Serum Levels of Arachidonic Acid, Interleukin-6, and C-Reactive Protein as Potential Indicators of Pulmonary Viral Infections: Comparative Analysis of Influenza A, Respiratory Syncytial Virus Infection, and COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Procedures
2.3. Statistical Analyses
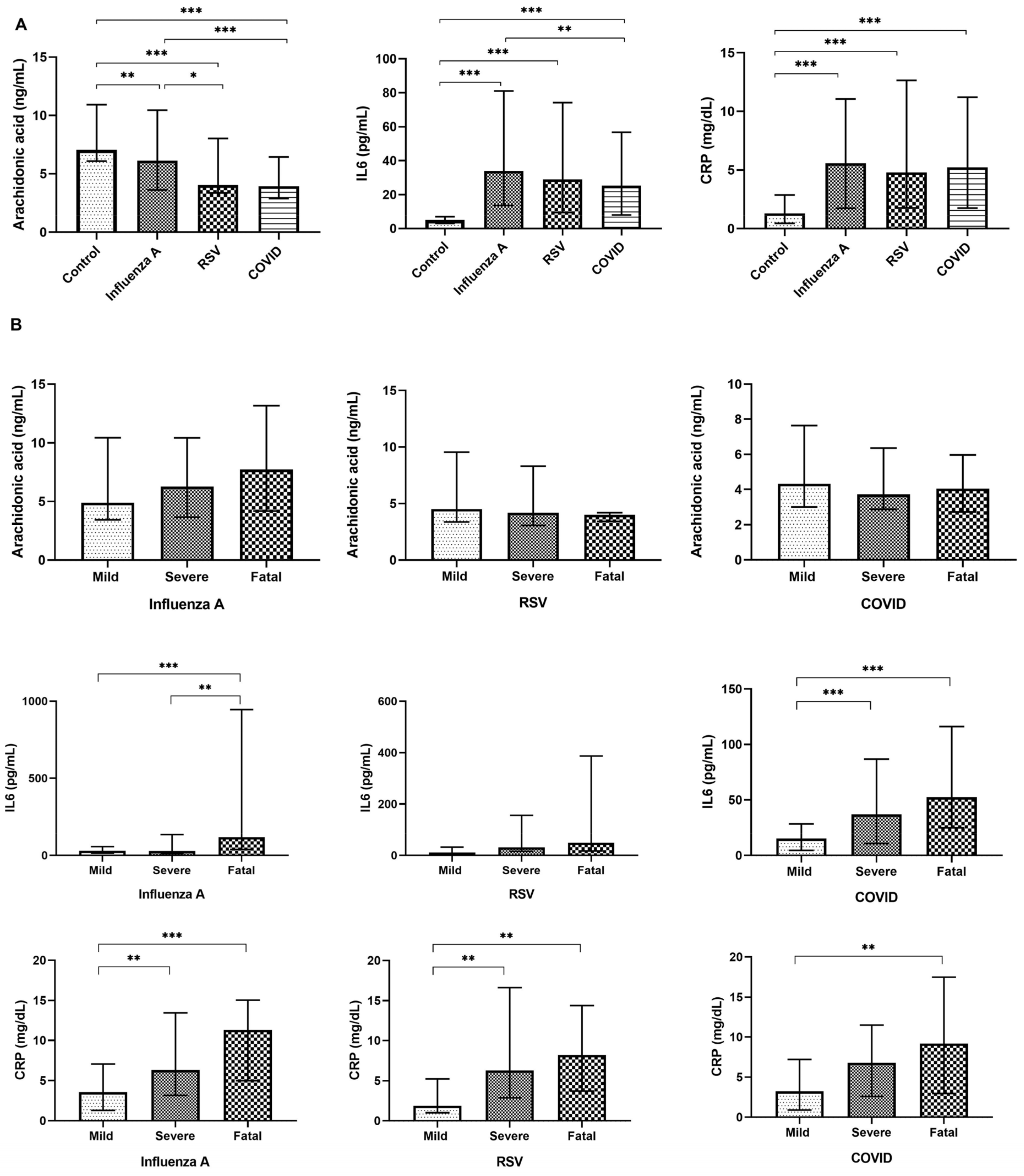
3. Results
3.1. Demographic and Clinical Characteristics of the Patients and the Control Group
3.2. Changes in the Circulating Levels of Selected Variables in Relation to the Disease
3.3. Serum Arachidonic Acid, IL-6, and CRP Concentrations as Disease Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Nair, H. Trends in the global burden of lower respiratory infections: The knowns and the unknowns. Lancet Infect. Dis. 2022, 22, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Drew, L. Research round-up: Respiratory syncytial virus. Nature 2023, 621, S66–S67. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.C.; Callahan, S.M.; Savchenko, K.G.; Stobart, C.C. A contemporary view of respiratory syncytial virus (RSV) biology and strain-specific differences. Pathogens 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Papanikolopoulou, A.; Vassiliu, S.; Theodoridou, K.; Nikolopoulou, G.; Sipsas, N.V. COVID-19 and respiratory virus co-infections: A systematic review of the literature. Viruses 2023, 15, 865. [Google Scholar] [CrossRef] [PubMed]
- Terliesner, N.; Unterwalder, N.; Edelmann, A.; Corman, V.; Knaust, A.; Rosenfeld, L.; Gratopp, A.; Ringe, H.; Martin, L.; von Bernuth, H.; et al. Viral infections in hospitalized children in Germany during the COVID-19 pandemic: Association with non-pharmaceutical interventions. Front. Pediatr. 2022, 10, 935483. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Armistead, I.; Messacar, K.; Alden, N.B.; Schmoll, E.; Austin, E.; Dominguez, S.R. Shifting epidemiology and severity of respiratory syncytial virus in children during the COVID-19 pandemic. JAMA Pediatr. 2023, 177, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Ambrosch, A.; Luber, D.; Klawonn, F.; Kabesch, M. Focusing on severe infections with the respiratory syncytial virus (RSV) in adults: Risk factors, symptomatology and clinical course compared to influenza A/B and the original SARS-CoV-2 strain. J. Clin. Virol. 2023, 161, 105399. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.M.; Diagne, M.M.; Sagne, S.N.; Tall, F.; Diouf, J.B.N.; Boiro, D.; Mendy, M.P.; Ndiaye, N.K.; Kiori, D.; Sy, S.; et al. Respiratory syncytial virus in pediatric patients with severe acute respiratory infections in Senegal: Findings from the 2022 sentinel surveillance season. Sci. Rep. 2023, 13, 20404. [Google Scholar] [CrossRef]
- Grangier, B.; Vacheron, C.H.; De Marignan, D.; Casalegno, J.S.; Couray-Targe, S.; Bestion, A.; Ader, F.; Richard, J.C.; Frobert, E.; Argaud, L.; et al. Comparison of mortality and outcomes of four respiratory viruses in the intensive care unit: A multicenter retrospective study. Sci. Rep. 2024, 14, 6690. [Google Scholar] [CrossRef]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global challenges to public health care systems during the COVID-19 pandemic: A review of pandemic measures and problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Boon, H.; Meinders, A.J.; van Hannen, E.J.; Tersmette, M.; Schaftenaar, E. Comparative analysis of mortality in patients admitted with an infection with influenza A/B virus, respiratory syncytial virus, rhinovirus, metapneumovirus or SARS-CoV-2. Influenza Other Respir Viruses 2024, 18, e13237. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better tests, better care: Improved diagnostics for infectious diseases. Clin. Infect. Dis. 2013, 57, S139–S170. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, W.I.; Mishra, N.; Briese, T. Screening for viral infections. Encycl. Virol. 2021, 91–97. [Google Scholar] [CrossRef]
- Weigele, B.A.; Orchard, R.C.; Jimenez, A.; Cox, G.W.; Alto, N.M. A systematic exploration of the interactions between bacterial effector proteins and host cell membranes. Nat. Commun. 2017, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal. Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Zhang, C.; Wang, Z.; Ni, Z.; Zhang, S.; Yang, S.; Huang, X.; Mo, L.; Li, J.; Lee, B.; et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 2021, 3, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lydic, T.A.; Goo, Y.H. Lipidomics unveils the complexity of the lipidome in metabolic diseases. Clin. Transl. Med. 2018, 7, 4. [Google Scholar] [CrossRef]
- Castañé, H.; Iftimie, S.; Baiges-Gaya, G.; Rodríguez-Tomàs, E.; Jiménez-Franco, A.; López-Azcona, A.F.; Garrido, P.; Castro, A.; Camps, J.; Joven, J. Machine learning and semi-targeted lipidomics identify distinct serum lipid signatures in hospitalized COVID-19-positive and COVID-19-negative patients. Metabolism 2022, 131, 155197. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Levinson, T.; Wasserman, A.; Shenhar-Tsarfaty, S.; Halutz, O.; Shapira, I.; Zeltser, D.; Rogowski, O.; Berliner, S.; Ziv-Baran, T. Comparative analysis of CRP as a biomarker of the inflammatory response intensity among common viral infections affecting the lungs: COVID-19 versus influenza A, influenza B and respiratory syncytial virus. Clin. Exp. Med. 2023, 23, 5307–5313. [Google Scholar] [CrossRef] [PubMed]
- NIH COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 12 April 2024).
- Gabaldó, X.; Juanpere, M.; Castañé, H.; Rodríguez-Tomàs, E.; López-Azcona, A.F.; Baiges-Gaya, G.; Castro, L.; Valverde-Díaz, E.; Muñoz-Blázquez, A.; Giménez-Cuenca, L.; et al. Usefulness of the measurement of serum paraoxonase-1 arylesterase activity in the diagnoses of COVID-19. Biomolecules 2022, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Theken, K.N.; Tang, S.Y.; Sengupta, S.; FitzGerald, G.A. The roles of lipids in SARS-CoV-2 viral replication and the host immune response. J. Lipid Res. 2021, 62, 100129. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Casari, I.; Manfredi, M.; Metharom, P.; Falasca, M. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 2021, 82, 101092. [Google Scholar] [CrossRef]
- Yan, B.; Chu, H.; Yang, D.; Sze, K.H.; Lai, P.M.; Yuan, S.; Shuai, H.; Wang, Y.; Kao, R.Y.; Chan, J.F.; et al. Characterization of the lipidomic profile of human coronavirus-infected cells: Implications for lipid metabolism remodeling upon coronavirus replication. Viruses 2019, 11, 73. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Nikkhoo, B.; Mohammadi, M.; Hasani, S.; Sigari, N.; Borhani, A.; Ramezani, C.; Charajoo, A.; Badri, S.; Rostami, F.; Etemadi, M.; et al. Elevated interleukin (IL)-6 as a predictor of disease severity among COVID-19 patients: A prospective cohort study. BMC Infect. Dis. 2023, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- Keddie, S.; Ziff, O.; Chou, M.K.L.; Taylor, R.L.; Heslegrave, A.; Garr, E.; Lakdawala, N.; Church, A.; Ludwig, D.; Manson, J.; et al. Laboratory biomarkers associated with COVID-19 severity and management. Clin. Immunol. 2020, 221, 108614. [Google Scholar] [CrossRef] [PubMed]
- Ulhaq, Z.S.; Soraya, G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020, 50, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Manti, S.; Staiano, A.; Orfeo, L.; Midulla, F.; Marseglia, G.L.; Ghizzi, C.; Zampogna, S.; Carnielli, V.P.; Favilli, S.; Ruggieri, M.; et al. UPDATE—2022 Italian guidelines on the management of bronchiolitis in infants. Ital. J. Pediatr. 2023, 49, 19. [Google Scholar] [CrossRef]

| Variable | Control Group n = 104 | Influenza A n = 172 | RSV n = 80 | COVID-19 n = 217 | p Value |
|---|---|---|---|---|---|
| Age | 50 (38–65) | 73 (53–73) | 75 (59–81) | 62 (47–76) | <0.001 |
| Male sex | 45 (43.3) | 83 (48.3) | 35 (43.8) | 112 (51.6) | 0.449 |
| Smoking | 33 (31.7) | 26 (15.1) | 7 (8.8) | 32 (14.7) | <0.001 |
| Alcohol | 45 (43.3) | 21 (12.2) | 11 (13.8) | 14 (6.5) | <0.001 |
| Initial admission department | |||||
| Emergency | N.A. | 100 (58.1) | 34 (42.5) | 81 (37.3) | <0.001 |
| Internal Medicine | N.A. | 42 (24.4) | 31 (38.8) | 59 (27.2) | |
| Geriatry | N.A. | 21 (12.2) | 8 (10.0) | 0 (0.0) | |
| Oncology | N.A. | 3 (1.7) | 4 (5.0) | 21 (9.7) | |
| Intensive Care Unit | N.A. | 1 (0.6) | 2 (2.5) | 0 (0.0) | |
| Surgery | N.A. | 1 (0.6) | 0 (0.0) | 0 (0.0) | |
| Traumatology | N.A. | 1 (0.6) | 0 (0.0) | 0 (0.0) | |
| Anesthesiology | N.A. | 1 (0.6) | 0 (0.0) | 0 (0.0) | |
| Gynecology | N.A. | 0 (0.0) | 1 (1.2) | 5 (2.3) | |
| Pneumology | N.A. | 1 (0.6) | 0 (0.0) | 2 (0.9) | |
| Other | N.A. | 1 (0.6) | 0 (0.0) | 49 (22.6) | |
| Symptoms | |||||
| Pneumonia | N.A. | 66 (38.4) | 20 (25.0) | 32 (14.7) | <0.001 |
| Bronchitis | N.A. | 49 (28.5) | 17 (21.3) | 0 (0.0) | <0.001 |
| Cough | N.A. | 114 (66.3) | 61 (76.3) | 95 (43.8) | <0.001 |
| Fever | N.A. | 101 (58.7) | 31 (38.8) | 109 (50.2) | 0.011 |
| Odynophagia | N.A. | 7 (4.1) | 3 (3.8) | 7 (3.2) | 0.905 |
| Headache | N.A. | 7 (4.1) | 1 (1.2) | 27 (12.4) | 0.001 |
| Anorexia or hyporexia | N.A. | 4 (2.3) | 2 (2.5) | 5 (2.3) | 0.995 |
| Myalgia | N.A. | 33 (19.2) | 13 (16.3) | 12 (5.5) | 0.001 |
| Arthralgia | N.A. | 23 (13.4) | 10 (12.5) | 10 (4.6) | 0.006 |
| Acute respiratory failure | N.A. | 85 (49.4) | 48 (60.0) | 13 (6.0) | <0.001 |
| Comorbidities | |||||
| Diabetes mellitus | N.A. | 38 (22.1) | 27 (33.8) | 57 (26.3) | 0.145 |
| Cardiovascular disease | N.A. | 100 (58.1) | 49 (61.3) | 111 (51.2) | 0.200 |
| Chronic liver disease | N.A. | 8 (4.7) | 3 (3.8) | 14 (6.5) | 0.579 |
| Chronic kidney disease | N.A. | 27 (15.7) | 18 (22.5) | 31 (14.3) | 0.228 |
| Chronic lung disease | N.A. | 50 (29.1) | 37 (46.3) | 25 (11.5) | <0.001 |
| Chronic neurological disease | N.A. | 36 (20.9) | 13 (16.3) | 31 (14.3) | 0.219 |
| Cancer | N.A. | 9 (5.2) | 7 (8.8) | 34 (15.7) | 0.003 |
| Interventions and treatments | |||||
| Intensive Care Unit admission | N.A. | 7 (4.1) | 6 (7.5) | 30 (13.8) | 0.004 |
| Non-invasive mechanical ventilation | N.A. | 8 (4.7) | 3 (3.8) | 9 (4.1) | 0.941 |
| Invasive mechanical ventilation | N.A. | 5 (2.9) | 1 (1.3) | 14 (6.5) | 0.078 |
| High-flow oxygen therapy | N.A. | 3 (1.7) | 0 (0.0) | 20 (9.2) | <0.001 |
| Conventional oxygen therapy | N.A. | 95 (55.2) | 44 (55.0) | 103 (47.5) | <0.001 |
| Anticoagulants | N.A. | 31 (18.0) | 17 (21.3) | 88 (40.6) | <0.001 |
| Corticosteroids | N.A. | 78 (45.3) | 52 (65.0) | 113 (52.1) | 0.007 |
| Disease severity | |||||
| Mild | N.A. | 80 (46.5) | 28 (35.0) | 93 (42.9) | 0.344 |
| Severe | N.A. | 68 (39.5) | 35 (43.8) | 82 (37.8) | |
| Fatal | N.A. | 24 (13.9) | 17 (21.3) | 42 (19.4) | |
| Deceased | N.A. | 17 (9.9) | 11 (13.8) | 19 (8.8) | 0.444 |
| Variable | B | 95% CI for B | β | t | p Value |
|---|---|---|---|---|---|
| Age | 0.014 | −0.041–0.069 | 0.039 | 0.502 | 0.616 |
| Male sex | −1.108 | −2.726–0.509 | −0.078 | −1.348 | 0.179 |
| Smoking | 0.595 | −0.573–1.764 | 0.095 | 1.002 | 0.317 |
| Alcohol | 0.338 | −0.804–1.480 | 0.056 | 0.583 | 0.560 |
| Disease * | −1.038 | −1.664–−0.411 | −0.203 | −3.260 | 0.001 |
| Interleukin-6 | 0.000 | −0.001–0.001 | 0.032 | 0.539 | 0.590 |
| C-reactive protein | 0.014 | −0.085–0.113 | 0.017 | 0.283 | 0.778 |
| Type 2 diabetes mellitus | −0.419 | −2.417–1.578 | −0.025 | −0.413 | 0.680 |
| Cardiovascular disease | −0.526 | −2.577–1.524 | −0.037 | −0.505 | 0.614 |
| Chronic liver disease | 0.099 | −3.453–3.651 | 0.003 | 0.055 | 0.956 |
| Chronic kidney disease | −1.184 | −3.567–1.199 | −0.061 | −0.978 | 0.329 |
| Chronic lung disease | −0.330 | −2.266–1.605 | −0.020 | −0.336 | 0.737 |
| Chronic neurological disease | 0.711 | −1.546–2.969 | 0.037 | 0.620 | 0.536 |
| Cancer | −1.068 | −3.707–1.571 | −0.046 | −0.796 | 0.426 |
| Severity ** | −0.057 | −1.447–1.334 | −0.006 | −0.080 | 0.936 |
| Deceased | −0.611 | −4.141–2.919 | −0.025 | −0.341 | 0.734 |
| Constant | 8.951 | 4.278–13.625 | 3.770 | <0.001 |
| Variable | B | 95% CI for B | β | t | p Value |
|---|---|---|---|---|---|
| Age | −4.832 | −11.232–1.568 | −0.115 | −1.486 | 0.138 |
| Male sex | 108.827 | −80.500–298.154 | 0.065 | 1.131 | 0.259 |
| Smoking | −2.108 | −138.983–134.767 | −0.003 | −0.030 | 0.976 |
| Alcohol | 18.935 | −114.661–152.531 | 0.026 | 0.279 | 0.780 |
| Disease * | −98.391 | −172.101–−24.681 | −0.163 | −2.627 | 0.009 |
| Arachidonic acid | 3.679 | −9.758–17.116 | 0.031 | 0.539 | 0.590 |
| C-reactive protein | 16.573 | 5.151–27.995 | 0.169 | 2.856 | 0.005 |
| Type 2 diabetes mellitus | −31.376 | −264.992–202.240 | −0.016 | −0.264 | 0.792 |
| Cardiovascular disease | 10.427 | −229.435–250.289 | 0.006 | 0.086 | 0.932 |
| Chronic liver disease | −95.142 | −510.319–320.035 | −0.025 | −0.451 | 0.652 |
| Chronic kidney disease | 114.124 | −163.616–392.864 | 0.050 | 0.806 | 0.421 |
| Chronic lung disease | −81.901 | −308.071–144.268 | −0.043 | −0.713 | 0.477 |
| Chronic neurological disease | −13.793 | −277.898–250.312 | −0.006 | −0.103 | 0.918 |
| Cancer | 29.816 | −279.067–338.698 | 0.011 | 0.190 | 0.849 |
| Severity ** | 310.316 | 151.692–468.940 | 0.270 | 3.850 | <0.001 |
| Deceased | −358.220 | −769.013–52.574 | −0.123 | −1.716 | 0.087 |
| Constant | −270.133 | −828.788–288.522 | −0.952 | 0.342 |
| Variable | B | 95% CI for B | β | t | p Value |
|---|---|---|---|---|---|
| Age | 0.045 | −0.019–0.108 | 0.104 | 1.385 | 0.167 |
| Male sex | 2.538 | 0.677–4.400 | 0.149 | 2.684 | 0.008 |
| Smoking | −0.294 | −1.653–1.064 | −0.039 | −0.426 | 0.670 |
| Alcohol | −0.408 | −1.734–0.918 | −0.056 | −0.605 | 0.545 |
| Disease * | −0.249 | −0.989–0.491 | −0.040 | −0.662 | 0.508 |
| Arachidonic acid | 0.019 | −0.114–0.153 | 0.016 | 0.283 | 0.778 |
| Interleukin-6 | 0.002 | 0.001–0.003 | 0.160 | 2.856 | 0.005 |
| Type 2 diabetes mellitus | 2.233 | −0.072–4.539 | 0.111 | 1.907 | 0.058 |
| Cardiovascular disease | −0.566 | −2.946–1.815 | −0.033 | −0.468 | 0.640 |
| Chronic liver disease | −0.479 | −4.602–3.644 | −0.012 | −0.229 | 0.819 |
| Chronic kidney disease | −0.487 | −3.257–2.283 | −0.021 | −0.346 | 0.729 |
| Chronic lung disease | −0.086 | −2.333–2.162 | −0.004 | −0.075 | 0.940 |
| Chronic neurological disease | 0.744 | −1.877–3.365 | 0.032 | 0.559 | 0.577 |
| Cancer | −0.690 | −3.756–2.376 | −0.025 | −0.443 | 0.658 |
| Severity ** | 1.985 | 0.387–3.583 | 0.169 | 2.444 | 0.015 |
| Deceased | 3.203 | −0.880–7.285 | 0.107 | 1.544 | 0.124 |
| Constant | −1.579 | −7.131–3.973 | −0.560 | 0.576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftimie, S.; Gabaldó-Barrios, X.; Penadés-Nadal, J.; Canela-Capdevila, M.; Piñana, R.; Jiménez-Franco, A.; López-Azcona, A.F.; Castañé, H.; Cárcel, M.; Camps, J.; et al. Serum Levels of Arachidonic Acid, Interleukin-6, and C-Reactive Protein as Potential Indicators of Pulmonary Viral Infections: Comparative Analysis of Influenza A, Respiratory Syncytial Virus Infection, and COVID-19. Viruses 2024, 16, 1065. https://doi.org/10.3390/v16071065
Iftimie S, Gabaldó-Barrios X, Penadés-Nadal J, Canela-Capdevila M, Piñana R, Jiménez-Franco A, López-Azcona AF, Castañé H, Cárcel M, Camps J, et al. Serum Levels of Arachidonic Acid, Interleukin-6, and C-Reactive Protein as Potential Indicators of Pulmonary Viral Infections: Comparative Analysis of Influenza A, Respiratory Syncytial Virus Infection, and COVID-19. Viruses. 2024; 16(7):1065. https://doi.org/10.3390/v16071065
Chicago/Turabian StyleIftimie, Simona, Xavier Gabaldó-Barrios, Joan Penadés-Nadal, Marta Canela-Capdevila, Rubén Piñana, Andrea Jiménez-Franco, Ana F. López-Azcona, Helena Castañé, María Cárcel, Jordi Camps, and et al. 2024. "Serum Levels of Arachidonic Acid, Interleukin-6, and C-Reactive Protein as Potential Indicators of Pulmonary Viral Infections: Comparative Analysis of Influenza A, Respiratory Syncytial Virus Infection, and COVID-19" Viruses 16, no. 7: 1065. https://doi.org/10.3390/v16071065
APA StyleIftimie, S., Gabaldó-Barrios, X., Penadés-Nadal, J., Canela-Capdevila, M., Piñana, R., Jiménez-Franco, A., López-Azcona, A. F., Castañé, H., Cárcel, M., Camps, J., Castro, A., & Joven, J. (2024). Serum Levels of Arachidonic Acid, Interleukin-6, and C-Reactive Protein as Potential Indicators of Pulmonary Viral Infections: Comparative Analysis of Influenza A, Respiratory Syncytial Virus Infection, and COVID-19. Viruses, 16(7), 1065. https://doi.org/10.3390/v16071065










