HCV Cascade of Care in HIV/HCV Co-Infected Individuals: Missed Opportunities for Micro-Elimination
Abstract
1. Introduction
2. Methods
- PWH with a positive anti-HCV test.
- Of those with anti-HCV(+), the percentage alive on 1 July 2017.
- Of those alive on 1 July 2017, the percentage retained in care (those disengaged from care were considered individuals who had never started DAAs and had not had a clinic visit up to two years before the clinic-specific database closure date).
- Of those in care, percentage with available data for chronic hepatitis C (CHC) infection (i.e., having initiated any treatment for HCV (DAAs or interferons) and/or having available HCV-RNA tests at/after a positive anti-HCV test and/or being positively genotyped).
- Among those with available data for CHC, the percentage of those with CHC. CHC was defined as initiating any treatment for HCV and/or having a positive HCV-RNA test and/or being positively genotyped. However, individuals who never initiated DAAs and were HCV-RNA-negative at their last HCV-RNA test after their anti-HCV positive test were assumed to have either experienced spontaneous clearance or been successfully treated with interferons. Thus, these individuals were not considered as having CHC.
- Of those with CHC, the percentage initiating DAAs.
- Among those initiating DAAs with available HCV-RNA tests at least 3 months after treatment completion, the percentage with SVR.
3. Results
3.1. Participants’ Characteristics
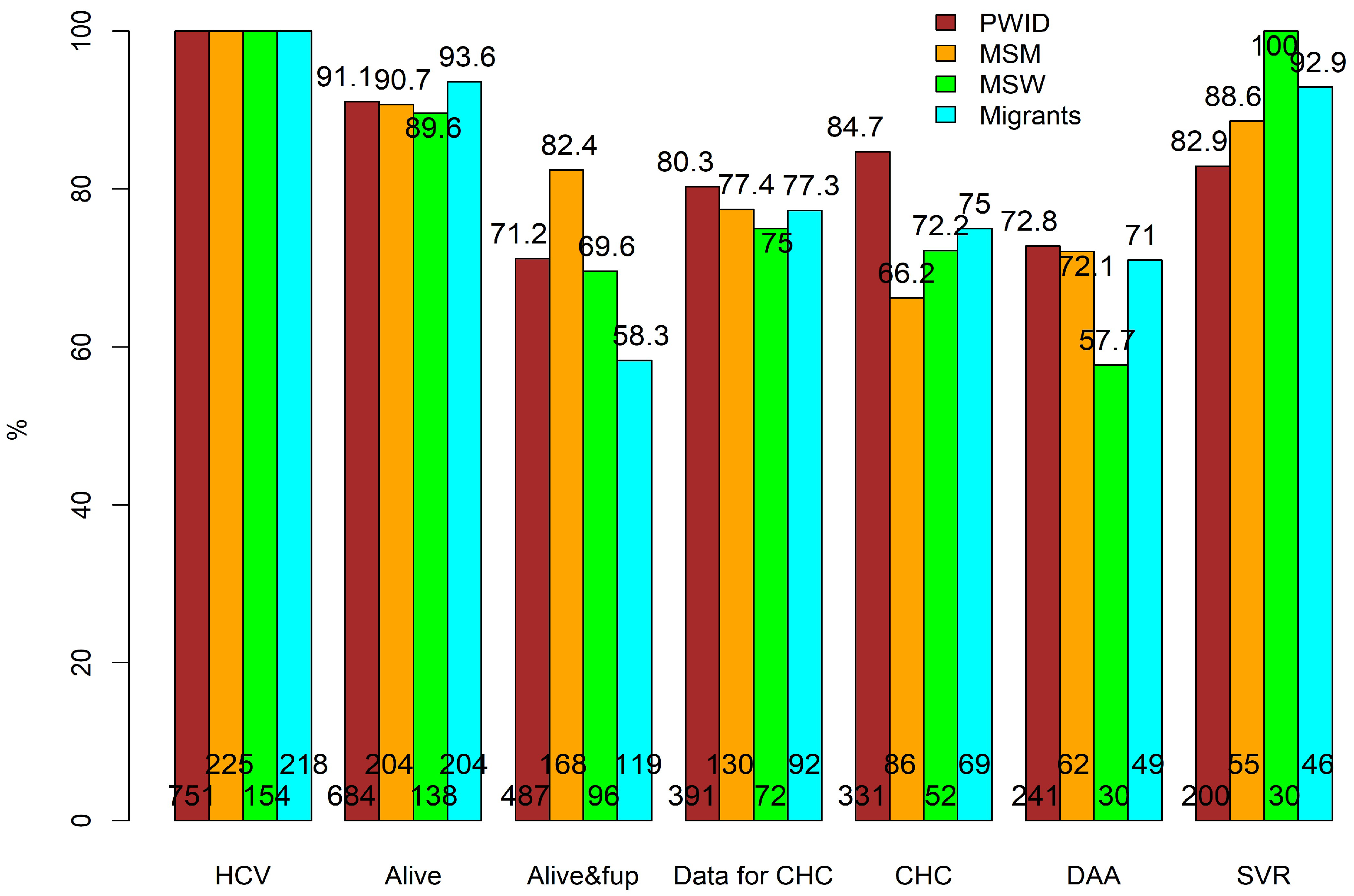
3.2. Overall CoC and Subgroup CoC Analyses
3.3. Mortality before DAA Treatment
3.4. Retention in Care before DAA Treatment
3.5. Availability of Data for CHC and CHC
3.6. Initiation of DAAs
3.7. SVR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Global Health Observatory. Available online: https://www.who.int/data/gho/data/themes/hiv-aids/hiv-aids (accessed on 3 March 2024).
- World Health Organization. Hepatitis C Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 3 March 2024).
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef] [PubMed]
- del Rio, C. Editorial: Can we end HIV as a public health problem globally? Progress towards achieving the UNAIDS 90-90-90 goals. Curr. Opin. HIV AIDS 2019, 14, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Goulis, J.; Sypsa, V.; Lionis, C.; Manolakopoulos, S.; Elefsiniotis, I.; Anagnostou, O.; Tsoulas, C.; Hatzakis, A.; Dalekos, G.N. Aiming towards hepatitis C virus elimination in Greece. Ann. Gastroenterol. 2019, 32, 321–329. [Google Scholar] [CrossRef] [PubMed]
- van Santen, D.K.; van der Helm, J.J.; Touloumi, G.; Pantazis, N.; Muga, R.; Gunsenheimer-Bartmeyer, B.; Gill, M.J.; Sanders, E.; Kelleher, A.; Zangerle, R.; et al. Effect of incident hepatitis C infection on CD4+ cell count and HIV RNA trajectories based on a multinational HIV seroconversion cohort. AIDS 2019, 33, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Gobran, S.T.; Pagliuzza, A.; Khedr, O.; Fert, A.; Chomont, N.; Bruneau, J.; Klein, M.B.; Ancuta, P.; Shoukry, N.H. DAA-mediated HCV cure reduces HIV DNA levels in HCV/HIV coinfected people. J. Virol. 2023, 97, e0110523. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; for the Icona Foundation Study Group; Cozzi-Lepri, A.; Tincati, C.; Calcagno, A.; Ceccherini-Silberstein, F.; De Luca, A.; Antinori, A.; Castagna, A.; Puoti, M.; et al. Immune activation and microbial translocation in liver disease progression in HIV/hepatitis co-infected patients: Results from the Icona Foundation study. BMC Infect. Dis. 2014, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.K.; Vickerman, P.; Dore, G.J.; Hickman, M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): The use of direct-acting antivirals as treatment for prevention. Curr. Opin. HIV AIDS 2015, 10, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Yehia, B.R.; Schranz, A.J.; Umscheid, C.A.; Re, V.L. The Treatment Cascade for Chronic Hepatitis C Virus Infection in the United States: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e101554. [Google Scholar] [CrossRef] [PubMed]
- Rizk, C.; Miceli, J.; Shiferaw, B.; Malinis, M.; Barakat, L.; Ogbuagu, O.; Villanueva, M. Implementing a Comprehensive Hepatitis C Virus (HCV) Clinic Within a Human Immunodeficiency Virus Clinic: A Model of Care for HCV Microelimination. Open Forum Infect. Dis. 2019, 6, ofz361. [Google Scholar] [CrossRef]
- Gardner, E.M.; McLees, M.P.; Steiner, J.F.; del Rio, C.; Burman, W.J. The Spectrum of Engagement in HIV Care and its Relevance to Test-and-Treat Strategies for Prevention of HIV Infection. Clin. Infect. Dis. 2011, 52, 793–800. [Google Scholar] [CrossRef]
- Bar, N.; Bensoussan, N.; Rabinowich, L.; Levi, S.; Houri, I.; Shor, D.B.-A.; Shibolet, O.; Mor, O.; Weitzman, E.; Turner, D.; et al. Barriers and Facilitators of Hepatitis C Care in Persons Coinfected with Human Immunodeficiency Virus. Int. J. Environ. Res. Public Health 2022, 19, 15237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Wegener, M.; Brooks, R.; Mininberg, L.; Helou, E.; Maughan, A.; Villanueva, M. Characterizing Persons With HIV/HCV Coinfection Who Remain Untreated for Hepatitis C at Four HIV Clinics in Connecticut (CT): Role of Multiple Overlapping Barriers at the Individual and Clinic System Levels. Health Promot. Pract. 2023, 24, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Haley, D.F.; Edmonds, A.; Ramirez, C.; French, A.L.; Tien, P.; Thio, C.L.; Witt, M.D.; Seaberg, E.C.; Plankey, M.W.; Cohen, M.H.; et al. Direct-Acting Antiviral Hepatitis C Treatment Cascade and Barriers to Treatment Initiation Among US Men and Women With and Without HIV. J. Infect. Dis. 2020, 223, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Gountas, I.; Sypsa, V.; Papatheodoridis, G.; Paraskevis, D.; Kalamitsis, G.; Anagnostou, O.; Antaraki, A.; Fotiou, A.; Hatzakis, A. A hepatitis C outbreak preceded the HIV outbreak among persons who inject drugs in Athens, Greece: Insights from a mathematical modelling study. J. Viral Hepat. 2019, 26, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Paraskevis, D.; Nikolopoulos, G.; Fotiou, A.; Tsiara, C.; Paraskeva, D.; Sypsa, V.; Lazanas, M.; Gargalianos, P.; Psichogiou, M.; Skoutelis, A.; et al. Economic Recession and Emergence of an HIV-1 Outbreak among Drug Injectors in Athens Metropolitan Area: A Longitudinal Study. PLoS ONE 2013, 8, e78941. [Google Scholar] [CrossRef] [PubMed]
- Hatzakis, A.; Sypsa, V.; Paraskevis, D.; Nikolopoulos, G.; Tsiara, C.; Micha, K.; Panopoulos, A.; Malliori, M.; Psichogiou, M.; Pharris, A.; et al. Design and baseline findings of a large-scale rapid response to an HIV outbreak in people who inject drugs in Athens, Greece: The ARISTOTLE programme. Addiction 2015, 110, 1453–1467. [Google Scholar] [CrossRef] [PubMed]
- Sypsa, V.; Roussos, S.; Tsirogianni, E.; Tsiara, C.; Paraskeva, D.; Chrysanthidis, T.; Chatzidimitriou, D.; Papadimitriou, E.; Paraskevis, D.; Goulis, I.; et al. A new outbreak of HIV infection among people who inject drugs during the COVID-19 pandemic in Greece. Int. J. Drug Policy 2023, 117, 104073. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, N.; Papastamopoulos, V.; Paparizos, V.; Metallidis, S.; Adamis, G.; Antoniadou, A.; Psichogiou, M.; Chini, M.; Sambatakou, H.; Sipsas, N.V.; et al. Long-term evolution of CD4+ cell count in patients under combined antiretroviral therapy. AIDS 2019, 33, 1645–1655. [Google Scholar] [CrossRef]
- Touloumi, G.; Thomadakis, C.; Pantazis, N.; Papastamopoulos, V.; Paparizos, V.; Metallidis, S.; Adamis, G.; Chini, M.; Psichogiou, M.; Chrysos, G.; et al. HIV continuum of care: Bridging cross-sectional and longitudinal analyses. AIDS 2021, 36, 583–591. [Google Scholar] [CrossRef]
- Basoulis, D.; Kostaki, E.G.; Paraskevis, D.; Hatzakis, A.; Psichogiou, M. Tracking missed opportunities for an early HIV diagnosis in a population of people living with HIV with known time of infection. Sex. Transm. Infect. 2021, 98, 79–84. [Google Scholar] [CrossRef]
- Kostaki, E.G.; Limnaios, S.; Adamis, G.; Xylomenos, G.; Chini, M.; Mangafas, N.; Lazanas, M.; Patrinos, S.; Metallidis, S.; Tsachouridou, O.; et al. Estimation of the determinants for HIV late presentation using the traditional definition and molecular clock-inferred dates: Evidence that older age, heterosexual risk group and more recent diagnosis are prognostic factors. HIV Med. 2022, 23, 1143–1152. [Google Scholar] [CrossRef]
- Roussos, S.; Angelopoulos, T.; Cholongitas, E.; Savvanis, S.; Papadopoulos, N.; Kapatais, A.; Chounta, A.; Ioannidou, P.; Deutsch, M.; Manolakopoulos, S.; et al. High levels of all-cause mortality among people who inject drugs from 2018 to 2022. Int. J. Drug Policy 2024, 126, 104356. [Google Scholar] [CrossRef]
- Yin, X.; Kong, L.; Du, P.; Jung, J. Effects of direct-acting antiviral treatment on reducing mortality among Medicare beneficiaries with HIV and HCV coinfection. AIDS Care 2022, 34, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- A Merriman, N.; Porter, S.B.; Brensinger, C.M.; Reddy, K.R.; Chang, K.-M. Racial Difference in Mortality among U.S. Veterans with HCV/HIV Coinfection. Am. J. Gastroenterol. 2006, 101, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M. Future Directions for HIV/HCV Care: Lessons Learned from Local Evaluation Projects in Texas and Connecticut and Implications for Practice and Health Promotion. Health Promot. Pract. 2023, 24, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Cachay, E.R.; Hill, L.; Wyles, D.; Colwell, B.; Ballard, C.; Torriani, F.; Mathews, W.C. The Hepatitis C Cascade of Care among HIV Infected Patients: A Call to Address Ongoing Barriers to Care. PLoS ONE 2014, 9, e102883. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.; Wegener, M.; Speers, S.; Nichols, L.; Sideleau, R.; Valeriano, T.; Buchelli, M.; Villanueva, M. Creating a Longitudinal HCV Care Cascade for Persons with HIV/HCV Coinfection in Selected HIV Clinics Using Data to Care Methods. Health Promot. Pract. 2023, 24, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Laut, K.; Resnati, C.; Del Campo, S.; Leen, C.; Falconer, K.; Trofimova, T.; Paduta, D.; Gatell, J.; Rauch, A.; et al. Uptake of hepatitis C virus treatment in HIV/hepatitis C virus-coinfected patients across Europe in the era of direct-acting antivirals. AIDS 2018, 32, 1995–2004. [Google Scholar] [CrossRef]
- Fursa, O.; Mocroft, A.; Lazarus, J.V.; Amele, S.; Lundgren, J.; Matulionyte, R.; Rasmussen, L.D.; Rockstroh, J.K.; Parczewski, M.; Jilich, D.; et al. The hepatitis C cascade of care in HIV/hepatitis C virus coinfected individuals in Europe: Regional and intra-regional differences. AIDS 2021, 36, 423–435. [Google Scholar] [CrossRef]
- Stanciu, C.; Muzica, C.M.; Girleanu, I.; Cojocariu, C.; Sfarti, C.; Singeap, A.-M.; Huiban, L.; Chiriac, S.; Cuciureanu, T.; Trifan, A. An update on direct antiviral agents for the treatment of hepatitis C. Expert Opin. Pharmacother. 2021, 22, 1729–1741. [Google Scholar] [CrossRef]
- Lodi, S.; Klein, M.; Rauch, A.; Epstein, R.; Wittkop, L.; Logan, R.; Rentsch, C.T.; Justice, A.C.; Touloumi, G.; Berenguer, J.; et al. Sustained virological response after treatment with direct antiviral agents in individuals with HIV and hepatitis C co-infection. J. Int. AIDS Soc. 2022, 25, e26048. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, D.; Choi, J.Y.; Kumarasamy, N.; Pujari, S.; Sun, L.P.; Merati, T.P.; Lee, M.P.; Van Kinh, N.; Kiertiburanakul, S.; Do, C.D.; et al. Viral hepatitis and the cascade of care among people living with HIV in the Asia-Pacific. HIV Med. 2022, 23, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Samur, H.; Martínez-Vernaza, S.; De la Hoz, A.; Barahona-Correa, J.; Ortiz, J.P.; Gualtero-Trujillo, S.; Rumbo-Romero, J.; Salazar, L.M.; Quintero, Y.S.; Valderrama-Beltrán, S. Comparative outcomes of direct-acting antiviral treatment in patients with HIV-Hepatitis C co-infection: Insights from a single center experience in Colombia. Infez. Med. 2023, 31, 374–383. [Google Scholar] [PubMed]
- Bruno, G.; Saracino, A.; Scudeller, L.; Fabrizio, C.; Dell’acqua, R.; Milano, E.; Milella, M.; Ladisa, N.; Monno, L.; Angarano, G. HCV mono-infected and HIV/HCV co-infected individuals treated with direct-acting antivirals: To what extent do they differ? Int. J. Infect. Dis. 2017, 62, 64–71. [Google Scholar] [CrossRef]
- Carvalho, L.; Pillai, S.; Daniels, E.; Sellers, P.; Whyte, R.; Eveson, L.; Foxton, M.; Nelson, M. Higher sustained virological response rates at 12 weeks in HIV-HCV co-infection; a tertiary centre experience. J. Infect. 2020, 80, 245–246. [Google Scholar] [CrossRef]
- Vourli, G.; Pharris, A.; Cazein, F.; Costagliola, D.; Dabis, F.; Del Amo, J.; Delpech, V.; Díaz, A.; Girardi, E.; Gourlay, A.; et al. Are European HIV cohort data within EuroCoord representative of the diagnosed HIV population? AIDS 2019, 33, 133–143. [Google Scholar] [CrossRef]

| MSM (N = 225; 18.5%) | PWID (N = 751; 61.9%) | MSW (N = 154; 12.7%) | Other/Unknown (N = 83; 6.8%) | Total (N = 1213) | p Value | |
|---|---|---|---|---|---|---|
| Migrant status | <0.001 | |||||
| No | 203 (90.2%) | 635 (84.6%) | 95 (61.7%) | 62 (74.7%) | 995 (82.0%) | |
| Yes | 22 (9.8%) | 116 (15.4%) | 59 (38.3%) | 21 (25.3%) | 218 (18.0%) | |
| Age at HCV diagnosis (years) | <0.001 | |||||
| Median (Q1, Q3) | 38.3 (32.3, 46.0) | 33.6 (28.9, 39.6) | 38.4 (31.5, 46.5) | 37.7 (29.5, 44.7) | 35.3 (29.8, 42.3) | |
| CD4 at HIV diagnosis (cells/) | <0.001 | |||||
| N-Miss | 2 | 18 | 3 | 10 | 33 | |
| Median (Q1, Q3) | 387.0 (171.0, 582.5) | 328.0 (164.0, 556.0) | 319.0 (112.5, 482.5) | 224.0 (79.0, 384.0) | 327.0 (147.0, 540.5) | |
| ART initiation | <0.001 | |||||
| No | 8 (3.6%) | 94 (12.5%) | 15 (9.7%) | 15 (18.1%) | 132 (10.9%) | |
| Yes | 217 (96.4%) | 657 (87.5%) | 139 (90.3%) | 68 (81.9%) | 1081 (89.1%) | |
| HCV genotype 1 | 0.032 | |||||
| N-Miss | 56 | 179 | 35 | 14 | 284 | |
| 1a | 12 (40.0%) | 47 (30.9%) | 3 (17.6%) | 2 (28.6%) | 64 (31.1%) | |
| 1b | 2 (6.7%) | 6 (3.9%) | 5 (29.4%) | 2 (28.6%) | 15 (7.3%) | |
| 3 | 12 (40.0%) | 75 (49.3%) | 7 (41.2%) | 3 (42.9%) | 97 (47.1%) | |
| 4 | 4 (13.3%) | 24 (15.8%) | 2 (11.8%) | 0 (0.0%) | 30 (14.6%) | |
| 1 Among those chronically infected with HCV | ||||||
| A. Mortality Model (N = 1213) | B. Loss-to-Follow-up Model (N = 1101) | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR 1 | 95% CI 1 | p 2 | HR 1 | 95% CI 1 | p 2 |
| Migrants | 0.71 | 0.38, 1.31 | 0.271 | 1.98 | 1.51, 2.60 | <0.001 |
| Mode of HIV infection | 0.064 | <0.001 | ||||
| MSM | — | — | — | — | ||
| PWID | 1.91 | 1.14, 3.22 | 0.015 | 2.35 | 1.61, 3.42 | <0.001 |
| MSW | 1.54 | 0.79, 3.04 | 0.208 | 1.83 | 1.16, 2.91 | 0.010 |
| Other/Unknown | 1.01 | 0.44, 2.30 | 0.978 | 1.70 | 0.98, 2.96 | 0.060 |
| Age at HCV diagnosis (per 10 years) | 1.15 | 0.96, 1.37 | 0.136 | 0.98 | 0.87, 1.11 | 0.743 |
| CD4 at HIV diagnosis (cells/μL) | 0.002 | 0.070 | ||||
| (0, 200) | — | — | — | — | ||
| (200, 350) | 0.60 | 0.37, 0.98 | 0.043 | 0.64 | 0.46, 0.90 | 0.011 |
| (350, 500) | 0.33 | 0.17, 0.63 | <0.001 | 0.76 | 0.54, 1.07 | 0.114 |
| >500 | 0.53 | 0.32, 0.86 | 0.011 | 0.82 | 0.61, 1.11 | 0.199 |
| Characteristic | OR 1 | 95% CI 1 | p 2 |
|---|---|---|---|
| Migrants | 1.49 | 0.80, 2.88 | 0.223 |
| Mode of HIV infection | 0.109 | ||
| MSM | — | — | |
| PWID | 1.10 | 0.61, 1.92 | 0.748 |
| MSW | 0.49 | 0.23, 1.06 | 0.071 |
| Other/Unknown | 1.15 | 0.37, 4.09 | 0.820 |
| Most recent CD4 before DAA treatment (cells/μL) | 0.035 | ||
| (0, 100) | — | — | |
| (100, 350) | 3.50 | 1.35, 9.15 | 0.010 |
| >350 | 2.51 | 1.04, 6.02 | 0.038 |
| % of time off ART before DAA (per 10%) | 0.64 | 0.50, 0.80 | <0.001 |
| Characteristic | OR 1 | 95% CI 1 | p 2 |
|---|---|---|---|
| Migrants | 1.56 | 0.40, 10.3 | 0.575 |
| Mode of HIV infection | 0.872 | ||
| MSM | — | — | |
| PWID | 0.66 | 0.18, 1.88 | 0.468 |
| MSW | NA 3 | 0.987 | |
| Other/Unknown | 1.12 | 0.14, 23.4 | 0.926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomadakis, C.; Basoulis, D.; Tsachouridou, O.; Protopapas, K.; Paparizos, V.; Astriti, M.; Chini, M.; Chrysos, G.; Marangos, M.; Panagopoulos, P.; et al. HCV Cascade of Care in HIV/HCV Co-Infected Individuals: Missed Opportunities for Micro-Elimination. Viruses 2024, 16, 885. https://doi.org/10.3390/v16060885
Thomadakis C, Basoulis D, Tsachouridou O, Protopapas K, Paparizos V, Astriti M, Chini M, Chrysos G, Marangos M, Panagopoulos P, et al. HCV Cascade of Care in HIV/HCV Co-Infected Individuals: Missed Opportunities for Micro-Elimination. Viruses. 2024; 16(6):885. https://doi.org/10.3390/v16060885
Chicago/Turabian StyleThomadakis, Christos, Dimitrios Basoulis, Olga Tsachouridou, Konstantinos Protopapas, Vasilios Paparizos, Myrto Astriti, Maria Chini, Georgios Chrysos, Markos Marangos, Periklis Panagopoulos, and et al. 2024. "HCV Cascade of Care in HIV/HCV Co-Infected Individuals: Missed Opportunities for Micro-Elimination" Viruses 16, no. 6: 885. https://doi.org/10.3390/v16060885
APA StyleThomadakis, C., Basoulis, D., Tsachouridou, O., Protopapas, K., Paparizos, V., Astriti, M., Chini, M., Chrysos, G., Marangos, M., Panagopoulos, P., Kofteridis, D., Sambatakou, H., Mastrogianni, E., Panatzis, N., Pechlivanidou, E., Psichοgiou, M., & Touloumi, G., on behalf of the Athens Multicenter AIDS Cohort Study (AMACS). (2024). HCV Cascade of Care in HIV/HCV Co-Infected Individuals: Missed Opportunities for Micro-Elimination. Viruses, 16(6), 885. https://doi.org/10.3390/v16060885








