Geminiviridae and Alphasatellitidae Diversity Revealed by Metagenomic Analysis of Susceptible and Tolerant Tomato Cultivars across Distinct Brazilian Biomes
Abstract
:1. Introduction
2. Material and Methods
2.1. Leaf Samples from Tomato Plants with Begomovirus-Like Symptoms
2.2. DNA Extraction and Molecular Marker Confirmation of the Presence of the Ty–1 and Ty–3 Introgressions
2.3. Enrichment of Circular DNAs by Rolling Circle Amplification—RCA and Confirmation of Begomovirus Infection
2.4. Preparation of Pools of Samples to High-Throughput Sequencing (HTS)
2.5. Viral Sequence Analyses
2.6. Detection of Viruses in Individual Samples by PCR with Virus-Specific Primers
2.7. PCR Conditions Used to Detect ssDNA Viruses and Subviral Agents in Individual Samples within Each Pool
2.8. Validation via Sanger Dideoxy Termination Sequencing of the Amplicons Obtained with Species-Specific PCR Primers
3. Results
3.1. Viral Diversity in the BP1 Pool (Composed of Samples from North, Northeast, and South Brazilian Regions)
3.2. Viral Diversity in the BP2 Pool (with Samples from Southeast and Central–West Regions)
3.3. Comparative Viral Diversity: BP1 Pool (North, Northeast, and South Regions) Versus BP2 Pool (Southeast and Central–West Regions)
3.4. PCR Detection with Species-Specific Primers of Geminiviruses and Subviral Pathogens in Individual Samples of the BP1 and BP2 Pools
3.4.1. Northern Region
3.4.2. Northeast Region
3.4.3. South Region
3.4.4. Southeast and Central–West Regions
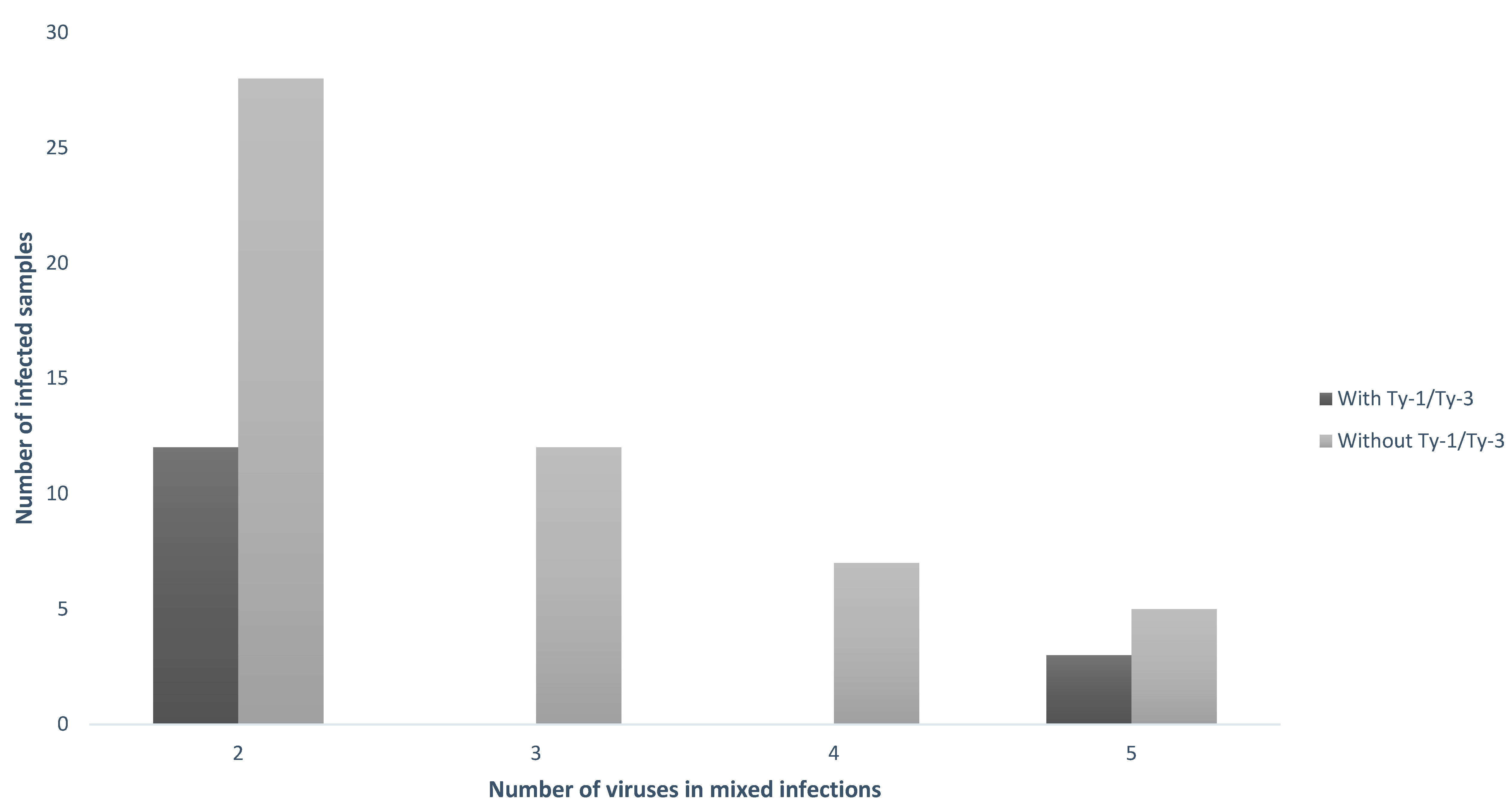
3.5. Comparative Diversity of Samples with Versus without the Ty–1/Ty–3 Introgressions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roumagnac, P.; Lett, J.M.; Fiallo-Olivé, E.; Navas-Castillo, J.; Zerbini, F.M.; Martin, D.P.; Varsani, A. Establishment of five new genera in the family Geminiviridae: Citlodavirus, Maldovirus, Mulcrilevirus, Opunvirus, and Topilevirus. Arch. Virol. 2022, 167, 695–710. [Google Scholar] [CrossRef]
- Rojas, M.R.; Macedo, M.; Malian, M. Soto-Aguilar, M.; Souza, J.; Briddon, R.; Kenyon, L. Rivera Bustamante, R.; Zerbini, F.; Adkins, S. World management of geminiviruses. Annu. Rev. Phytopathol. 2018, 56, 637–677. [Google Scholar] [CrossRef]
- Brown, J.K.; Zerbini, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.C.F.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A.; et al. Revision of begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef] [PubMed]
- Sunter, G.; Hartitz, M.D.; Hormuzdi, S.G.; Brough, C.L.; Bisaro, D.M. Genetic analysis of tomato golden mosaic virus: ORF AL2 is required for coat protein accumulation while ORF AL3 is necessary for efficient DNA replication. Virology 1990, 179, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hanley-Bowdoin, L.; Bejarano, E.R.; Robertson, D.; Mansoor, S. Geminiviruses: Masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 2013, 11, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, F.; Meng, Q.; Yu, H.; Wu, G.; Li, M.; Qing, L. The C5 protein encoded by Ageratum leaf curl Sichuan virus is a virulence factor and contributes to the virus infection. Mol. Plant Pathol. 2021, 22, 1149–1158. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Hu, T.; Zhou, X. Identification of two distinct begomoviruses infecting Malvastrum coromandelianum. Phytopathology Res. 2021, 3, 8. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Lozano-Duran, R.; Hu, T.; Zhou, X. Identification of a novel C6 protein encoded by tomato leaf curl China virus. Phytopatholy. Res. 2022, 4, 46. [Google Scholar] [CrossRef]
- Liu, H.; Chang, Z.; Zhao, S.; Gong, P.; Zhang, M.; Lozano-Durán, R.; Yan, H.; Zhou, X.; Li, F. Functional identification of a novel C7 protein of tomato yellow leaf curl virus. Virology 2023, 585, 117–126. [Google Scholar] [CrossRef]
- Noueiry, A.O.; Lucas, W.J.; Gilbertson, R.L. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 1994, 76, 925–932. [Google Scholar] [CrossRef]
- Fontes, E.P.; Eagle, P.A.; Sipe, P.S.; Luckow, V.A.; Hanley-Bowdoin, L. Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J. Biol. Chem. 1994, 269, 8459–8465. [Google Scholar] [CrossRef] [PubMed]
- Argüello-Astorga, G.; Ruiz-Medrano, R. An iteron-related domain is associated to Motif 1 in the replication proteins of geminiviruses: Identification of potential interacting amino acid-base pairs by a comparative approach. Arch. Virol. 2001, 146, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Argüello-Astorga, G.; Lopez-Ochoa, L.; Kong, L.J.; Orozco, B.M.; Settlage, S.B.; Hanley-Bowdoin, L. A novel motif in geminivirus replication proteins interacts with the plant retinoblastoma-related protein. J. Virol. 2004, 78, 4817–4826. [Google Scholar] [CrossRef] [PubMed]
- Cantú-Iris, M.; Pastor-Palacios, G.; Mauricio-Castillo, J.A.; Bañuelos-Hernández, B.; Avalos-Calleros, J.A.; Juárez-Reyes, A.; Rivera-Bustamante, R.; Argüello-Astorga, G.R. Analysis of a new begomovirus unveils a composite element conserved in the CP gene promoters of several Geminiviridae genera: Clues to comprehend the complex regulation of late genes. PLoS ONE 2019, 14, e0210485. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Song, Y.; Wang, Y.; Zhou, X. Functional analysis of a novel βV1 gene identified in a geminivirus betasatellite. Sci. China Life Sci. 2020, 63, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.G.; Zerbini, F.M.; Navas-Castillo, J.; Fiallo-Olivé, E. Revealing the complexity of sweepovirus-deltasatellite–plant host interactions: Expanded natural and experimental helper virus range and effect dependence on virus-host combination. Microorganisms 2021, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Zarreen, F.; Chakraborty, S. Roles of two distinct alphasatellites modulating geminivirus pathogenesis. Virol. J. 2021, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- Matyis, J.C.; Silva, D.M.; Oliveira, A.A.R.; Costa, A.S. Purificação e morfologia do vírus do mosaico dourado do tomateiro. Summa Phytopathol. 1975, 1, 267–274. [Google Scholar]
- Fernandes, D.S.; Okuma, D.; Pantoja-Gomez, L.M.; Cuenca, A.; Corrêa, A.S. Bemisia tabaci MEAM1 still remains the dominant species in open field crops in Brazil. Brazilian J. Biol. 2022, 84, e256949. [Google Scholar] [CrossRef]
- Reis, L.N.A.; Fonseca, M.E.N.; Ribeiro, S.G.; Naito, F.Y.B.; Boiteux, L.S.; Pereira-Carvalho, R.C. Metagenomics of neotropical single-stranded DNA viruses in tomato cultivars with and without the Ty–1 gene. Viruses 2020, 12, 819. [Google Scholar] [CrossRef]
- Reis, L.N.A.; Boiteux, L.S.; Fonseca, M.E.N.; Rojas, M.R.; Gilbertson, R.L.; Pereira-Carvalho, R.C. Tomato golden net virus and tomato yellow net virus: Two novel New World begomoviruses with monopartite genomes. Arch. Virol. 2023, 168, 235. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Silva-Lobo, V.; Santana, F.; Fonseca, M.; Boiteux, L. Inheritance of resistance to the bipartite Tomato chlorotic mottle begomovirus derived from Lycopersicon esculentum cv. ‘Tyking’. Euphytica 2005, 143, 27–33. [Google Scholar] [CrossRef]
- Boiteux, L.; Oliveira, V.; Silva, C.; Makishima, N.; Inoue-Nagata, A.; Fonseca, M.; Giordano, L. Reaction of tomato hybrids carrying the Ty–1 locus to Brazilian bipartite begomovirus species. Hort. Bras. 2007, 25, 20–23. [Google Scholar] [CrossRef]
- García-Cano, E.; Resende, R.O.; Boiteux, L.S.; Giordano, L.B.; Fernández-Muñoz, R.; Moriones, E. Phenotypic expression, stability, and inheritance of a recessive resistance to monopartite begomoviruses associated with tomato yellow leaf curl disease in tomato. Phytopathology 2008, 98, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Carvalho, R.C.; Boiteux, L.S.; Fonseca, M.E.N.; Díaz-Pendón, J.A.; Moriones, E.; Fernández-Muñoz, R.; Charchar, J.M.; Resende, R.O. Multiple resistance to Meloidogyne spp. and to bipartite and monopartite Begomovirus spp. in wild Solanum (Lycopersicon) accessions. Plant Dis. 2010, 94, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Carvalho, R.C.; Díaz-Pendón, J.A.; Fonseca, M.E.N.; Boiteux, L.S.; Fernández-Muñoz, R.; Moriones, E.; Resende, R.O. Recessive resistance derived from tomato cv. Tyking-limits drastically the spread of tomato yellow leaf curl virus. Viruses 2015, 7, 2518–2533. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, M.G.; Hutton, S.F.; Ibrahem, R.M.; Kormelink, R.; Visser, R.G.; Scott, J.W.; Edwards, J.D.; Bai, Y. The tomato yellow leaf curl virus resistance genes Ty–1 and Ty–3 are allelic and code for DFDGD-class RNA–dependent RNA polymerases. PLoS Genet. 2013, 9, e1003399. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.I.; Jones, A.T. Responses of plants to viruses: Proposals for the use of terms. Phytopathology 1983, 73, 127–128. [Google Scholar] [CrossRef]
- Butterbach, P.; Verlaan, M.G.; Dullemans, A.; Lohuis, D.; Visser, R.G.; Bai, Y.; Kormelink, R. Tomato yellow leaf curl virus resistance by Ty–1 involves increased cytosine methylation of viral genomes and is compromised by cucumber mosaic virus infection. Proc. Natl. Acad. Sci. USA 2014, 35, 12942–12947. [Google Scholar] [CrossRef]
- Boiteux, L.S.; Fonseca, M.E.N.; Simon, P.W. Effects of plant tissue and DNA purification method on randomly amplified polymorphic DNA-based genetic fingerprinting analysis in carrot. J. Am. Soc. Hor. Sci. 1999, 124, 32–38. [Google Scholar] [CrossRef]
- Maxwell, D.M.C.; Salus, M.; Montes, L.; Mejía, L. Tagging Begomovirus Resistance Gene. Available online: www.plantpath.wisc.edu (accessed on 20 January 2024).
- Inoue-Nagata, A.K.; Albuquerque, L.; Rocha, W.; Nagata, T. A simple method for cloning the complete begomovirus genome using the bacteriophage-29 DNA polymerase. J. Virol. Methods 2004, 116, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.R.; Gilbertson, R.; Russell, D.; Maxwell, D. Use of degenerate primers in the polymerase chain reaction to detect whitefly-transmitted geminiviruses. Plant Dis. 1993, 77, 340–347. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Nery, F.M.; Melo, F.L.; Boiteux, L.S.; Ribeiro, S.G.; Resende, R.O.; Orilio, A.F.; Batista, J.G.; Lima, M.F.; Pereira-Carvalho, R.C. Molecular characterization of Hovenia dulcis-associated virus 1 (HDaV1) and 2 (HDaV2): New tentative species within the order Picornavirales. Viruses 2020, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Menzel, P.; Ng, K.; Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nature Com. 2016, 7, 11257. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.G.; Ambrozevícius, L.P.; Ávila, A.C.; Bezerra, I.C.; Calegario, R.F.; Fernandes, J.J.; Lima, M.F.; Mello, R.N.; Rocha, H.; Zerbini, F.M. Distribution and genetic diversity of tomato-infecting begomoviruses in Brazil. Arch. Virol. 2003, 148, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.R.; Albuquerque, L.C.; Giordano, L.B.; Boiteux, L.S.; Avila, A.C.; Inoue-Nagata, A.K. Diversity and prevalence of Brazilian bipartite Begomovirus species associated to tomatoes. Virus Genes 2008, 36, 251–258. [Google Scholar] [CrossRef]
- Cotrim, A.A.; Krause-Sakate, R.; Narita, N.; Zerbini, F.M.; Pavan, M.A. Diversidade genética de begomovírus em cultivos de tomateiro no Centro-Oeste Paulista. Summa Phytopathol. 2007, 33, 300–303. [Google Scholar] [CrossRef]
- Calegario, R.; Ferreira, S.; Andrade, C.; Zerbini, F. Caracterização de um isolado do begomovírus Sida micrantha mosaic virus (SiMMV) obtido de tomateiro. Fitopatol. Bras. 2004, 29, 150. [Google Scholar]
- Duarte, M.F.; Pereira-Carvalho, R.C.; Reis, L.N.A.; Rojas, M.R.; Gilbertson, R.L.; Costa, H.; Boiteux, L.S.; Fonseca, M.E.N. Natural infection of tomatoes (Solanum lycopersicum) by Euphorbia yellow mosaic virus isolates across four Brazilian states. Plant Dis. 2021, 105, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.G.; Martin, D.P.; Lacorte, C.; Simões, I.C.; Orlandini, D.R.S.; Inoue-Nagata, A.K. Molecular and biological characterization of tomato chlorotic mottle virus suggests that recombination underlies the evolution and diversity of Brazilian tomato begomoviruses. Phytopathology 2007, 97, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Mituti, T.; Moura, M.F.; Macedo, M.A.; Silva, T.N.; Pinto, L.R.; Costa, H.; Krause-Sakate, R.; Inoue-Nagata, A.K.; Nunes, G.G.; Lima, M.F.; et al. Survey of begomoviruses and the crinivirus, tomato chlorosis virus, in solanaceous in Southeast/Midwest of Brazil. Trop. Plant Pathol. 2019, 44, 468–472. [Google Scholar] [CrossRef]
- Reis, L.N.A.; Boiteux, L.S.; Fonseca, M.E.N.; Pereira–Carvalho, R.C. Tomato yellow vein streak virus and tomato golden vein virus: A reappraisal of the classification status of two South American Begomovirus species based upon genome-wide pairwise identity of multiple isolates. Virus Genes 2021, 57, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.C.; Varsani, A.; Fernandes, F.R.; Pinheiro, B.; Martin, D.P.; Ferreira, P.T.O.; Lemos, T.O.; Inoue–Nagata, A.K. Further characterization of tomato-infecting begomoviruses in Brazil. Arch. Virol. 2012, 157, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.F.; Fonseca, M.E.; Costa, H.; Fernandes, N.A.; Reis, A.; Boiteux, L.S.; Pereira-Carvalho, R.C. Diversity of tomato-infecting begomoviruses and spatiotemporal dynamics of an endemic viral species of the Brazilian Atlantic rain forest biome. Virus Genes 2021, 57, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Vaghi-Medina, C.G.; Teppa, E.; Bornancini, V.A.; Flores, C.R.; Marino-Buslje, C.; López Lambertini, P.M. Tomato apical leaf curl virus: A novel, monopartite geminivirus detected in tomatoes in Argentina. Front. Microbiol. 2018, 8, 2665. [Google Scholar] [CrossRef]
- ICTV International Committee on Taxonomy of Viruses. Available online: http://www.ictvonline.org (accessed on 15 January 2024).
- Lett, J.M.; De Bruyn, A.; Hoareau, M.; Ouattara, A.; Claverie, S.; Dalmon, A.; Laplace, D.; Lefeuvre, P.; Hostachy, B. Tomato chlorotic mottle Guyane virus: A novel tomato-infecting bipartite begomovirus from French Guiana. Arch. Virol. 2015, 160, 2887–2890. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Nishimura, Y.; Shimizu, Y.; Nishiyama, H.; Yoshikawa, G.; Uehara, H.; Hingamp, P.; Goto, S.; Ogata, H. Linking virus genomes with host taxonomy. Viruses 2016, 8, 66. [Google Scholar] [CrossRef]
- Sastry, K.S.; Mandal, B.; Hammond, J.; Scott, S.W.; Briddon, R.W. Encyclopedia of Plant Viruses and Viroids, 1st ed.; Springer: New Delhi, India, 2019; p. 2936. [Google Scholar]
- Kitajima, E.W. An annotated list of plant viruses and viroids described in Brazil (1926–2018). Biota Neot. 2020, 20, e20190932. [Google Scholar] [CrossRef]
- GenBank. Available online: www.ncbi.nlm.nih.gov/ (accessed on 20 January 2024).
- Seal, S.; VandenBosch, F.; Jeger, M. Factors influencing begomovirus evolution and their increasing global significance: Implications for sustainable control. Crit. Rev. Plant Sci. 2006, 25, 23–46. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Navas-Castillo, J. The Role of Extensive Recombination in the Evolution of Geminiviruses. In Viral Fitness and Evolution, 1st ed.; Domingo, E., Schuster, P., Elena, S.F., Perales, C., Domingo, E., Eds.; Springer: Cham, Switzerland, 2023; Volume 439, pp. 139–166. [Google Scholar]
- Maclot, F.; Candresse, T.; Filloux, D.; Malmstrom, C.M.; Roumagnac, P.; Van-der-Vlugt, R.; Massart, S. Illuminating an ecological blackbox: Using high throughput sequencing to characterize the plant virome across scales. Front. Microbiol. 2020, 11, 578064. [Google Scholar] [CrossRef] [PubMed]
- Quadros, A.F.; Silva, J.P.; Xavier, C.A.; Zerbini, F.M.; Boari, A.J. Two new begomoviruses infecting tomato and Hibiscus sp. in the Amazon region of Brazil. Arch. Virol. 2019, 164, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Calegario, R.F.; Ferreira, S.S.; Andrade, E.C.; Zerbini, F.M. Characterization of tomato yellow spot virus, a novel tomato-infecting begomovirus in Brazil. Pes. Agr. Bras. 2007, 42, 1335–1343. [Google Scholar] [CrossRef]
- Ferro, M.M.; Ramos-Sobrinho, R.; Silva, J.T.; Assunção, I.P.; Lima, G.S. Genetic structure of populations of the begomoviruses Tomato mottle leaf curl virus and Sida mottle Alagoas virus infecting tomato (Solanum lycopersicum) and Sida spp., respectively. Trop. Plant Pathol. 2017, 42, 39–45. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Ann. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, R.S.; Ribeiro, G.C.; Lamas, N.S.; Ribeiro, S.G.; Costa, A.F.; Boiteux, L.S.; Fonseca, M.E.N. First report of Sida micrantha mosaic virus infecting Oxalis species in Brazil. Plant Dis. 2018, 102, 1862. [Google Scholar] [CrossRef]
- Pereira-Silva, J.; Boiteux, L.S.; Fonseca, M.E.N.; Reis, L.N.A.; Souza, A.S.; Nery, F.M.B.; Madeira, N.R.; Pereira-Carvalho, R.C. Novel natural hosts of tomato severe rugose virus (ToSRV) in the Fabaceae, Solanaceae, and Oxalidaceae families. J. Plant Dis. Prot. 2022, 129, 425–431. [Google Scholar] [CrossRef]
- Lima, A.T.M.; Pereira, C.D.O.; Alfenas, P.F.; Paula, M.B.; Mello, R.N.; Zerbini, F.M. Primeiro relato de infecção pelo geminivírus tomato severe rugose virus (ToSRV) em tomateiro no estado de Santa Catarina. Fitopatol. Bras. 2006, 31, 223–224. [Google Scholar]
- Fernandes-Acioli, N.A.N.; Boiteux, L.S.; Fonseca, M.D.N.; Segnana, L.R.G.; Kitajima, E.W. Report of tomato yellow spot virus infecting Leonurus sibiricus in Paraguay and within tomato fields in Brazil. Plant Dis. 2014, 98, 1445. [Google Scholar] [CrossRef]
- Gorayeb, E.S.; Nascimento, S.C.; Santos, A.N.M.R.; Batalhon, L.; Albuquerque, M.R.M.; Oliveira, V.G.F.; Souza, V.B.; Bogo, A.; Silva, F.N. Survey of viruses and vectors in tomato plants, alternative hosts and weeds in the state of Santa Catarina, Brazil. Plant Pathol. 2024, 73, 444–454. [Google Scholar] [CrossRef]
- Silva, F.N.; Lima, A.; Rocha, C.; Castillo-Urquiza, G.; Alves-Júnior, M.; Zerbini, F. Recombination and pseudorecombination driving the evolution of the begomoviruses tomato severe rugose virus (ToSRV) and tomato rugose mosaic virus (ToRMV): Two recombinant DNA–A components sharing the same DNA–B. Virol. J. 2014, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Tovar, R.; Navas-Castillo, J. Deciphering the biology of deltasatellites from the New World: Maintenance by New World begomoviruses and whitefly transmission. New Phytol. 2016, 212, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Paprotka, T.; Metzler, V.; Jeske, H. The first DNA 1-like α satellites in association with New World begomoviruses in natural infections. Virology 2010, 404, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, R.S.; Lamas, N.S.; Lacorte, C.; Lacerda, A.L.M.; Varsani, A.; Ribeiro, S.G. A novel geminivirus identified in tomato and cleome plants sampled in Brazil. Virus Res. 2017, 240, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.G.; Melo, F.L.; Pereira-Carvalho, R.C.; Alves-Freitas, D.M.T.; Ribeiro, S.G. First report of tomato apical leaf curl virus infecting tomato in Brazil. Plant Dis. 2019, 103, 1443. [Google Scholar] [CrossRef]
- Fortes, I.M.; Fernández-Muñoz, R.; Moriones, E. The crinivirus tomato chlorosis virus compromises the control of tomato yellow leaf curl virus in tomato plants by the Ty–1 gene. Phytopathology 2023, 113, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- González-Arcos, M.; Fonseca, M.E.N.; Arruabarrena, A.; Lima, M.F.; Michereff-Filho, M.; Moriones, E.; Fernandez-Munhoz, R.; Boiteux, L.S. Identification of genetic sources with attenuated tomato chlorosis virus-induced symptoms in Solanum (section Lycopersicon) germplasm. Euphytica 2018, 214, 178. [Google Scholar] [CrossRef]
- Prabhandakavi, P.; Pogiri, R.; Kumar, R.; Acharya, S.; Esakky, R.; Chakraborty, M.; Pinnamaneni, R.; Palicherla, S.R. Pyramiding Ty-1/Ty–3, Ty-2, ty-5 and ty-6 genes into tomato hybrid to develop resistance against tomato leaf curl viruses and recurrent parent genome recovery by ddRAD sequencing method. J. Plant Biochem. Biotech. 2021, 30, 462–476. [Google Scholar] [CrossRef]

| Code of the Contigs | Read Coverage | Assembled Genome Size (nts) | BLASTn Coverage (%) | Identity (%) | E-Value | Virus Description * | GeneBank Accession Number |
|---|---|---|---|---|---|---|---|
| 40 | 227,279 | 2593 | 100 | 99.96 | 0 | Tomato severe rugose virus DNA–A 1 | MW573989.1 |
| * | 17,827 | 2660 | 100 | 99.85 | 0 | Tomato yellow leaf deformation dwarf virus DNA–A1 | NC_055586.1 |
| 38 | 131,012 | 2630 | 100 | 99.73 | 0 | Tomato chlorotic mottle Guyane virus DNA–A 1 | MK878452.1 |
| 5 | 31,367 | 2694 | 99 | 99.52 | 0 | Tomato rugose yellow leaf curl virus DNA–A 1 | KU682839.1 |
| 1098 | 9959 | 2661 | 100 | 98.99 | 0 | Tomato yellow spot virus DNA–A 3 | KX348172.1 |
| 63 | 277,339 | 2634 | 100 | 98.97 | 0 | Tomato mottle leaf curl virus DNA–A 4 | KX896408.1 |
| 66 | 55,010 | 2603 | 100 | 98.47 | 0 | Chino del tomate Amazonas virus DNA–A 1 | NC_038443.1 |
| 7 | 170,461 | 2623 | 99 | 98.25 | 0 | Tomato golden leaf distortion virus DNA–A 3 | HM357456.2 |
| 125 | 205,526 | 2631 | 100 | 97.00 | 0 | Tomato mottle leaf curl virus DNA–A 6 | MT215005.1 |
| 144 | 156,309 | 2623 | 99 | 96.85 | 0 | Tomato golden leaf distortion virus DNA–A 2 | HM357456.2 |
| 140 | 77,529 | 2627 | 100 | 96.66 | 0 | Tomato mottle leaf curl virus DNA–A 5 | JF803247.1 |
| 44 | 194,632 | 2632 | 100 | 95.79 | 0 | Tomato mottle leaf curl virus DNA–A 4 | KX896414.1 |
| 552 | 4407 | 2686 | 100 | 95.38 | 0 | Sida micrantha mosaic virus DNA–A 2 | KC706535.1 |
| 105 | 152,241 | 2623 | 99 | 95.18 | 0 | Tomato golden leaf distortion virus DNA–A 1 | HM357456.2 |
| 109 | 14,715 | 2661 | 100 | 95.11 | 0 | Tomato yellow spot virus DNA–A 1 | KX348172.1 |
| 21 | 44,698 | 2622 | 99 | 94.82 | 0 | Tomato bright yellow mottle virus DNA–A 1 | NC_038468.1 |
| 6958 | 2854 | 2643 | 99 | 94.54 | 0 | Sida yellow blotch virus DNA–A 1 | MT103998.1 |
| 67 | 254,814 | 2631 | 100 | 92.49 | 0 | Tomato mottle leaf curl virus DNA–A 1 | KX896414.1 |
| 68 | 129,337 | 2631 | 100 | 91.66 | 0 | Tomato mottle leaf curl virus DNA–A 1 | JF803250.1 |
| 205 | 32,299 | 2685 | 100 | 91.01 | 0 | Sida micrantha mosaic virus DNA–A 1 | EU908733.1 |
| New species #1 | 41,497 | 2604 | 100 | 90.40 | 0 | Tomato interveinal chlorosis virus DNA–A 2 | NC_038469.1 |
| 8679 | 82 | 2139 | 100 | 90.27 | 0 | Tomato-associated geminivirus 1 1 | MN527305.1 |
| New species #2 | 153,967 | 2631 | 100 | 90.17 | 0 | Tomato mottle leaf curl virus DNA–A1 | MT215005.1 |
| New species #3 | 7992 | 2657 | 99 | 87.10 | 0 | Tomato bright yellow mottle virus DNA–A1 | NC_038468.1 |
| New species #4 | 24,589 | 2612 | 97 | 82.80 | 0 | Tomato golden leaf distortion virus DNA–A1 | HM357456.2 |
| Code of the Contigs | Read Coverage | Assembled Genome Size (nts) | BLASTn Coverage (%) | Identity (%) | E-Value | Virus Description * | GeneBank Accession Number |
|---|---|---|---|---|---|---|---|
| 10 | 129,354 | 2593 | 100 | 99.92 | 0 | Tomato chlorotic mottle Guyane virus DNA–B 2 | MK878451.1 |
| 98 | 3725 | 2609 | 100 | 99.00 | 0 | Tomato yellow leaf deformation dwarf virus DNA–B 2 | NC_060089.1 |
| 81 | 18,916 | 2535 | 100 | 97.09 | 0 | Chino del tomate Amazonas virus DNA–B 2 | MG675220.1 |
| 2034 | 92,661 | 2662 | 83 | 96.76 | 0 | Tomato chlorotic mottle Guyane virus DNA–B 1 | MK878451.1 |
| 1156 | 15,163 | 2634 | 100 | 93.70 | 0 | Tomato yellow spot virus DNA–B 3 | KX348205.1 |
| 2913 | 82,057 | 2597 | 100 | 91.62 | 0 | Tomato chlorotic mottle Guyane virus DNA–B 1 | MK878451.1 |
| 1778 | 1475 | 2583 | 100 | 88.31 | 0 | Tomato crinkle leaf yellows virus DNA–B 3 | JN419011.1 |
| 299 | 2808 | 2619 | 99 | 83.98 | 0 | Tomato yellow leaf deformation dwarf virus DNA–B 3 | NC_060089.1 |
| 93 | 10,358 | 2565 | 85 | 82.05 | 0 | Tomato interveinal chlorosis virus-2 DNA–B 2 | MK087039.1 |
| 8 | 14,354 | 2656 | 96 | 77.95 | 0 | Tomato rugose yellow leaf curl virus DNA–B 1 | JN381822.1 |
| Code of the Contigs | Read Coverage | Assembled Genome Size (nts) | BLASTn Coverage (%) | Identity (%) | E-Value | Virus Description * | GeneBank Accession Number |
|---|---|---|---|---|---|---|---|
| 38 | 46,535 | 1322 | 100 | 99.92 | 0 | Alphasatellitidae sp.1 | MT214093.1 |
| 107 | 574,158 | 2591 | 100 | 99.88 | 0 | Tomato severe rugose virus DNA–A 5 | MT733811.1 |
| 78 | 73,475 | 2631 | 100 | 99.81 | 0 | Tomato mottle leaf curl virus DNA–A 3 | MT733813.1 |
| 255 | 1205 | 2628 | 100 | 99.51 | 0 | Euphorbia yellow mosaic virus DNA–A 1 | MN782438.1 |
| 52 | 206,835 | 2622 | 100 | 99.50 | 0 | Tomato chlorotic mottle virus DNA–A 2 | MT733804.1 |
| 79 | 57,409 | 2561 | 100 | 99.45 | 0 | Tomato golden vein virus DNA–A 4 | KC706652.1 |
| 827 | 601,302 | 2698 | 99 | 99.32 | 0 | Tomato rugose mosaic virus DNA–A 1 | MT215006.1 |
| 34 | 113,869 | 2561 | 100 | 98.83 | 0 | Tomato golden vein virus DNA–A 1 | KC706646.1 |
| 14432 | 113 | 2636 | 100 | 98.79 | 0 | Tomato yellow net virus DNA–A 2 | MT214096.1 |
| 47 | 12,561 | 2610 | 100 | 98.74 | 0 | Euphorbia yellow mosaic virus DNA–A 2 | KY559437.1 |
| 211 | 245,699 | 2631 | 100 | 98.29 | 0 | Tomato mottle leaf curl virus DNA–A 3 | MT733813.1 |
| 29 | 335,413 | 2606 | 100 | 98.05 | 0 | Tomato rugose mosaic virus DNA–A 2 | MT215006.1 |
| 934 | 1694 | 2574 | 100 | 97.98 | 0 | Tomato-associated geminivirus 2 1 | MN527305.1 |
| 37 | 154,963 | 2631 | 100 | 97.95 | 0 | Tomato mottle leaf curl virus DNA–A 2 | MT214088.1 |
| * | 26,055 | 2560 | 100 | 97.89 | 0 | Tomato yellow vein streak virus DNA–A 1 | KC136337.1 |
| 7 | 232,163 | 2622 | 100 | 97.83 | 0 | Tomato chlorotic mottle virus DNA–A 3 | MT733804.1 |
| 268 | 4530 | 2671 | 99 | 97.78 | 0 | Sida micrantha mosaic virus DNA–A 4 | JX415194.1 |
| 73 | 138,272 | 2561 | 100 | 97.77 | 0 | Tomato golden vein virus DNA–A 4 | JF803259.1 |
| * | 4822 | 2667 | 100 | 97.76 | 0 | Sida yellow mosaic virus DNA–A 1 | AY090558.1 |
| 101 | 264,219 | 2631 | 100 | 96.92 | 0 | Tomato mottle leaf curl virus DNA–A 5 | MT215005.1 |
| 50 | 286,731 | 2637 | 95 | 96.83 | 0 | Tomato rugose mosaic virus DNA–A 1 | MT215006.1 |
| 74 | 3857 | 2560 | 100 | 96.76 | 0 | Tomato yellow vein streak virus DNA–A 1 | MN508216.1 |
| 40 | 229,047 | 2631 | 100 | 95.79 | 0 | Tomato mottle leaf curl virus DNA–A 4 | MT733813.1 |
| 307 | 94,086 | 2676 | 100 | 95.70 | 0 | Sida micrantha mosaic virus DNA–A 1 | KC706535.1 |
| 201 | 10,481 | 1365 | 100 | 94.59 | 0 | Euphorbia yellow mosaic alphasatellite 1 | FN436008.1 |
| 49 | 203,042 | 2602 | 100 | 93.45 | 0 | Tomato chlorotic mottle virus DNA–A 1 | MT733804.1 |
| 23 | 145,276 | 2631 | 100 | 94.19 | 0 | Tomato mottle leaf curl virus DNA–A 1 | JF803247.1 |
| * | 431,707 | 2676 | 100 | 94.18 | 0 | Sida micrantha mosaic virus DNA–A 1 | MT214092.1 |
| * | 204,035 | 2618 | 100 | 93.81 | 0 | Tomato interveinal chlorosis virus DNA–A 1 | JF803253.1 |
| 56 | 412,517 | 2631 | 100 | 93.06 | 0 | Tomato mottle leaf curl virus DNA–A 1 | MT733813.1 |
| 3065 | 123,209 | 2572 | 100 | 92.76 | 0 | Tomato severe rugose virus DNA–A 1 | HQ606468.1 |
| 357 | 36,971 | 2675 | 100 | 91.76 | 0 | Sida micrantha mosaic virus DNA–A 1 | MT214092.1 |
| 84 | 21,005 | 2556 | 100 | 91.68 | 0 | Tomato golden vein virus DNA–A 1 | KC706653.1 |
| New species #5 | 369,619 | 2561 | 100 | 89.03 | 0 | Tomato golden vein virus DNA–A 1 | MN928612.1 |
| 45 | 18,143 | 2879 | 100 | 85.21 | 0 | Tomato apical leaf curl virus 2 | MT135209.1 |
| Code of the Contigs | Read Coverage | Assembled Genome Size (nts) | BLASTn Coverage (%) | Identity (%) | E-Value | Virus Description * | GeneBank Accession Number |
|---|---|---|---|---|---|---|---|
| 103 | 143,673 | 2571 | 100 | 99.73 | 0 | Tomato severe rugose virus DNA–B 4 | MT215002.1 |
| 82 | 102,342 | 2597 | 100 | 99.58 | 0 | Tomato chlorotic mottle virus DNA–B 6 | MT214087.1 |
| 16 | 137 | 2570 | 100 | 99.57 | 0 | Tomato rugose mosaic virus DNA–B 7 | MT215007.1 |
| 14 | 47,649 | 2533 | 100 | 99.33 | 0 | Tomato golden vein virus DNA–B 3 | MN928611.1 |
| 122 | 48,514 | 2551 | 100 | 98.55 | 0 | Tomato golden vein virus DNA–B 9 | MT733807.1 |
| 132 | 142,262 | 2554 | 100 | 98.26 | 0 | Tomato severe rugose virus DNA–B 2 | MT214085.1 |
| 121 | 147,477 | 2571 | 93 | 97.00 | 0 | Tomato severe rugose virus DNA–B 1 | MT215002.1 |
| * | 45,881 | 2543 | 100 | 96.58 | 0 | Tomato mild leaf curl virus DNA–B 1 | DQ336352.1 |
| 27613 | 4 | 318 | 100 | 96.54 | 0 | Sida micrantha mosaic virus DNA–B 1 | AJ557452.1 |
| 3050 | 90,728 | 2597 | 85 | 90.55 | 0 | Tomato rugose mosaic virus DNA–B 1 | MT214091.1 |
| 21 | 37,396 | 2527 | 97 | 95.50 | 0 | Tomato golden vein virus DNA–B 1 | KC706660.1 |
| 499 | 2740 | 2556 | 100 | 94.97 | 0 | Tomato yellow vein streak virus DNA–B 1 | MN508217.1 |
| Virus Acronyms * (Number of Positive Samples) | Codes of the Isolates with Positive PCR Detection per Pathogen per Geographical Region | ||||
|---|---|---|---|---|---|
| North | Northeast | South | Southeast | Central–West | |
| ToSRV (15 + 20 + 19 = 54) | – | – | PR–143; PR–173; PR–174; RS–033; RS–012; RS–013; RS–014; RS–015; SC–001; SC–002; SC–015; SC–030; SC–032; SC–044; and SC–051 | MG–013; MG–014; MG–108; MG–109; MG–267; MG–292; SP–003; SP–006; SP–008; SP–017; SP–111; SP–205; SP–206; SP–154; SP–173; SP–201; SP–239; SP–254; SP–259; and SP–274 | DF–663; DF–034; DF–209; DF–487; GO–121; GO–033; GO–034; GO–126; GO–127; GO–204; GO–208; GO–211; GO–212; GO–218; GO–589; GO–604; GO–605; GO–617; and GO–618 |
| ToMoLCV (2 + 23 + 9 + 14 + 10 = 58) | AM–012 and RR–003 | BA–034; BA–035; BA–050; BA–100; BA–128; BA–134; BA–143; BA–173; BA–174; CE–001; CE–011; CE–012; PB–025; PB–027; PE–027; PE–028; PE–011; PE–012; PE–099; PE–100; PE–104; PE–105; and PE–121 | PR–144; PR–173; PR–174; RS–015; RS–071; RS–095; SC–002; SC–015; and SC–030 | MG–013; MG–014; MG–109; MG–292; MG–381; SP–003; SP–056; SP–058; SP–111; SP–213; SP–205; SP–154; SP–173; and SP–201 | DF–027; DF–024; DF–054; DF–154; DF–487; GO–495; GO–604; GO–605; GO–617; and GO–618 |
| ToCMoV (16 + 11 = 27) | – | – | – | MG–046; MG– 013; MG–014; MG–084; MG–109; MG–267; MG–292; MG–381; SP–004; SP–006; SP–008; SP–017; SP–056; SP–205; SP–239; and SP–254 | DF–027; DF–024; DF–034; DF–044; DF–054; DF–057; DF–170; DF–209; GO–121; GO–126; and GO–127 |
| TGVV (8 + 8 = 16) | – | – | – | MG–046; MG–013; MG–014; MG–108; MG–109; SP–003; SP–017; and SP–206 | DF–027; DF–024; DF–170; DF–209; GO–121; GO–126; GO–127; and GO–218 |
| SimMV (5 + 2 + 1 + 2 + 11 = 21) | AM–010; RR–003; RR–004; TO–045; and TO–046 | BA–100 and PE–011 | PR–143 | MG–267 and SP–173 | DF–024; DF–034; DF–044; DF–054; DF–170; DF–209; GO–121; GO–033; GO–126; GO–127; and GO–204 |
| EuYMV (1 + 3 = 4) | – | – | – | SP–003 | DF–170; GO–204; and GO–208 |
| ToCMoGV (1) | AM–035 | – | – | – | – |
| ToYSV (3) | RR–003; RR–004; and TO–046 | – | – | – | – |
| ToBYMoV (1) | TO–167 | – | – | – | – |
| New species #1 (3) | – | CE–001; PE–011; and PE–012 | – | – | – |
| New species #2 (2) | – | – | PR–173 and PR–174 | – | – |
| New species #3 (1) | TO–167 | – | – | – | – |
| New species #4 (1) | – | – | PR–144 | – | – |
| New species #5 (5) | – | – | – | – | DF–209, GO–121, GO–126, GO–127, and GO–218 |
| Alfasatellite (3) | – | – | – | – | DF–024, DF–027, and DF–057 |
| ToALCV (1) | – | – | – | SP–173 | – |
| TAGV (1) | – | – | – | – | GO–495 |
| Virus Acronyms (Total of Positive Samples) | Codes of the Isolates with Positive PCR Detection per Pathogen per Geographical Region | ||||
|---|---|---|---|---|---|
| North | Northeast | South | Southeast | Central–West | |
| ToSRV (1 + 6 + 7 = 14) | – | – | PR–112 | MG–268; MG–291; SP–018; SP–156; SP–240; and SP–252 | DF–216; DF–235; DF–338; DF–530; DF–546; DF–528; and DF–541 |
| ToMoLCV (1+ 11 = 12) | – | – | – | SP–172 | DF–155; DF–235; DF–530; DF–546; DF–528; DF–541; GO–124; GO–229; GO–342; GO–499; and GO–526 |
| ToCMoV (3 + 3 = 6) | – | – | – | MG–268; SP–066; and SP–252 | DF–216; DF–235; and GO–124 |
| TGVV (1 + 4 = 5) | – | – | – | SP–018 | DF–216; DF–235; GO–124; and GO–229 |
| ToYNV (1) | – | – | – | – | GO–342 |
| SimMV (5) | – | – | – | – | DF–216; DF–235; DF–338; GO–005; and GO–124 |
| EuYMV (1) | – | – | – | SP–066 | – |
| New species #5 (3) | – | – | – | – | DF–216, DF–235; and GO–124 |
| ToALCV ToALCV (1) | – | – | – | SP–172 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.A.d.; Reis, L.d.N.A.d.; Fonseca, M.E.d.N.; Melo, F.F.S.; Boiteux, L.S.; Pereira-Carvalho, R.d.C. Geminiviridae and Alphasatellitidae Diversity Revealed by Metagenomic Analysis of Susceptible and Tolerant Tomato Cultivars across Distinct Brazilian Biomes. Viruses 2024, 16, 899. https://doi.org/10.3390/v16060899
Oliveira IAd, Reis LdNAd, Fonseca MEdN, Melo FFS, Boiteux LS, Pereira-Carvalho RdC. Geminiviridae and Alphasatellitidae Diversity Revealed by Metagenomic Analysis of Susceptible and Tolerant Tomato Cultivars across Distinct Brazilian Biomes. Viruses. 2024; 16(6):899. https://doi.org/10.3390/v16060899
Chicago/Turabian StyleOliveira, Izaías Araújo de, Luciane de Nazaré Almeida dos Reis, Maria Esther de Noronha Fonseca, Felipe Fochat Silva Melo, Leonardo Silva Boiteux, and Rita de Cássia Pereira-Carvalho. 2024. "Geminiviridae and Alphasatellitidae Diversity Revealed by Metagenomic Analysis of Susceptible and Tolerant Tomato Cultivars across Distinct Brazilian Biomes" Viruses 16, no. 6: 899. https://doi.org/10.3390/v16060899





