The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy
Abstract
1. Introduction
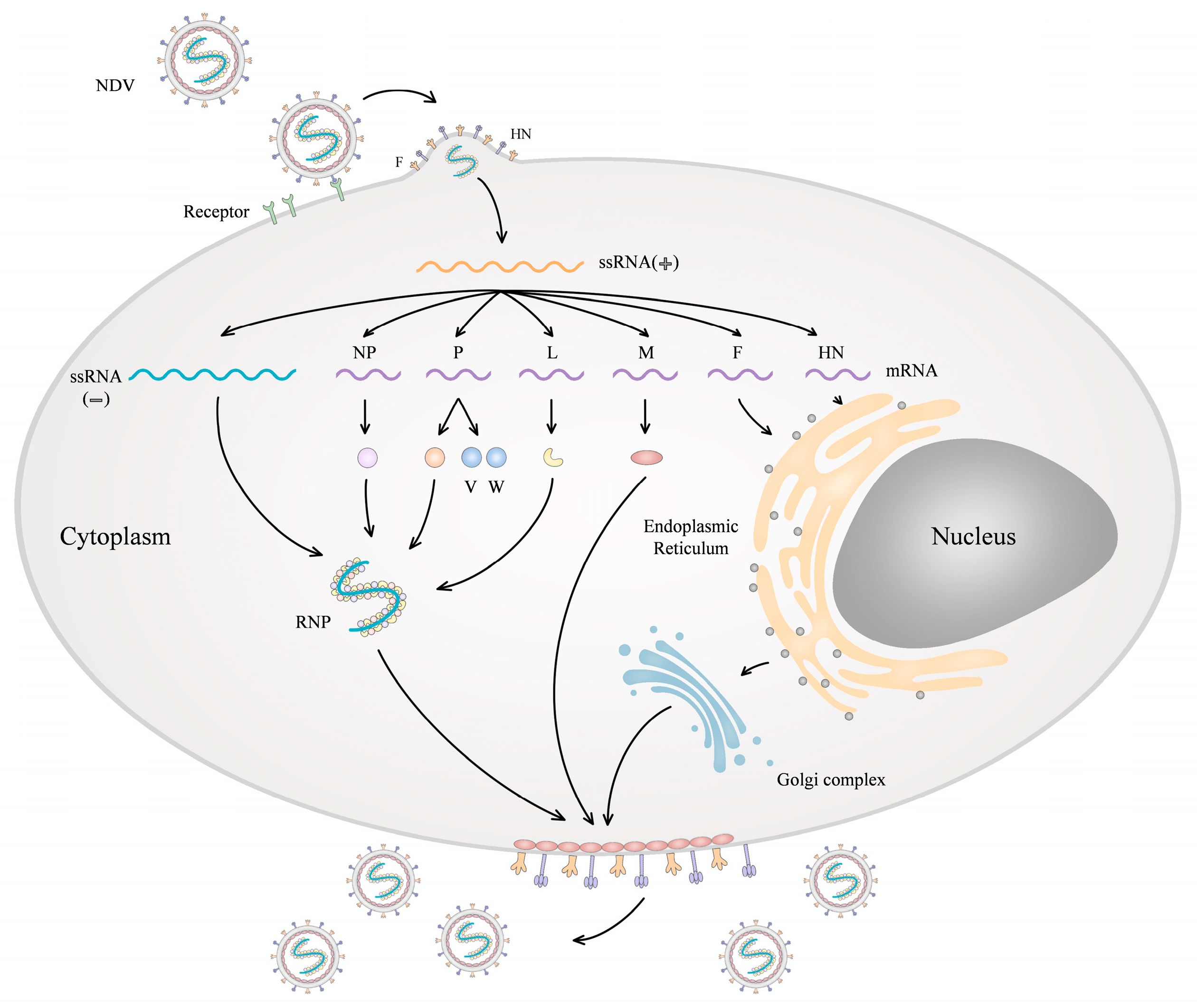
2. Molecular Biology of NDV
3. Advantages of NDV as a Vaccine Carrier
4. Advantages of NDV as an Oncolytic Agent
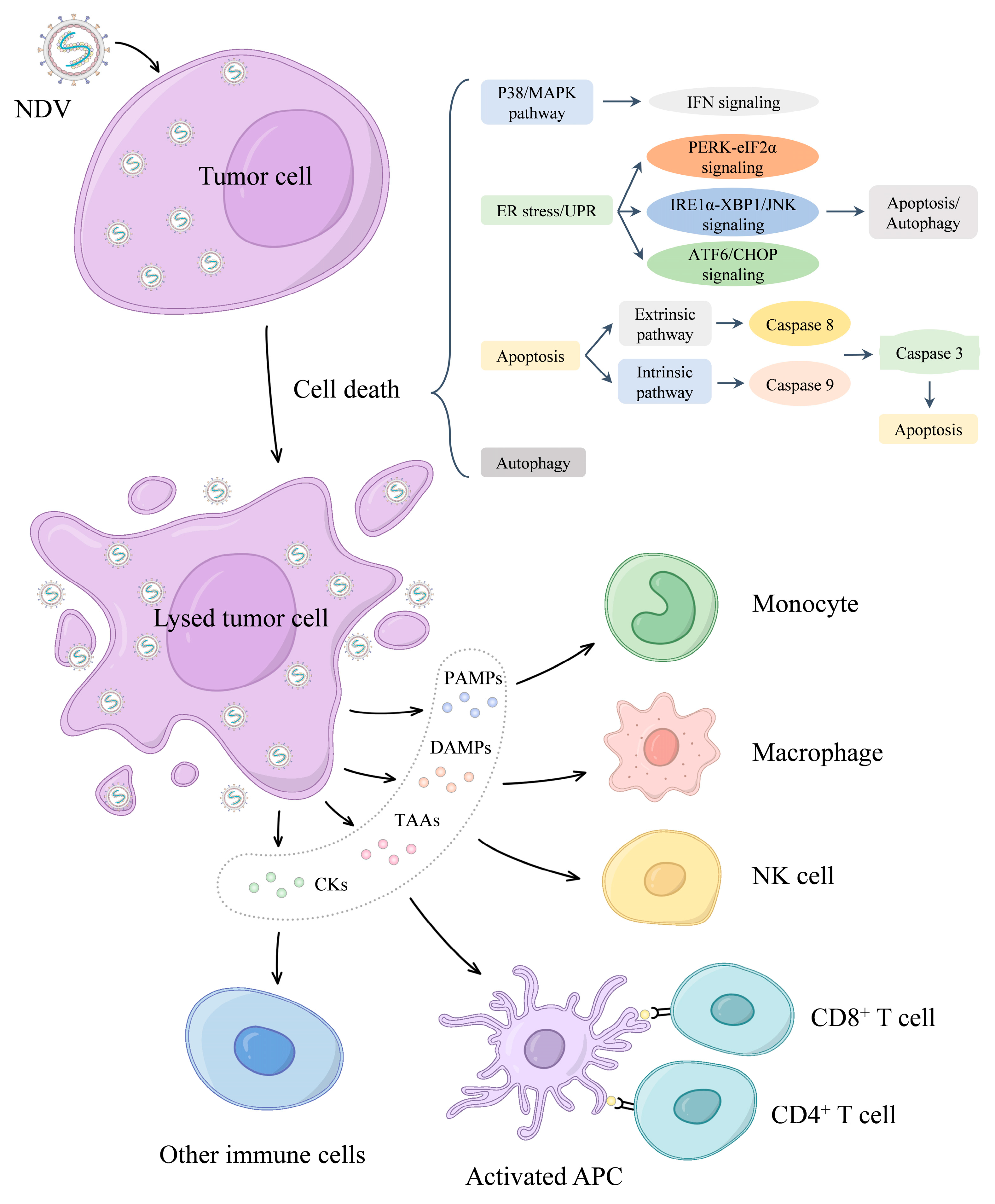
5. Oncolytic Mechanism of NDV
5.1. Tumor-Selective Viral Replication
5.2. NDV Mediates Oncolysis
5.2.1. Apoptosis
5.2.2. Autophagy
5.2.3. NDV Activates the Antitumor Immune Response
6. The Application of NDV in Vaccine Vector and Tumor Therapy
6.1. Application of NDV as a Vaccine Carrier in Infectious Diseases
6.2. Application of NDV in Tumor Therapy
| NDV Strains | Types of Cancer | Phase | Patients (n) | References |
|---|---|---|---|---|
| 73-T | Melanoma | II | 83 | [123,124] |
| MTH-68/H | Advanced chemorefractory cancers | II | 59 | [125] |
| MTH-68/H | Glioblastoma multiforme (GBM), high-grade glioma | No data | 4 | [126] |
| PV701 | Advanced solid cancers | I | 113 | [119,127] |
| HUJ | GBM | I/II | 14 | [128] |
| ATV-NDV | Early breast cancer, metastatic breast cancer, and metastatic ovarian cancer | I | 121 | [132] |
| ATV-NDV | Locally advanced renal cell carcinoma | II | 208 | [133] |
| ATV-NDV | Head and neck squamous cell carcinoma (HNSCC) | I | 20 | [134] |
| ATV-NDV | GBM | II | 110 | [135] |
| ATV-NDV | Colorectal resected carcinoma | II | 57 | [136] |
| ATV-NDV | Colorectal resected carcinoma | I | 51 | [137] |
| ATV-NDV | Colorectal cancer | III | 310 | [138] |
| ATV-NDV-αHN-αCD28 | Colorectal cancer | I | 40 | [139] |
| MEDI5395 (rNDV-GM-CSF) | Advanced solid tumors | I | 188 | NCT03889275 |
| MEDI9253 (rNDV-IL 12) | Solid tumors | I | 86 | NCT04613492 |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Burman, B.; Pesci, G.; Zamarin, D. Newcastle Disease Virus at the Forefront of Cancer Immunotherapy. Cancers 2020, 12, 3552. [Google Scholar] [CrossRef]
- Cuadrado-Castano, S.; Sanchez-Aparicio, M.T.; Garcia-Sastre, A.; Villar, E. The therapeutic effect of death: Newcastle disease virus and its antitumor potential. Virus Res. 2015, 209, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Yin, Y.; Song, C.; Tan, L.; Qiu, X.; Liao, Y.; Liu, W.; Meng, S.; Sun, Y.; Ding, C. Newcastle disease virus induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. iScience 2021, 24, 102837. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yu, S.; Yang, S.; Qiu, X.; Meng, C.; Tan, L.; Song, C.; Liao, Y.; Liu, W.; Sun, Y.; et al. Newcastle Disease virus infection activates PI3K/Akt/mTOR and p38 MAPK/Mnk1 pathways to benefit viral mRNA translation via interaction of the viral NP protein and host eIF4E. PLoS Pathog. 2020, 16, e1008610. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Shergold, A.; Elder, M.J.; Whitworth, J.; Cheng, X.; Jin, H.; Wilkinson, R.W.; Harper, J.; Carroll, D.K. Oncolytic Newcastle disease virus activation of the innate immune response and priming of antitumor adaptive responses in vitro. Cancer Immunol. Immunother. 2020, 69, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Peeters, B.P.; de Leeuw, O.S.; Koch, G.; Gielkens, A.L. Rescue of Newcastle disease virus from cloned cDNA: Evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 1999, 73, 5001–5009. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Huang, Z.; Samal, S.K. Recovery of a virulent strain of newcastle disease virus from cloned cDNA: Expression of a foreign gene results in growth retardation and attenuation. Virology 2000, 278, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.; Zhao, L.; Ledda, A.; Liu, W.; Ding, C.; Nair, V.A.-O.X.; Ferretti, L. Patterns of RNA Editing in Newcastle Disease Virus Infections. Viruses 2020, 12, 1249. [Google Scholar] [CrossRef]
- Huang, Z.; Krishnamurthy, S.; Panda, A.; Samal, S.K. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J. Virol. 2003, 77, 8676–8685. [Google Scholar] [CrossRef]
- Park, M.S.; Garcia-Sastre, A.; Cros, J.F.; Basler, C.F.; Palese, P. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 2003, 77, 9522–9532. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.L.; Zhang, H.; Nan, W.L.; Xie, C.Z.; Ha, Z.; Chen, X.; Xu, X.H.; Qian, J.; Qiu, X.S.; Ge, J.Y.; et al. Lentogenic NDV V protein inhibits IFN responses and represses cell apoptosis. Vet. Microbiol. 2021, 261, 109181. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, J.; Teng, Q.; Li, X.; Bu, Y.; Zhang, G. Mechanisms and consequences of Newcastle disease virus W protein subcellular localization in the nucleus or mitochondria. J. Virol. 2021, 95, e02087-20. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhang, Y.; Zhan, Y.; Yuan, Y.; Sun, Y.; Qiu, X.; Meng, C.; Song, C.; Liao, Y.; Ding, C. Newcastle disease virus employs macropinocytosis and Rab5a-dependent intracellular trafficking to infect DF-1 cells. Oncotarget 2016, 7, 86117–86133. [Google Scholar] [CrossRef]
- El-Sayed, A.; Harashima, H. Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.M.; Field, G.M.; Sauvron, J.M.; Mirza, A.M.; Deng, R.; Mahon, P.J.; Langedijk, J.P. Structural and functional relationship between the receptor recognition and neuraminidase activities of the Newcastle disease virus hemagglutinin-neuraminidase protein: Receptor recognition is dependent on neuraminidase activity. J. Virol. 2001, 75, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Abolnik, C.; Afonso, C.L.; Albina, E.; Bahl, J.; Berg, M.; Briand, F.X.; Brown, I.H.; Choi, K.S.; Chvala, I.; et al. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect. Genet. Evol. 2019, 74, 103917. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, O.S.; Koch, G.; Hartog, L.; Ravenshorst, N.; Peeters, B.P.H. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin-neuraminidase protein. J. Gen. Virol. 2005, 86, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Huang, Z.; Elankumaran, S.; Rockemann, D.D.; Samal, S.K. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathog. 2004, 36, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Huo, N.; Wang, W.; Guo, Y.; Wei, Q.; Wang, X.; Zhang, S.; Yang, Z.; Xiao, S.A.-O. Comprehensive analysis of amino acid sequence diversity at the F protein cleavage site of Newcastle disease virus in fusogenic activity. PLoS ONE 2017, 12, e0183923. [Google Scholar] [CrossRef]
- de Leeuw, O.S.; Hartog, L.; Koch, G.; Peeters, B.P.H. Effect of fusion protein cleavage site mutations on virulence of Newcastle disease virus: Non-virulent cleavage site mutants revert to virulence after one passage in chicken brain. J. Gen. Virol. 2003, 84, 475–484. [Google Scholar] [CrossRef]
- Schirrmacher, V. Fifty Years of Clinical Application of Newcastle Disease Virus: Time to Celebrate! Biomedicines 2016, 4, 16. [Google Scholar] [CrossRef]
- Cassel, W.F.; Garrett, R.E. Newcastle disease virus as an antineoplastic agent. Cancer 1965, 18, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Dai, C.; Zhang, Y.; Zhao, Y.; Wang, Y.; Ru, G. Development of Molecular Mechanisms and Their Application on Oncolytic Newcastle Disease Virus in Cancer Therapy. Front. Mol. Biosci. 2022, 9, 889403. [Google Scholar] [CrossRef] [PubMed]
- Al-Ziaydi, A.G.; Al-Shammari, A.M.; Hamzah, M.I.; Kadhim, H.S.; Jabir, M.S. Hexokinase inhibition using D-Mannoheptulose enhances oncolytic newcastle disease virus-mediated killing of breast cancer cells. Cancer Cell Int. 2020, 20, 420. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, A.M.; Abdullah, A.H.; Allami, Z.M.; Yaseen, N.Y. 2-Deoxyglucose and Newcastle Disease Virus Synergize to Kill Breast Cancer Cells by Inhibition of Glycolysis Pathway Through Glyceraldehyde3-Phosphate Downregulation. Front. Mol. Biosci. 2019, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Reichard, K.W.; Lorence, R.M.; Cascino, C.J.; Peeples, M.E.; Walter, R.J.; Fernando, M.B.; Reyes, H.M.; Greager, J.A. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 1992, 52, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Romero, N.; Palacin-Aliana, I.; Esteban-Rubio, S.; Madurga, R.; Rius-Rocabert, S.; Carrion-Navarro, J.; Presa, J.; Cuadrado-Castano, S.; Sanchez-Gomez, P.; Garcia-Sastre, A.; et al. Newcastle Disease Virus (NDV) Oncolytic Activity in Human Glioma Tumors Is Dependent on CDKN2A-Type I IFN Gene Cluster Codeletion. Cells 2020, 9, 1405. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Takimoto, T.; Scroggs, R.A.; Portner, A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 2006, 80, 5145–5155. [Google Scholar] [CrossRef]
- Fiola, C.; Peeters, B.; Fournier, P.; Arnold, A.; Bucur, M.; Schirrmacher, V. Tumor selective replication of Newcastle disease virus: Association with defects of tumor cells in antiviral defence. Int. J. Cancer 2006, 119, 328–338. [Google Scholar] [CrossRef]
- Schirrmacher, V. Molecular Mechanisms of Anti-Neoplastic and Immune Stimulatory Properties of Oncolytic Newcastle Disease Virus. Biomedicines 2022, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V.; van Gool, S.; Stuecker, W. Breaking Therapy Resistance: An Update on Oncolytic Newcastle Disease Virus for Improvements of Cancer Therapy. Biomedicines 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Wilden, H.; Schirrmacher, V. Importance of retinoic acid-inducible gene I and of receptor for type I interferon for cellular resistance to infection by Newcastle disease virus. Int. J. Oncol. 2012, 40, 287–298. [Google Scholar] [PubMed]
- Wilden, H.; Fournier, P.; Zawatzky, R.; Schirrmacher, V. Expression of RIG-I, IRF3, IFN-beta and IRF7 determines resistance or susceptibility of cells to infection by Newcastle Disease Virus. Int. J. Oncol. 2009, 34, 971–982. [Google Scholar] [PubMed]
- Washburn, B.; Schirrmacher, V. Human tumor cell infection by Newcastle Disease Virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int. J. Oncol. 2002, 21, 85–93. [Google Scholar] [CrossRef]
- Lenza, M.P.; Atxabal, U.; Oyenarte, I.; Jiménez-Barbero, J.; Ereño-Orbea, J. Current Status on Therapeutic Molecules Targeting Siglec Receptors. Cells 2020, 9, 2691. [Google Scholar] [CrossRef]
- Mansour, M.; Palese, P.; Zamarin, D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J. Virol. 2011, 85, 6015–6023. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Yaacov, B.; Shiloach, T.; Eliahoo, E.; Kadouri, L.; Lotem, M.; Perlman, R.; Zakay-Rones, Z.; Panet, A.; Ben-Yehuda, D. The oncolytic activity of Newcastle disease virus NDV-HUJ on chemoresistant primary melanoma cells is dependent on the proapoptotic activity of the inhibitor of apoptosis protein Livin. J. Virol. 2010, 84, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, J.; Puehler, F.; Mumberg, D.; Boukamp, P.; Beier, R. Rac1 is required for oncolytic NDV replication in human cancer cells and establishes a link between tumorigenesis and sensitivity to oncolytic virus. Oncogene 2010, 29, 2205–2216. [Google Scholar] [CrossRef]
- Mustafa, Z.; Shamsuddin, H.S.; Ideris, A.; Ibrahim, R.; Jaafar, H.; Ali, A.M.; Abdullah, J.M. Viability reduction and Rac1 gene downregulation of heterogeneous ex-vivo glioma acute slice infected by the oncolytic Newcastle disease virus strain V4UPM. Biomed. Res. Int. 2013, 2013, 248507. [Google Scholar] [CrossRef]
- Kent, L.N.; Leone, G. The broken cycle: E2F dysfunction in cancer. Nat. Rev. Cancer 2019, 19, 326–338. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, P.V.; Tiwari, A.K.; Ratta, B.; Bais, M.V.; Chaturvedi, U.; Palia, S.K.; Sharma, B.; Chauhan, R.S. Time course of Newcastle disease virus-induced apoptotic pathways. Virus Res. 2009, 144, 350–354. [Google Scholar] [CrossRef]
- Molouki, A.; Hsu, Y.T.; Jahanshiri, F.; Abdullah, S.; Rosli, R.; Yusoff, K. The matrix (M) protein of Newcastle disease virus binds to human bax through its BH3 domain. Virol. J. 2011, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Castano, S.; Ayllon, J.; Mansour, M.; de la Iglesia-Vicente, J.; Jordan, S.; Tripathi, S.; García-Sastre, A.; Villar, E. Enhancement of the proapoptotic properties of newcastle disease virus promotes tumor remission in syngeneic murine cancer models. Mol. Cancer Ther. 2015, 14, 1247–1258. [Google Scholar] [CrossRef]
- Bai, F.L.; Yu, Y.H.; Tian, H.; Ren, G.P.; Wang, H.; Zhou, B.; Han, X.H.; Yu, Q.Z.; Li, D.S. Genetically engineered Newcastle disease virus expressing interleukin-2 and TNF-related apoptosis-inducing ligand for cancer therapy. Cancer Biol. Ther. 2014, 15, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Kazimirsky, G.; Jiang, W.; Slavin, S.; Ziv-Av, A.; Brodie, C. Mesenchymal stem cells enhance the oncolytic effect of Newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL. Stem Cell Res. Ther. 2016, 7, 149. [Google Scholar] [CrossRef]
- Ch’ng, W.C.; Abd-Aziz, N.; Ong, M.H.; Stanbridge, E.J.; Shafee, N. Human renal carcinoma cells respond to Newcastle disease virus infection through activation of the p38 MAPK/NF-κB/IκBα pathway. Cell. Oncol. 2015, 38, 279–288. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, W.; Niu, Q.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; Ding, C. eIF2α-CHOP-BCl-2/JNK and IRE1α-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus. Cell Death Dis. 2019, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Elankumaran, S.; Rockemann, D.; Samal, S.K. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 2006, 80, 7522–7534. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Fournier, P.; Schirrmacher, V. Induction of interferon-alpha and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology 2002, 297, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, H.X.; Mao, X.; Fang, H.; Wang, H.; Li, Y.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; et al. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection. Oncotarget 2017, 8, 43201–43217. [Google Scholar] [CrossRef] [PubMed]
- Ghrici, M.; El Zowalaty, M.; Omar, A.R.; Ideris, A. Newcastle disease virus Malaysian strain AF2240 induces apoptosis in MCF-7 human breast carcinoma cells at an early stage of the virus life cycle. Int. J. Mol. Med. 2013, 31, 525–532. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Sun, L.; Li, C.; Hu, N.; Sheng, Y.; Chen, Z.; Li, X.; Chi, B.; Jin, N. Anti-tumor effects of an oncolytic adenovirus expressing hemagglutinin-neuraminidase of Newcastle disease virus in vitro and in vivo. Viruses 2014, 6, 856–874. [Google Scholar] [CrossRef] [PubMed]
- Molouki, A.; Yusoff, K. NDV-induced apoptosis in absence of Bax; evidence of involvement of apoptotic proteins upstream of mitochondria. Virol. J. 2012, 9, 179. [Google Scholar] [CrossRef]
- Shan, P.; Tang, B.; Xie, S.; Zhang, Z.; Fan, J.; Wei, Z.; Song, C. NDV-D90 inhibits 17β-estradiol-mediated resistance to apoptosis by differentially modulating classic and nonclassic estrogen receptors in breast cancer cells. J. Cell Biochem. 2021, 122, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Lu, H.; Cui, Y.; Hou, X.; Huang, C.; Liu, G. Overexpression of p53 delivered using recombinant NDV induces apoptosis in glioma cells by regulating the apoptotic signaling pathway. Exp. Ther. Med. 2018, 15, 4522–4530. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Liu, H.; Wang, X.; Chu, Z.; Hu, R.; Ren, J.; Xiao, S.; Zhang, S.; Wang, X.; et al. High level expression of ISG12(1) promotes cell apoptosis via mitochondrial-dependent pathway and so as to hinder Newcastle disease virus replication. Vet. Microbiol. 2019, 228, 147–156. [Google Scholar] [CrossRef]
- Wang, C.; Chu, Z.; Liu, W.; Pang, Y.; Gao, X.; Tang, Q.; Ma, J.; Lu, K.; Adam, F.E.A.; Dang, R.; et al. Newcastle disease virus V protein inhibits apoptosis in DF-1 cells by downregulating TXNL1. Vet. Res. 2018, 49, 102. [Google Scholar] [CrossRef] [PubMed]
- Peker, N.; Gozuacik, D. Autophagy as a Cellular Stress Response Mechanism in the Nervous System. J. Mol. Biol. 2020, 432, 2560–2588. [Google Scholar] [CrossRef]
- Russell, R.C.; Yuan, H.X.; Guan, K.L. Autophagy regulation by nutrient signaling. Cell Res. 2014, 24, 42–57. [Google Scholar] [CrossRef]
- Meng, C.; Zhou, Z.; Jiang, K.; Yu, S.; Jia, L.; Wu, Y.; Liu, Y.; Meng, S.; Ding, C. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch. Virol. 2012, 157, 1011–1018. [Google Scholar] [CrossRef]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; De Vleeschouwer, S.; Agostinis, P.; Graf, N.; Van Gool, S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, S.; Ding, N.; Meng, C.; Meng, S.; Zhang, S.; Zhan, Y.; Qiu, X.; Tan, L.; Chen, H.; et al. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J. Virol. 2014, 88, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, Y.J.; Zhang, F.Q.; Zhang, X.R.; Qiu, X.S.; Yu, L.P.; Wu, Y.T.; Ding, C. Newcastle disease virus NP and P proteins induce autophagy via the endoplasmic reticulum stress-related unfolded protein response. Sci. Rep. 2016, 6, 24721. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Rehman, Z.U.; Shi, M.; Yang, B.; Qu, Y.; Yang, X.F.; Shao, Q.; Meng, C.; Yang, Z.; Gao, X.; et al. Syncytia generated by hemagglutinin-neuraminidase and fusion proteins of virulent Newcastle disease virus induce complete autophagy by activating AMPK-mTORC1-ULK1 signaling. Vet. Microbiol. 2019, 230, 283–290. [Google Scholar] [CrossRef]
- Bu, X.; Zhao, Y.; Zhang, Z.; Wang, M.; Li, M.; Yan, Y. Recombinant Newcastle disease virus (rL-RVG) triggers autophagy and apoptosis in gastric carcinoma cells by inducing ER stress. Am. J. Cancer Res. 2016, 6, 924–936. [Google Scholar]
- Yan, Y.; Shao, X.; Gu, W.; Zhang, A.; Bu, X.; Liang, B. Recombinant virus expressing hIFN-λ1 (rL-hIFN-λ1) has important effects on endoplasmic reticulum stress, autophagy and apoptosis in small cell lung cancer. Transl. Cancer Res. 2020, 9, 5209–5217. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tang, N.; Liu, P.; Sun, Y.; Lu, S.; Liu, W.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; et al. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy 2022, 18, 1503–1521. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, Z.; Chen, F.; Kong, X.; Liu, H.; Jiang, K.; Liu, W.; Hu, M.; Zhang, X.; Ding, C.; et al. Newcastle disease virus induces apoptosis in cisplatin-resistant human lung adenocarcinoma A549 cells in vitro and in vivo. Cancer Lett. 2012, 317, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yuan, R.; Xiang, B.; Zhao, X.; Gao, P.; Dai, X.; Liao, M.; Ren, T. Newcastle disease virus-induced autophagy mediates antiapoptotic signaling responses in vitro and in vivo. Oncotarget 2017, 8, 73981–73993. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar] [PubMed]
- Jarahian, M.; Watzl, C.; Fournier, P.; Arnold, A.; Djandji, D.; Zahedi, S.; Cerwenka, A.; Paschen, A.; Schirrmacher, V.; Momburg, F. Activation of natural killer cells by newcastle disease virus hemagglutinin-neuraminidase. J. Virol. 2009, 83, 8108–8121. [Google Scholar] [CrossRef] [PubMed]
- Washburn, B.; Weigand, M.A.; Grosse-Wilde, A.; Janke, M.; Stahl, H.; Rieser, E.; Sprick, M.R.; Schirrmacher, V.; Walczak, H. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J. Immunol. 2003, 170, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V.; Bai, L.; Umansky, V.; Yu, L.; Xing, Y.; Qian, Z. Newcastle disease virus activates macrophages for anti-tumor activity. Int. J. Oncol. 2000, 16, 363–373. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, C.; Shi, X.; Xu, D.; Li, M.; Cui, J.; Li, W.; Xu, J.; Jin, H. Dendritic cells loaded with the lysate of tumor cells infected with Newcastle Disease Virus trigger potent anti-tumor immunity by promoting the secretion of IFN-γ and IL-2 from T cells. Oncol. Lett. 2018, 16, 1180–1188. [Google Scholar] [CrossRef]
- Elankumaran, S.; Chavan, V.; Qiao, D.; Shobana, R.; Moorkanat, G.; Biswas, M.; Samal, S.K. Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy. J. Virol. 2010, 84, 3835–3844. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Heath, W.R. The CD8+ dendritic cell subset. Immunol. Rev. 2010, 234, 18–31. [Google Scholar] [CrossRef]
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016. [Google Scholar] [CrossRef]
- Buijs, P.R.; van Eijck, C.H.; Hofland, L.J.; Fouchier, R.A.; van den Hoogen, B.G. Different responses of human pancreatic adenocarcinoma cell lines to oncolytic Newcastle disease virus infection. Cancer Gene Ther. 2014, 21, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ni, J.; Cao, Y.; Liu, X. Newcastle Disease Virus as a Vaccine Vector for 20 Years: A Focus on Maternally Derived Antibody Interference. Vaccines 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Samal, S.K. Innovation in Newcastle Disease Virus Vectored Avian Influenza Vaccines. Viruses 2019, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Fulber, J.P.C.; Kamen, A.A. Development and Scalable Production of Newcastle Disease Virus-Vectored Vaccines for Human and Veterinary Use. Viruses 2022, 14, 975. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.B.; Yusoff, K.; Ideris, A.; Hair-Bejo, M.; Jibril, A.H.; Peeters, B.P.H.; Omar, A.R. Exploring the Prospects of Engineered Newcastle Disease Virus in Modern Vaccinology. Viruses 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.P.; Yadav, K.; Pathak, D.C.; Ramamurthy, N.; D’Silva, A.L.; Marriappan, A.K.; Ramakrishnan, S.; Vakharia, V.N.; Chellappa, M.M.; Dey, S. Recombinant Newcastle Disease Virus (NDV) Expressing Sigma C Protein of Avian Reovirus (ARV) Protects against Both ARV and NDV in Chickens. Pathogens 2019, 8, 145. [Google Scholar] [CrossRef]
- Abozeid, H.H.; Paldurai, A.; Varghese, B.P.; Khattar, S.K.; Afifi, M.A.; Zouelfakkar, S.; El-Deeb, A.H.; El-Kady, M.F.; Samal, S.K. Development of a recombinant Newcastle disease virus-vectored vaccine for infectious bronchitis virus variant strains circulating in Egypt. Vet. Res. 2019, 50, 12. [Google Scholar] [CrossRef]
- Shirvani, E.; Paldurai, A.; Manoharan, V.K.; Varghese, B.P.; Samal, S.K. A Recombinant Newcastle Disease Virus (NDV) Expressing S Protein of Infectious Bronchitis Virus (IBV) Protects Chickens against IBV and NDV. Sci. Rep. 2018, 8, 11951. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, Y.; Wang, Z.; Yao, L.; Li, L.; Shang, Y.; Wang, H.; Zhang, R.; Shao, H.; Luo, Q.; et al. Characterization of a Recombinant Thermostable Newcastle Disease Virus (NDV) Expressing Glycoprotein gB of Infectious Laryngotracheitis Virus (ILTV) Protects Chickens against ILTV Challenge. Viruses 2023, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Song, M.; Song, C.; Zhang, Y.; Wang, X.; Huang, Q.; Wang, B.; Yang, P.; Zhao, S.; Li, Y.; et al. Single-Dose Vaccination of Recombinant Chimeric Newcastle Disease Virus (NDV) LaSota Vaccine Strain Expressing Infectious Bursal Disease Virus (IBDV) VP2 Gene Provides Full Protection against Genotype VII NDV and IBDV Challenge. Vaccines 2021, 9, 1483. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Cao, X.L.; Wang, Y.X.; Guo, X.C.; Liu, J.M.; Xue, Z.Q.; Li, H.J.; Wang, W.; Zhang, T.T.; Li, Q.; et al. Development and evaluation of a bivalent vaccine based on recombinant newcastle disease virus expressing infectious bursal disease virus VP2L-CH3-CH4 in SPF chickens. Vet. Microbiol. 2024, 288, 109950. [Google Scholar] [CrossRef]
- Tian, K.Y.; Guo, H.F.; Li, N.; Zhang, Y.H.; Wang, Z.; Wang, B.; Yang, X.; Li, Y.T.; Zhao, J. Protection of chickens against hepatitis-hydropericardium syndrome and Newcastle disease with a recombinant Newcastle disease virus vaccine expressing the fowl adenovirus serotype 4 fiber-2 protein. Vaccine 2020, 38, 1989–1997. [Google Scholar] [CrossRef]
- Sun, M.; Dong, J.; Li, L.; Lin, Q.; Sun, J.; Liu, Z.; Shen, H.; Zhang, J.; Ren, T.; Zhang, C. Recombinant Newcastle disease virus (NDV) expressing Duck Tembusu virus (DTMUV) pre-membrane and envelope proteins protects ducks against DTMUV and NDV challenge. Vet. Microbiol. 2018, 218, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cong, Y.; Yin, R.; Feng, N.; Yang, S.; Xia, X.; Xiao, Y.; Wang, W.; Liu, X.; Hu, S.; et al. Generation and evaluation of a recombinant genotype VII Newcastle disease virus expressing VP3 protein of Goose parvovirus as a bivalent vaccine in goslings. Virus Res. 2015, 203, 77–83. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Liu, G.; Chen, Z.; Jia, R. Generation and evaluation of a recombinant goose origin Newcastle disease virus expressing Cap protein of goose origin avastrovirus as a bivalent vaccine in goslings. Poult. Sci. 2019, 98, 4426–4432. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.; Kekungu, P.; Barman, N.N.; Kumar, S. Evaluation of surface glycoproteins of classical swine fever virus as immunogens and reagents for serological diagnosis of infections in pigs: A recombinant Newcastle disease virus approach. Arch. Virol. 2019, 164, 3007–3017. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, F.; Li, Z.; Zhao, G.; Xie, C.; Ha, Z.; Zhang, J.; Han, J.; Xiao, P.; Zhuang, X.; et al. Construction and immunological evaluation of recombinant Newcastle disease virus vaccines expressing highly pathogenic porcine reproductive and respiratory syndrome virus GP3/GP5 proteins in pigs. Vet. Microbiol. 2019, 239, 108490. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, J.; Wen, Z.; Chen, W.; Wang, X.; Liu, R.; Bu, Z. Characterization of a recombinant Newcastle disease virus expressing the glycoprotein of bovine ephemeral fever virus. Arch. Virol. 2017, 162, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, X.; Tian, M.; Gao, Y.; Wen, Z.; Yu, G.; Zhou, W.; Zu, S.; Bu, Z. Recombinant Newcastle disease viral vector expressing hemagglutinin or fusion of canine distemper virus is safe and immunogenic in minks. Vaccine 2015, 33, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, X.; Tao, L.; Wen, Z.; Feng, N.; Yang, S.; Xia, X.; Yang, C.; Chen, H.; Bu, Z. Newcastle disease virus-vectored rabies vaccine is safe, highly immunogenic, and provides long-lasting protection in dogs and cats. J. Virol. 2011, 85, 8241–8252. [Google Scholar] [CrossRef] [PubMed]
- Tcheou, J.; Raskin, A.; Singh, G.; Kawabata, H.; Bielak, D.; Sun, W.; González-Domínguez, I.; Sather, D.N.; García-Sastre, A.; Palese, P.; et al. Safety and Immunogenicity Analysis of a Newcastle Disease Virus (NDV-HXP-S) Expressing the Spike Protein of SARS-CoV-2 in Sprague Dawley Rats. Front. Immunol. 2021, 12, 791764. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; An, Y.H.; Jang, J.J.; Jeon, J.H.; Jang, S.H.; Jang, H. The human ACE-2 receptor binding domain of SARS-CoV-2 express on the viral surface of the Newcastle disease virus as a non-replicating viral vector vaccine candidate. PLoS ONE 2022, 17, e0263684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, P.; Bai, S.; Lv, M.; Wang, J.; Chen, W.; Yu, Q.; Wu, J. Heterologous prime-boost regimens with HAdV-5 and NDV vectors elicit stronger immune responses to Ebola virus than homologous regimens in mice. Arch. Virol. 2021, 166, 3333–3341. [Google Scholar] [CrossRef]
- Khattar, S.K.; DeVico, A.L.; LaBranche, C.C.; Panda, A.; Montefiori, D.C.; Samal, S.K. Enhanced Immune Responses to HIV-1 Envelope Elicited by a Vaccine Regimen Consisting of Priming with Newcastle Disease Virus Expressing HIV gp160 and Boosting with gp120 and SOSIP gp140 Proteins. J. Virol. 2016, 90, 1682–1686. [Google Scholar] [CrossRef]
- Nath, B.; Vandna; Saini, H.M.; Prasad, M.; Kumar, S. Evaluation of Japanese encephalitis virus E and NS1 proteins immunogenicity using a recombinant Newcastle disease virus in mice. Vaccine 2020, 38, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Viktorova, E.G.; Khattar, S.K.; Kouiavskaia, D.; Laassri, M.; Zagorodnyaya, T.; Dragunsky, E.; Samal, S.; Chumakov, K.; Belov, G.A. Newcastle Disease Virus-Based Vectored Vaccine against Poliomyelitis. J. Virol. 2018, 92, e00976-18. [Google Scholar] [CrossRef]
- Song, K.Y.; Wong, J.; Gonzalez, L.; Sheng, G.; Zamarin, D.; Fong, Y. Antitumor efficacy of viral therapy using genetically engineered Newcastle disease virus [NDV(F3aa)-GFP] for peritoneally disseminated gastric cancer. J. Mol. Med. 2010, 88, 589–596. [Google Scholar] [CrossRef]
- Bu, X.; Li, M.; Zhao, Y.; Liu, S.; Wang, M.; Ge, J.; Bu, Z.; Yan, Y. Genetically engineered Newcastle disease virus expressing human interferon-λ1 induces apoptosis in gastric adenocarcinoma cells and modulates the Th1/Th2 immune response. Oncol. Rep. 2016, 36, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liang, B.; Zhang, J.; Liu, Y.; Bu, X. Apoptotic induction of lung adenocarcinoma A549 cells infected by recombinant RVG Newcastle disease virus (rL-RVG) in vitro. Mol. Med. Rep. 2015, 11, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, S.; Wang, T.; Li, Y.; Jiang, K.; Lin, G.; Ma, Y.; Barr, M.P.; Song, F.; Zhang, G.; et al. Oncolytic newcastle disease virus triggers cell death of lung cancer spheroids and is enhanced by pharmacological inhibition of autophagy. Am. J. Cancer Res. 2015, 5, 3612–3623. [Google Scholar] [PubMed]
- Keshavarz, M.; Nejad, A.S.M.; Esghaei, M.; Bokharaei-Salim, F.; Dianat-Moghadam, H.; Keyvani, H.; Ghaemi, A. Oncolytic Newcastle disease virus reduces growth of cervical cancer cell by inducing apoptosis. Saudi J. Biol. Sci. 2020, 27, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shao, X.; Gu, L.; Jiang, K.; Wang, S.; Chen, J.; Fang, J.; Guo, X.; Yuan, M.; Shi, J.; et al. Targeting STAT3 enhances NDV-induced immunogenic cell death in prostate cancer cells. J. Cell Mol. Med. 2020, 24, 4286–4297. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhong, L.P.; He, J.; Huang, Y.; Zhao, Y.X. Application of Newcastle disease virus in the treatment of colorectal cancer. World J. Clin. Cases 2019, 7, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, J.M.; Mustafa, Z.; Ideris, A. Newcastle disease virus interaction in targeted therapy against proliferation and invasion pathways of glioblastoma multiforme. Biomed. Res. Int. 2014, 2014, 386470. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Sun, T.-K.; Chen, M.-S.; Munir, M.; Liu, H.-J. Oncolytic viruses-modulated immunogenic cell death, apoptosis and autophagy linking to virotherapy and cancer immune response. Front. Cell. Infect. Microbiol. 2023, 13, 1142172. [Google Scholar] [CrossRef] [PubMed]
- Csatary, L.K.; Moss, R.W.; Beuth, J.; Töröcsik, B.; Szeberenyi, J.; Bakacs, T. Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H). Anticancer Res. 1999, 19, 635–638. [Google Scholar]
- Pecora, A.L.; Rizvi, N.; Cohen, G.I.; Meropol, N.J.; Sterman, D.; Marshall, J.L.; Goldberg, S.; Gross, P.; O’Neil, J.D.; Groene, W.S.; et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 2002, 20, 2251–2266. [Google Scholar] [CrossRef]
- Yaacov, B.; Eliahoo, E.; Lazar, I.; Ben-Shlomo, M.; Greenbaum, I.; Panet, A.; Zakay-Rones, Z. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008, 15, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V.; Fournier, P. Newcastle disease virus: A promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol. Biol. 2009, 542, 565–605. [Google Scholar] [PubMed]
- Wheelock, E.F.; Dingle, J.H. Observations on the repeated administration of viruses to a patient with acute leukemia. N. Engl. J. Med. 1964, 24, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cassel, W.A.; Murray, D.R. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med. Oncol. Tumor Pharmacother. 1992, 9, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Batliwalla, F.M.; Bateman, B.A.; Serrano, D.; Murray, D.; Macphail, S.; Maino, V.C.; Ansel, J.C.; Gregersen, P.K.; Armstrong, C.A. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol. Med. 1998, 4, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Csatary, L.K.; Eckhardt, S.; Bukosza, I.; Czegledi, F.; Fenyvesi, C.; Gergely, P.; Bodey, B.; Csatary, C.M. Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect. Prev. 1993, 17, 619–627. [Google Scholar] [PubMed]
- Csatary, L.K.; Gosztonyi, G.; Szeberenyi, J.; Fabian, Z.; Liszka, V.; Bodey, B.; Csatary, C.M. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J. Neurooncol 2004, 67, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Lorence, R.M.; Pecora, A.L.; Major, P.P.; Hotte, S.J.; Laurie, S.A.; Roberts, M.S.; Groene, W.S.; Bamat, M.K. Overview of phase I studies of intravenous administration of PV701, an oncolytic virus. Curr. Opin. Mol. Ther. 2003, 5, 618–624. [Google Scholar] [PubMed]
- Freeman, A.I.; Zakay-Rones, Z.; Gomori, J.M.; Linetsky, E.; Rasooly, L.; Greenbaum, E.; Rozenman-Yair, S.; Panet, A.; Libson, E.; Irving, C.S.; et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef]
- Kalyanasundram, J.; Hamid, A.; Yusoff, K.; Chia, S.L. Newcastle disease virus strain AF2240 as an oncolytic virus: A review. Acta Trop. 2018, 183, 126–133. [Google Scholar] [CrossRef]
- Najmuddin, S.; Amin, Z.M.; Tan, S.W.; Yeap, S.K.; Kalyanasundram, J.; Ani, M.A.C.; Veerakumarasivam, A.; Chan, S.C.; Chia, S.L.; Yusoff, K.; et al. Cytotoxicity study of the interleukin-12-expressing recombinant Newcastle disease virus strain, rAF-IL12, towards CT26 colon cancer cells in vitro and in vivo. Cancer Cell Int. 2020, 20, 278. [Google Scholar] [CrossRef]
- Syed Najmuddin, S.U.F.; Amin, Z.M.; Tan, S.W.; Yeap, S.K.; Kalyanasundram, J.; Veerakumarasivam, A.; Chan, S.C.; Chia, S.L.; Yusoff, K.; Alitheen, N.B. Oncolytic effects of the recombinant Newcastle disease virus, rAF-IL12, against colon cancer cells in vitro and in tumor-challenged NCr-Foxn1nu nude mice. PeerJ 2020, 8, e9761. [Google Scholar] [CrossRef]
- Ahlert, T.; Sauerbrei, W.; Bastert, G.; Ruhland, S.; Bartik, B.; Simiantonaki, N.; Schumacher, J.; Häcker, B.; Schumacher, M.; Schirrmacher, V. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J. Clin. Oncol. 1997, 15, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, H.H.; Anton, P.; Atzpodien, J. Adjuvant treatment of locally advanced renal cancer with autologous virus-modified tumor vaccines. World J. Urol. 1995, 13, 171–173. [Google Scholar] [CrossRef]
- Karcher, J.; Dyckhoff, G.; Beckhove, P.; Reisser, C.; Brysch, M.; Ziouta, Y.; Helmke, B.H.; Weidauer, H.; Schirrmacher, V.; Herold-Mende, C. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res. 2004, 64, 8057–8061. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.H.; Bonsanto, M.M.; Beckhove, P.; Brysch, M.; Geletneky, K.; Ahmadi, R.; Schuele-Freyer, R.; Kremer, P.; Ranaie, G.; Matejic, D.; et al. Antitumor vaccination of patients with glioblastoma multiforme: A pilot study to assess feasibility, safety, and clinical benefit. J. Clin. Oncol. 2004, 22, 4272–4281. [Google Scholar] [CrossRef]
- Ockert, D.; Schirrmacher, V.; Beck, N.; Stoelben, E.; Ahlert, T.; Flechtenmacher, J.; Hagmüller, E.; Buchcik, R.; Nagel, M.; Saeger, H.D. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin. Cancer Res. 1996, 2, 21–28. [Google Scholar]
- Schulze, T.; Kemmner, W.; Weitz, J.; Wernecke, K.D.; Schirrmacher, V.; Schlag, P.M. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: Results of a prospective randomized trial. Cancer Immunol. Immunother. 2009, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, H.; Sun, T.M.; Yao, W.Q.; Chen, L.L.; Jin, Y.; Li, C.L.; Meng, F.J. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J. Gastroenterol. 2003, 9, 495–498. [Google Scholar] [CrossRef]
- Schirrmacher, V.; Schlude, C.; Weitz, J.; Beckhove, P. Strong T-cell costimulation can reactivate tumor antigen-specific T cells in late-stage metastasized colorectal carcinoma patients: Results from a phase I clinical study. Int. J. Oncol. 2015, 46, 71–77. [Google Scholar] [CrossRef]
- Numpadit, S.; Ito, C.; Nakaya, T.; Hagiwara, K. Investigation of oncolytic effect of recombinant Newcastle disease virus in primary and metastatic oral melanoma. Med. Oncol. 2023, 40, 138. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Tian, G.; Liu, Y.; He, J.; Gao, X.; Yu, Y.; Liu, X.; Zhang, X.; Sun, T.; Liu, S.; et al. Recombinant Newcastle Disease Virus Encoding IL-12 and/or IL-2 as Potential Candidate for Hepatoma Carcinoma Therapy. Technol. Cancer Res. Treat. 2016, 15, Np83–Np94. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, Q.; Mei, Y.; Liu, Y.; Zhao, L. Newcastle disease virus co-expressing interleukin 7 and interleukin 15 modified tumor cells as a vaccine for cancer immunotherapy. Cancer Sci. 2018, 109, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yi, C.; Yang, X.; Xu, J.; Sun, Q.; Liu, Y.; Zhao, L. Tumor Cells Modified with Newcastle Disease Virus Expressing IL-24 as a Cancer Vaccine. Mol. Ther. Oncolytics 2019, 14, 213–221. [Google Scholar] [CrossRef]
- Huang, F.Y.; Wang, J.Y.; Dai, S.Z.; Lin, Y.Y.; Sun, Y.; Zhang, L.; Lu, Z.; Cao, R.; Tan, G.H. A recombinant oncolytic Newcastle virus expressing MIP-3α promotes systemic antitumor immunity. J. Immunother. Cancer 2020, 8, e000330. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Liu, T.; Jiang, S.; Cao, Y.; Kang, K.; Su, H.; Ren, G.; Wang, Z.; Xiao, W.; Li, D. Oncolytic Newcastle disease virus expressing the co-stimulator OX40L as immunopotentiator for colorectal cancer therapy. Gene Ther. 2023, 30, 64–74. [Google Scholar] [CrossRef]
- Nettelbeck, D.M.; Leber, M.F.; Altomonte, J.; Angelova, A.; Beil, J.; Berchtold, S.; Delic, M.; Eberle, J.; Ehrhardt, A.; Engeland, C.E.; et al. Virotherapy in Germany-Recent Activities in Virus Engineering, Preclinical Development, and Clinical Studies. Viruses 2021, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, G.; Palese, P.; Goff, P.H. Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma. EBioMedicine 2019, 49, 96–105. [Google Scholar] [CrossRef]
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Tian, J.; Zhao, J.; Zhao, Y.; Zhang, G. The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy. Viruses 2024, 16, 886. https://doi.org/10.3390/v16060886
Yang H, Tian J, Zhao J, Zhao Y, Zhang G. The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy. Viruses. 2024; 16(6):886. https://doi.org/10.3390/v16060886
Chicago/Turabian StyleYang, Huiming, Jiaxin Tian, Jing Zhao, Ye Zhao, and Guozhong Zhang. 2024. "The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy" Viruses 16, no. 6: 886. https://doi.org/10.3390/v16060886
APA StyleYang, H., Tian, J., Zhao, J., Zhao, Y., & Zhang, G. (2024). The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy. Viruses, 16(6), 886. https://doi.org/10.3390/v16060886







