D., Yang, D., Dai, H., Wu, J., Meng, S., Guo, J., Wang, Z., & Shen, S. (2024). Quantitation of Enterovirus A71 Empty and Full Particles by Sedimentation Velocity Analytical Ultracentrifugation. Viruses, 16(4), 573. https://doi.org/10.3390/v16040573





