Exploring Dengue Dynamics: A Multi-Scale Analysis of Spatio-Temporal Trends in Ibagué, Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Data
2.2. Methodology
2.2.1. Variable Selection and Transformation
2.2.2. Model Fitting
2.2.3. Aggregation Level Comparison
3. Results
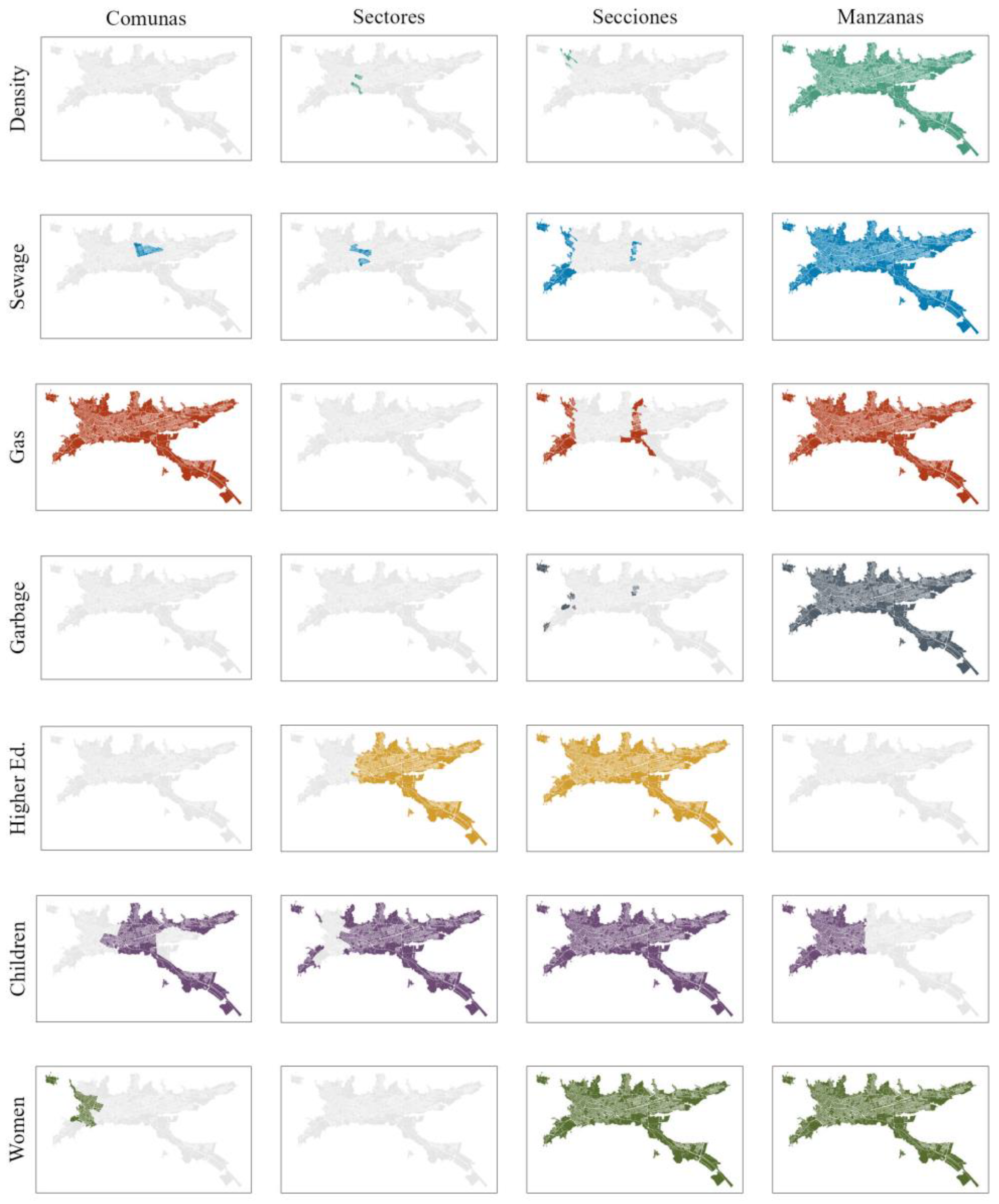
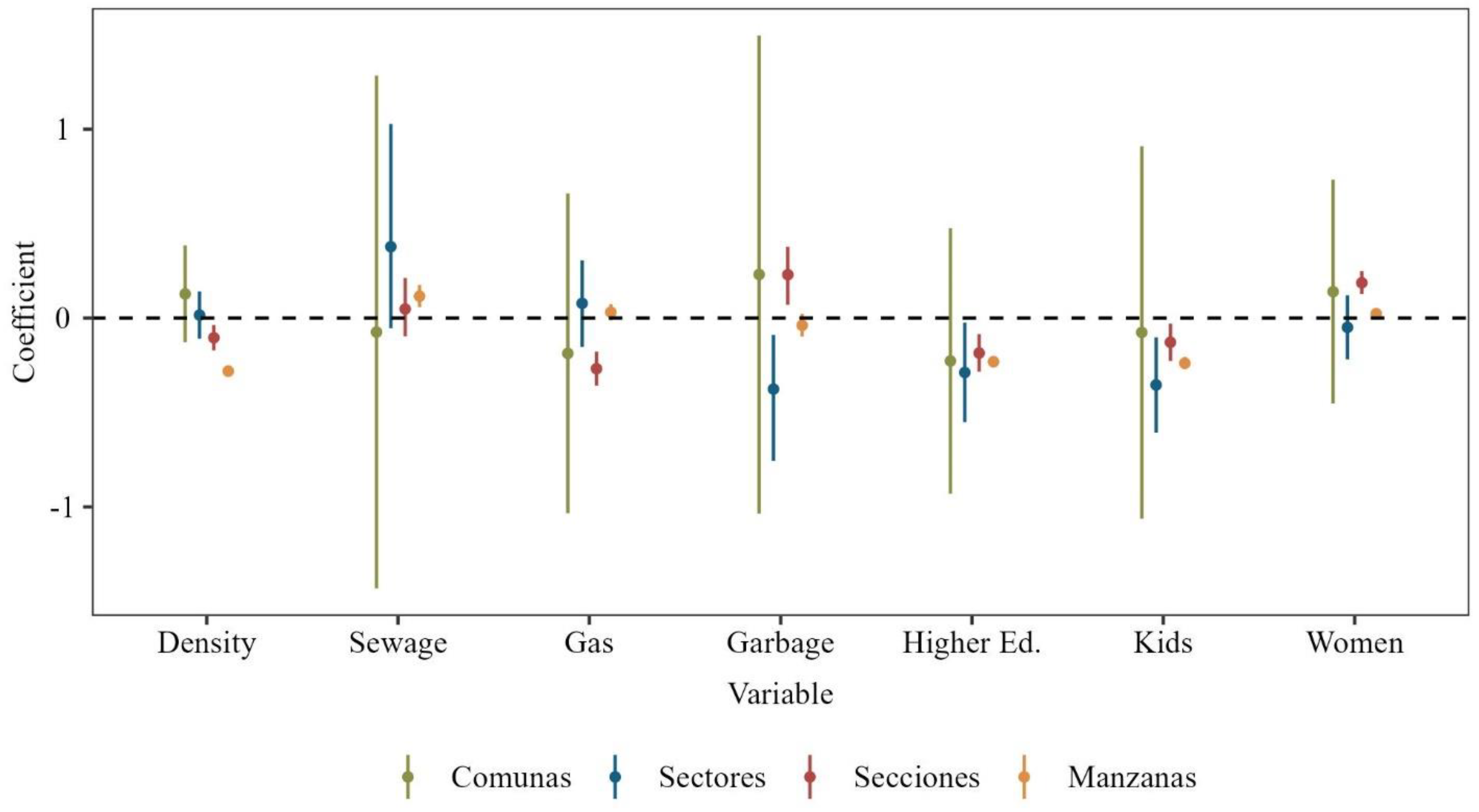
3.1. Variable Selection
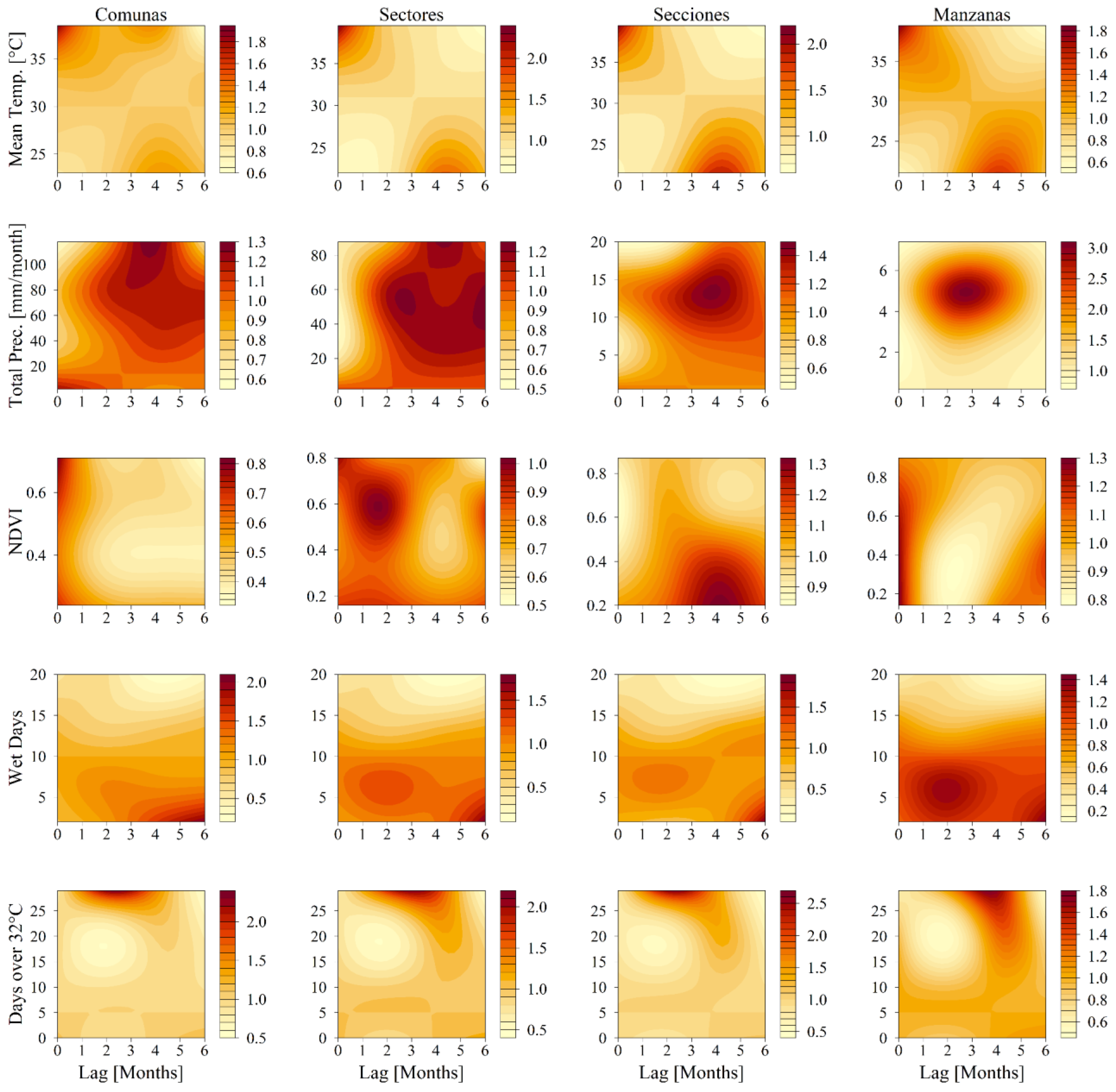
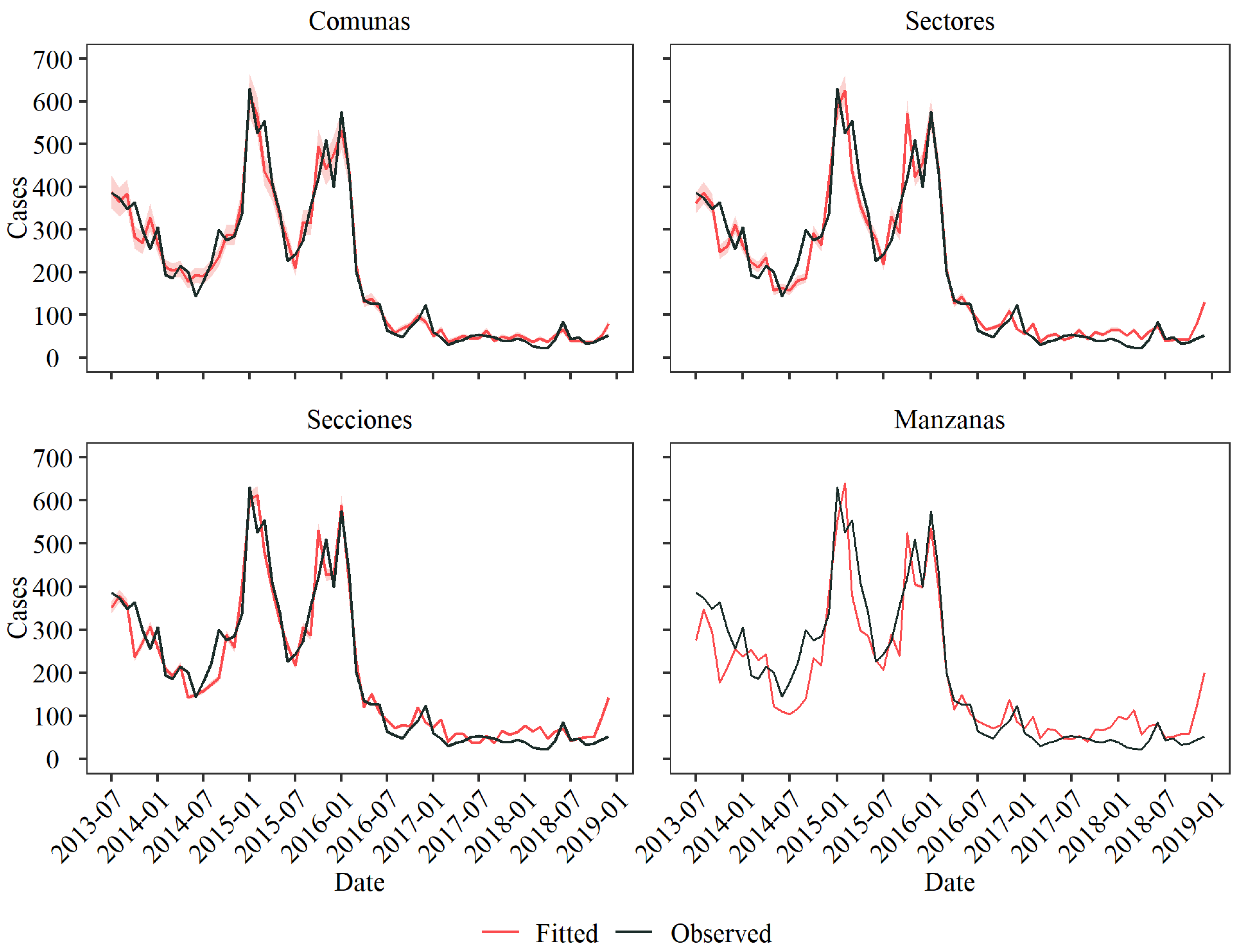
3.2. Model Fitting and Comparison
3.3. Level Comparison
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurane, I. Dengue Hemorrhagic Fever with Special Emphasis on Immunopathogenesis. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 28 October 2022).
- Silva, M.M.O.; Rodrigues, M.S.; Paploski, I.A.D.; Kikuti, M.; Kasper, A.M.; Cruz, J.S.; Queiroz, T.L.; Tavares, A.S.; Santana, P.M.; Araújo, J.M.G.; et al. Accuracy of Dengue Reporting by National Surveillance System, Brazil. Emerg. Infect. Dis. 2016, 22, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Barbosa, H.; Medina-Moreno, S.; Zapata, J.C.; Chua, J.V. Dengue Infections in Colombia: Epidemiological Trends of a Hyperendemic Country. TropicalMed 2020, 5, 156. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Salud “Estamos en año epidémico para Dengue”: INS. Available online: https://www.ins.gov.co/Noticias/Paginas/INS-Estamos-en-a%C3%B1o-epid%C3%A9mico-para-Dengue.aspx (accessed on 8 April 2024).
- Padilla, J.C.; Rojas, D.P.; Sáenz Gómez, R. Dengue en Colombia: Epidemiología de la Reemergencia a la Hiperendemia; Primera edición en español; Los Autores: Bogotá, Colombia, 2012; ISBN 978-958-46-0661-7. [Google Scholar]
- Guha-Sapir, D.; Schimmer, B. Dengue Fever: New Paradigms for a Changing Epidemiology. Emerg. Themes Epidemiol. 2005, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P.; Lathrop, S.; Bunning, M.; Biggerstaff, B.; Singer, D.; Tiwari, T.; Baber, L.; Amador, M.; Thirion, J.; Hayes, J.; et al. Texas Lifestyle Limits Transmission of Dengue Virus. Emerg. Infect. Dis. 2003, 9, 86–89. [Google Scholar] [CrossRef] [PubMed]
- da Queiroz, E.R.S.; de Andrade Medronho, R. Spatial Analysis of the Incidence of Dengue, Zika and Chikungunya and Socioeconomic Determinants in the City of Rio de Janeiro, Brazil. Epidemiol. Infect. 2021, 149, e188. [Google Scholar] [CrossRef]
- Alcaldía Municipal de Ibagué—Ibagué Vibra. Available online: https://ibague.gov.co/portal/seccion/contenido/index.php?type=3&cnt=53 (accessed on 28 October 2022).
- Carrasquilla, M.C.; Ortiz, M.I.; León, C.; Rondón, S.; Kulkarni, M.A.; Talbot, B.; Sander, B.; Vásquez, H.; Cordovez, J.M.; González, C.; et al. Entomological Characterization of Aedes Mosquitoes and Arbovirus Detection in Ibagué, a Colombian City with Co-Circulation of Zika, Dengue and Chikungunya Viruses. Parasites Vectors 2021, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Faruk, M.O.; Jannat, S.N.; Rahman, M.S. Impact of Environmental Factors on the Spread of Dengue Fever in Sri Lanka. Int. J. Environ. Sci. Technol. 2022, 19, 10637–10648. [Google Scholar] [CrossRef]
- Rose, N.H.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.B.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.-P.; et al. Climate and Urbanization Drive Mosquito Preference for Humans. Curr. Biol. 2020, 30, 3570–3579.e6. [Google Scholar] [CrossRef]
- Lee, S.A.; Jarvis, C.I.; Edmunds, W.J.; Economou, T.; Lowe, R. Spatial Connectivity in Mosquito-Borne Disease Models: A Systematic Review of Methods and Assumptions. J. R. Soc. Interface. 2021, 18, 20210096. [Google Scholar] [CrossRef]
- Kuddus, M.A.; Tynan, E.; McBryde, E. Urbanization: A Problem for the Rich and the Poor? Public Health Rev. 2020, 41, 1. [Google Scholar] [CrossRef]
- Vatanpour, N.; Malvandi, A.M.; Hedayati Talouki, H.; Gattinoni, P.; Scesi, L. Impact of Rapid Urbanization on the Surface Water’s Quality: A Long-Term Environmental and Physicochemical Investigation of Tajan River, Iran (2007–2017). Env. Sci. Pollut. Res. 2020, 27, 8439–8450. [Google Scholar] [CrossRef]
- Romeo-Aznar, V.; Picinini Freitas, L.; Gonçalves Cruz, O.; King, A.A.; Pascual, M. Fine-Scale Heterogeneity in Population Density Predicts Wave Dynamics in Dengue Epidemics. Nat. Commun. 2022, 13, 996. [Google Scholar] [CrossRef]
- Pickett, K.E. Multilevel Analyses of Neighbourhood Socioeconomic Context and Health Outcomes: A Critical Review. J. Epidemiol. Community Health 2001, 55, 111–122. [Google Scholar] [CrossRef]
- Diez Roux, A.V. Investigating Neighborhood and Area Effects on Health. Am. J. Public Health 2001, 91, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Waller, L.A.; Gotway, C.A. Applied Spatial Statistics for Public Health Data; Wiley series in probability and statistics; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-471-38771-8. [Google Scholar]
- Chaix, B.; Merlo, J.; Evans, D.; Leal, C.; Havard, S. Neighbourhoods in Eco-Epidemiologic Research: Delimiting Personal Exposure Areas. A Response to Riva, Gauvin, Apparicio and Brodeur. Soc. Sci. Med. 2009, 69, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Diez Roux, A.V.; Mair, C. Neighborhoods and Health: Neighborhoods and Health. Ann. N. Y. Acad. Sci. 2010, 1186, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Jaya, I.G.N.M.; Folmer, H. Bayesian Spatiotemporal Mapping of Relative Dengue Disease Risk in Bandung, Indonesia. J. Geogr. Syst. 2020, 22, 105–142. [Google Scholar] [CrossRef]
- Rotejanaprasert, C.; Ekapirat, N.; Areechokchai, D.; Maude, R.J. Bayesian Spatiotemporal Modeling with Sliding Windows to Correct Reporting Delays for Real-Time Dengue Surveillance in Thailand. Int. J. Health Geogr. 2020, 19, 4. [Google Scholar] [CrossRef]
- Sani, A.; Abapihi, B.; Mukhsar, M.; Kadir, K. Relative Risk Analysis of Dengue Cases Using Convolution Extended into Spatio-Temporal Model. J. Appl. Stat. 2015, 42, 2509–2519. [Google Scholar] [CrossRef]
- Telle, O.; Nikolay, B.; Kumar, V.; Benkimoun, S.; Pal, R.; Nagpal, B.; Paul, R.E. Social and Environmental Risk Factors for Dengue in Delhi City: A Retrospective Study. PLoS Negl. Trop. Dis. 2021, 15, e0009024. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Lee, S.A.; O’Reilly, K.M.; Brady, O.J.; Bastos, L.; Carrasco-Escobar, G.; de Castro Catão, R.; Colón-González, F.J.; Barcellos, C.; Carvalho, M.S.; et al. Combined Effects of Hydrometeorological Hazards and Urbanisation on Dengue Risk in Brazil: A Spatiotemporal Modelling Study. Lancet Planet. Health 2021, 5, e209–e219. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.R.; Eastin, M.D.; Paul, R.; Casas, I.; Delmelle, E.M. Space–Time Conditional Autoregressive Modeling to Estimate Neighborhood-Level Risks for Dengue Fever in Cali, Colombia. Am. J. Trop. Med. Hyg. 2020, 103, 2040–2053. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Morrison, A.C. Vector Dynamics and Transmission of Dengue Virus: Implications for Dengue Surveillance and Prevention Strategies. In Dengue Virus; Rothman, A.L., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 338, pp. 115–128. ISBN 978-3-642-02214-2. [Google Scholar]
- Departamento Administrativo Nacional de Estadística. Manual De Uso Del Marco Geoestadístico Nacional En El Proceso Estadístico; Departamento Administrativo Nacional de Estadística: Bogotá, Colombia, 2018; p. 40. [Google Scholar]
- McMillen, D.P. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships. Am. J. Agric. Econ. 2004, 86, 554–556. [Google Scholar] [CrossRef]
- Rue, H.; Martino, S.; Chopin, N. Approximate Bayesian Inference for Latent Gaussian Models by Using Integrated Nested Laplace Approximations. J. R. Stat. Soc. Ser. B Stat. Methodol. 2009, 71, 319–392. [Google Scholar] [CrossRef]
- Kyle, J.L.; Harris, E. Global Spread and Persistence of Dengue. Annu. Rev. Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Departamento Administrativo Nacional de Estadística. La Información del DANE en la Toma de Decisiones Regionales: Ibagué, Colombia; Departamento Administrativo Nacional de Estadística: Bogotá, Colombia, 2020. [Google Scholar]
- Departamento Administrativo Nacional de Estadística. Geoportal DANE. Available online: https://geoportal.dane.gov.co/servicios/descarga-y-metadatos/descarga-mgn-marco-geoestadistico-nacional/ (accessed on 23 November 2022).
- MOD11A1.061 Terra Land Surface Temperature and Emissivity Daily Global 1km | Earth Engine Data Catalog. Available online: https://developers.google.com/earth-engine/datasets/catalog/MODIS_061_MOD11A1 (accessed on 8 January 2024).
- MOD13Q1.061 Terra Vegetation Indices 16-Day Global 250m | Earth Engine Data Catalog. Available online: https://developers.google.com/earth-engine/datasets/catalog/MODIS_061_MOD13Q1 (accessed on 8 January 2024).
- Funk, C.; Peterson, P.; Landsfeld, M.; Pedreros, D.; Verdin, J.; Shukla, S.; Husak, G.; Rowland, J.; Harrison, L.; Hoell, A.; et al. The Climate Hazards Infrared Precipitation with Stations—A New Environmental Record for Monitoring Extremes. Sci. Data 2015, 2, 150066. [Google Scholar] [CrossRef]
- Kache, P.; Ruiz-Carrascal, D.; Lowe, R.; Stewart-Ibarra, A.M.; Seto, K.C.; Diuk-Wasser, M.; Santos-Vega, M. Climate Extremes Increase Dengue Risk along Elevation and Socio-Economic Gradients in Colombia. Nat. Clim. Chang. 2024. under review. [Google Scholar]
- Gollini, I.; Lu, B.; Charlton, M.; Brunsdon, C.; Harris, P. GWmodel: An R. Package for Exploring Spatial Heterogeneity Using Geographically Weighted Models. J. Stat. Soft. 2015, 63, 1–50. [Google Scholar] [CrossRef]
- Lu, B.; Harris, P.; Charlton, M.; Brunsdon, C. The GWmodel R Package: Further Topics for Exploring Spatial Heterogeneity Using Geographically Weighted Models. Geo-Spat. Inf. Sci. 2014, 17, 85–101. [Google Scholar] [CrossRef]
- Thapa, R.B.; Estoque, R.C. Geographically Weighted Regression in Geospatial Analysis. In Progress in Geospatial Analysis; Murayama, Y., Ed.; Springer: Tokyo, Japan, 2012; pp. 85–96. ISBN 978-4-431-53999-5. [Google Scholar]
- Wheeler, D.C.; Páez, A. Geographically Weighted Regression. In Handbook of Applied Spatial Analysis; Fischer, M.M., Getis, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 461–486. ISBN 978-3-642-03646-0. [Google Scholar]
- Cazelles, B.; Chavez, M.; Berteaux, D.; Ménard, F.; Vik, J.O.; Jenouvrier, S.; Stenseth, N.C. Wavelet Analysis of Ecological Time Series. Oecologia 2008, 156, 287–304. [Google Scholar] [CrossRef]
- Gouhier, T.C.; Grinsted, A.; Simko, V. R Package Biwavelet: Conduct Univariate and Bivariate Wavelet Analyses. Available online: https://github.com/tgouhier/biwavelet (accessed on 8 January 2024).
- Lowe, R.; Gasparrini, A.; Van Meerbeeck, C.J.; Lippi, C.A.; Mahon, R.; Trotman, A.R.; Rollock, L.; Hinds, A.Q.J.; Ryan, S.J.; Stewart-Ibarra, A.M. Nonlinear and Delayed Impacts of Climate on Dengue Risk in Barbados: A Modelling Study. PLoS Med. 2018, 15, e1002613. [Google Scholar] [CrossRef]
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package Dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef]
- Besag, J.; York, J.; Molliè, A. Bayesian Image Restoration, with Two Applications in Spatial Statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Martino, S.; Rue, H. Implementing Approximate Bayesian Inference Using Integrated Nested Laplace Approximation: A Manual for the Inla Program; Department of Mathematical Sciences, NTNU: Trondheim, Norway, 2009. [Google Scholar]
- Martins, T.G.; Simpson, D.; Lindgren, F.; Rue, H. Bayesian Computing with INLA: New Features. Comput. Stat. Data Anal. 2013, 67, 68–83. [Google Scholar] [CrossRef]
- Schmidt, W.-P.; Suzuki, M.; Dinh Thiem, V.; White, R.G.; Tsuzuki, A.; Yoshida, L.-M.; Yanai, H.; Haque, U.; Huu Tho, L.; Anh, D.D.; et al. Population Density, Water Supply, and the Risk of Dengue Fever in Vietnam: Cohort Study and Spatial Analysis. PLoS Med. 2011, 8, e1001082. [Google Scholar] [CrossRef] [PubMed]
- Romeo-Aznar, V.; Paul, R.; Telle, O.; Pascual, M. Mosquito-Borne Transmission in Urban Landscapes: The Missing Link between Vector Abundance and Human Density. Proc. R. Soc. B 2018, 285, 20180826. [Google Scholar] [CrossRef]
- Rasid, H.; Mallsk, A.U. Living on the Edge of Stagnant Water: An Assessment of Environmental Impacts of Construction-Phase Drainage Congestion along Dhaka City Flood Control Embankment, Bangladesh. Environ. Manag. 1996, 20, 89–98. [Google Scholar] [CrossRef]
- Chitolina, R.F.; Anjos, F.A.; Lima, T.S.; Castro, E.A.; Costa-Ribeiro, M.C.V. Raw Sewage as Breeding Site to Aedes (Stegomyia) Aegypti (Diptera, Culicidae). Acta Trop. 2016, 164, 290–296. [Google Scholar] [CrossRef]
- Foster, W.A.; Walker, E.D. MOSQUITOES (Culicidae). In Medical and Veterinary Entomology; Elsevier: San Diego, CA, USA, 2002; pp. 203–262. ISBN 978-0-12-510451-7. [Google Scholar]
- Sur, D.; Von Seidlein, L.; Manna, B.; Dutta, S.; Deb, A.K.; Sarkar, B.L.; Kanungo, S.; Deen, J.L.; Ali, M.; Kim, D.R.; et al. The Malaria and Typhoid Fever Burden in the Slums of Kolkata, India: Data from a Prospective Community-Based Study. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 725–733. [Google Scholar] [CrossRef]
- Krystosik, A.; Njoroge, G.; Odhiambo, L.; Forsyth, J.E.; Mutuku, F.; LaBeaud, A.D. Solid Wastes Provide Breeding Sites, Burrows, and Food for Biological Disease Vectors, and Urban Zoonotic Reservoirs: A Call to Action for Solutions-Based Research. Front. Public Health 2020, 7, 405. [Google Scholar] [CrossRef]
- Klafke, F.; Barros, V.G.; Henning, E. Solid Waste Management and Aedes Aegypti Infestation Interconnections: A Regression Tree Application. Waste Manag. Res. 2023, 41, 1684–1696. [Google Scholar] [CrossRef]
- Naqvi, S.A.A.; Sajjad, M.; Tariq, A.; Sajjad, M.; Waseem, L.A.; Karuppannan, S.; Rehman, A.; Hassan, M.; Al-Ahmadi, S.; Hatamleh, W.A. Societal Knowledge, Attitude, and Practices towards Dengue and Associated Factors in Epidemic-Hit Areas: Geoinformation Assisted Empirical Evidence. Heliyon 2024, 10, e23151. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Quijano, F.A.; Martínez-Vega, R.A.; Rodriguez-Morales, A.J.; Rojas-Calero, R.A.; Luna-González, M.L.; Díaz-Quijano, R.G. Association between the Level of Education and Knowledge, Attitudes and Practices Regarding Dengue in the Caribbean Region of Colombia. BMC Public Health 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Urrea, V.; Ochoa, A.; Mesa, O. Seasonality of Rainfall in Colombia. Water Resour. Res. 2019, 55, 4149–4162. [Google Scholar] [CrossRef]
- Santoso, A.; Mcphaden, M.J.; Cai, W. The Defining Characteristics of ENSO Extremes and the Strong 2015/2016 El Niño. Rev. Geophys. 2017, 55, 1079–1129. [Google Scholar] [CrossRef]
- Elsinga, J.; Lizarazo, E.F.; Vincenti, M.F.; Schmidt, M.; Velasco-Salas, Z.I.; Arias, L.; Bailey, A.; Tami, A. Health Seeking Behaviour and Treatment Intentions of Dengue and Fever: A Household Survey of Children and Adults in Venezuela. PLoS Negl. Trop. Dis. 2015, 9, e0004237. [Google Scholar] [CrossRef]
- Duong, V.; Lambrechts, L.; Paul, R.E.; Ly, S.; Lay, R.S.; Long, K.C.; Huy, R.; Tarantola, A.; Scott, T.W.; Sakuntabhai, A.; et al. Asymptomatic Humans Transmit Dengue Virus to Mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 14688–14693. [Google Scholar] [CrossRef]

| Model | ||||
|---|---|---|---|---|
| Level | Metric | Spatial | Temporal | Spatio-Temporal |
| Comunas | DIC | 5345 | 4861 | 4860 |
| WAIC | 5358 | 4863 | 4863 | |
| Sectores | DIC | 14,646 | 13,874 | 13,871 |
| WAIC | 14,656 | 13,874 | 13,869 | |
| Secciones | DIC | 33,942 | 32,206 | 32,163 |
| WAIC | 33,871 | 32,185 | 32,139 | |
| Manzanas | DIC | 89,900 | 84,728 | 84,051 |
| WAIC | 89,845 | 84,667 | 83,988 | |
| Comunas | Sectores | Secciones | Manzanas | |
|---|---|---|---|---|
| RMSE | 32.69 | 45.80 | 42.34 | 66.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, J.; Tabares, A.; Santos-Vega, M. Exploring Dengue Dynamics: A Multi-Scale Analysis of Spatio-Temporal Trends in Ibagué, Colombia. Viruses 2024, 16, 906. https://doi.org/10.3390/v16060906
Otero J, Tabares A, Santos-Vega M. Exploring Dengue Dynamics: A Multi-Scale Analysis of Spatio-Temporal Trends in Ibagué, Colombia. Viruses. 2024; 16(6):906. https://doi.org/10.3390/v16060906
Chicago/Turabian StyleOtero, Julian, Alejandra Tabares, and Mauricio Santos-Vega. 2024. "Exploring Dengue Dynamics: A Multi-Scale Analysis of Spatio-Temporal Trends in Ibagué, Colombia" Viruses 16, no. 6: 906. https://doi.org/10.3390/v16060906
APA StyleOtero, J., Tabares, A., & Santos-Vega, M. (2024). Exploring Dengue Dynamics: A Multi-Scale Analysis of Spatio-Temporal Trends in Ibagué, Colombia. Viruses, 16(6), 906. https://doi.org/10.3390/v16060906







