Immuno-Haematologic Aspects of Dengue Infection: Biologic Insights and Clinical Implications
Abstract
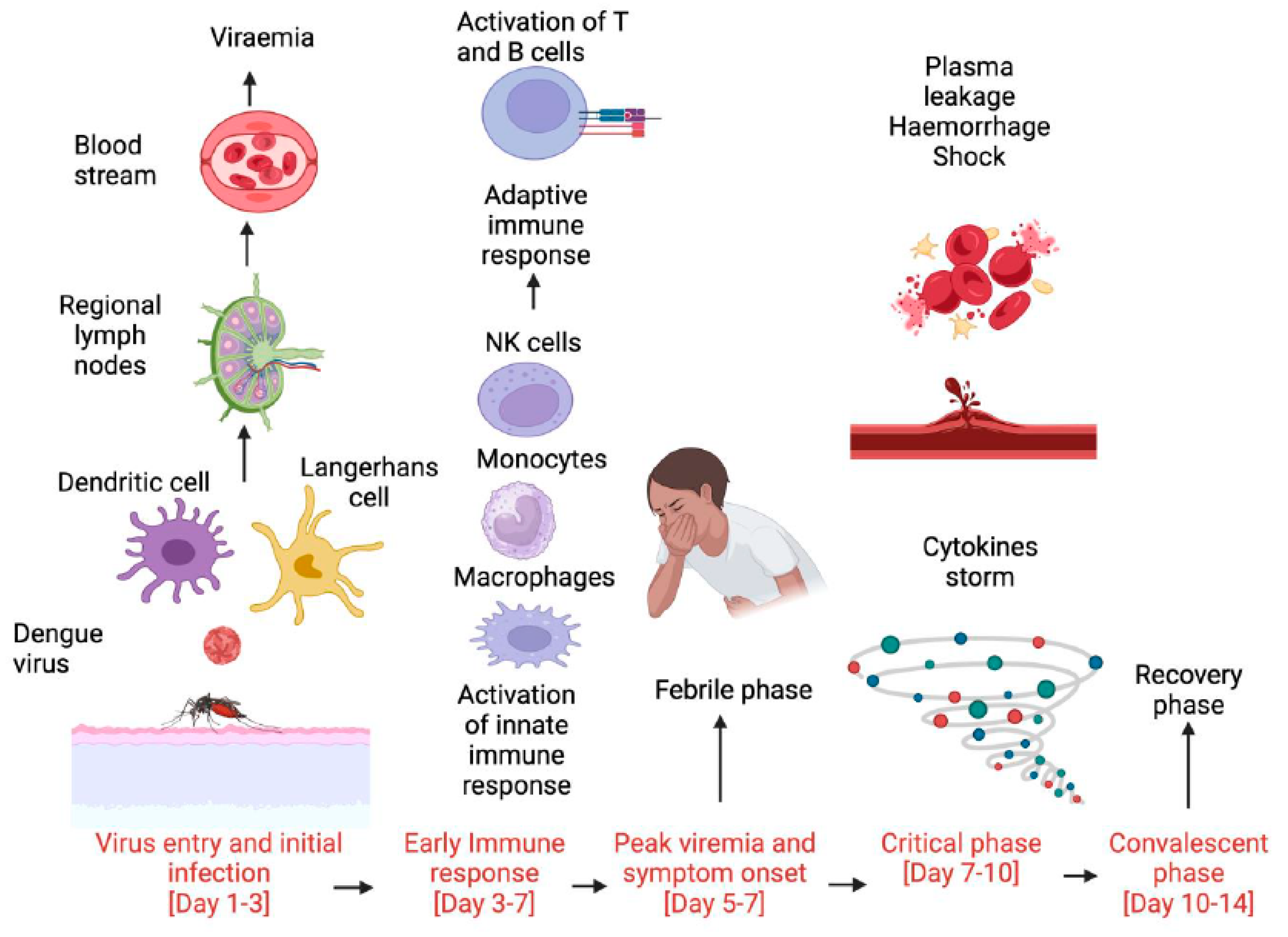
:1. Introduction
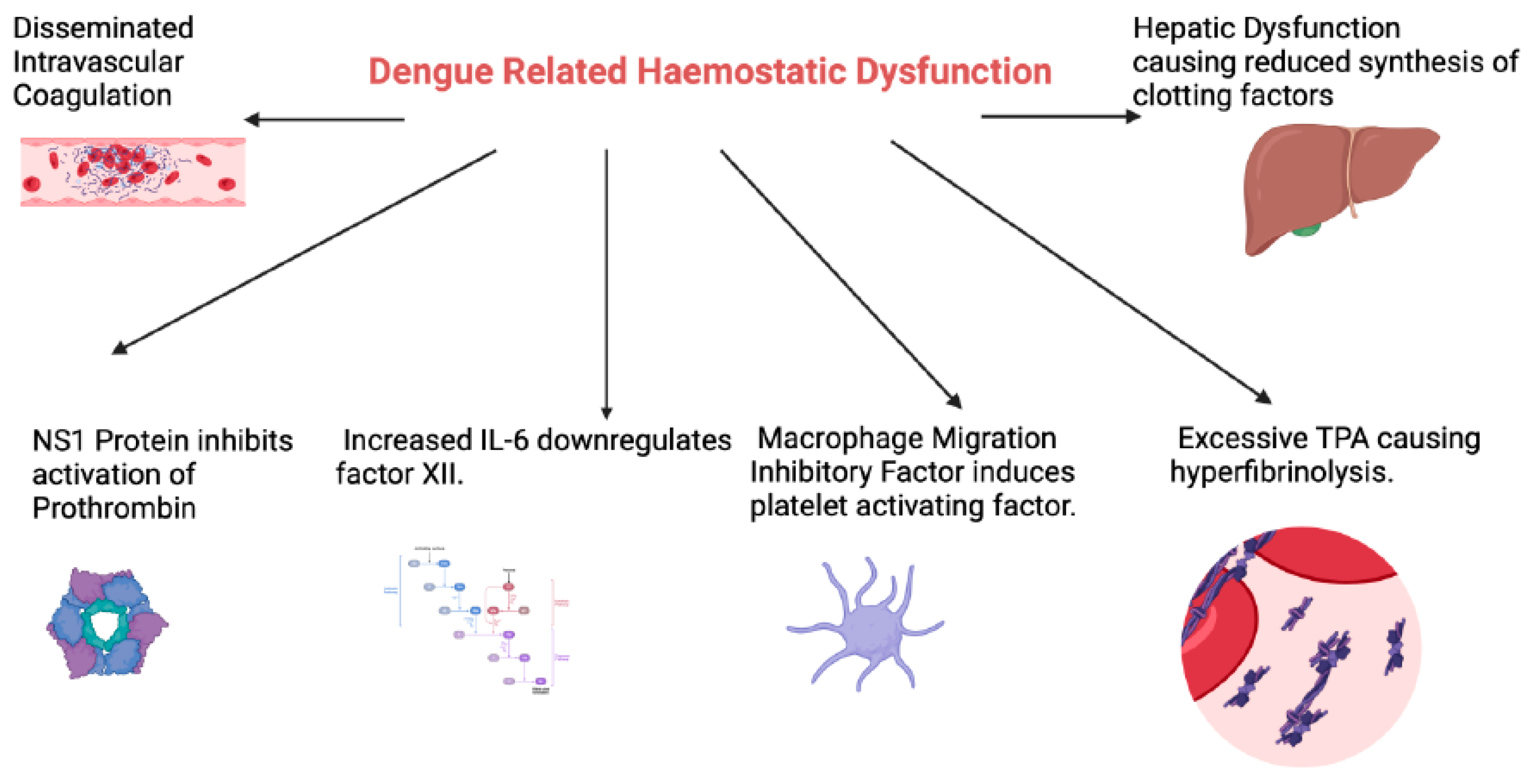
2. Haemostatic Abnormalities in Dengue Infection
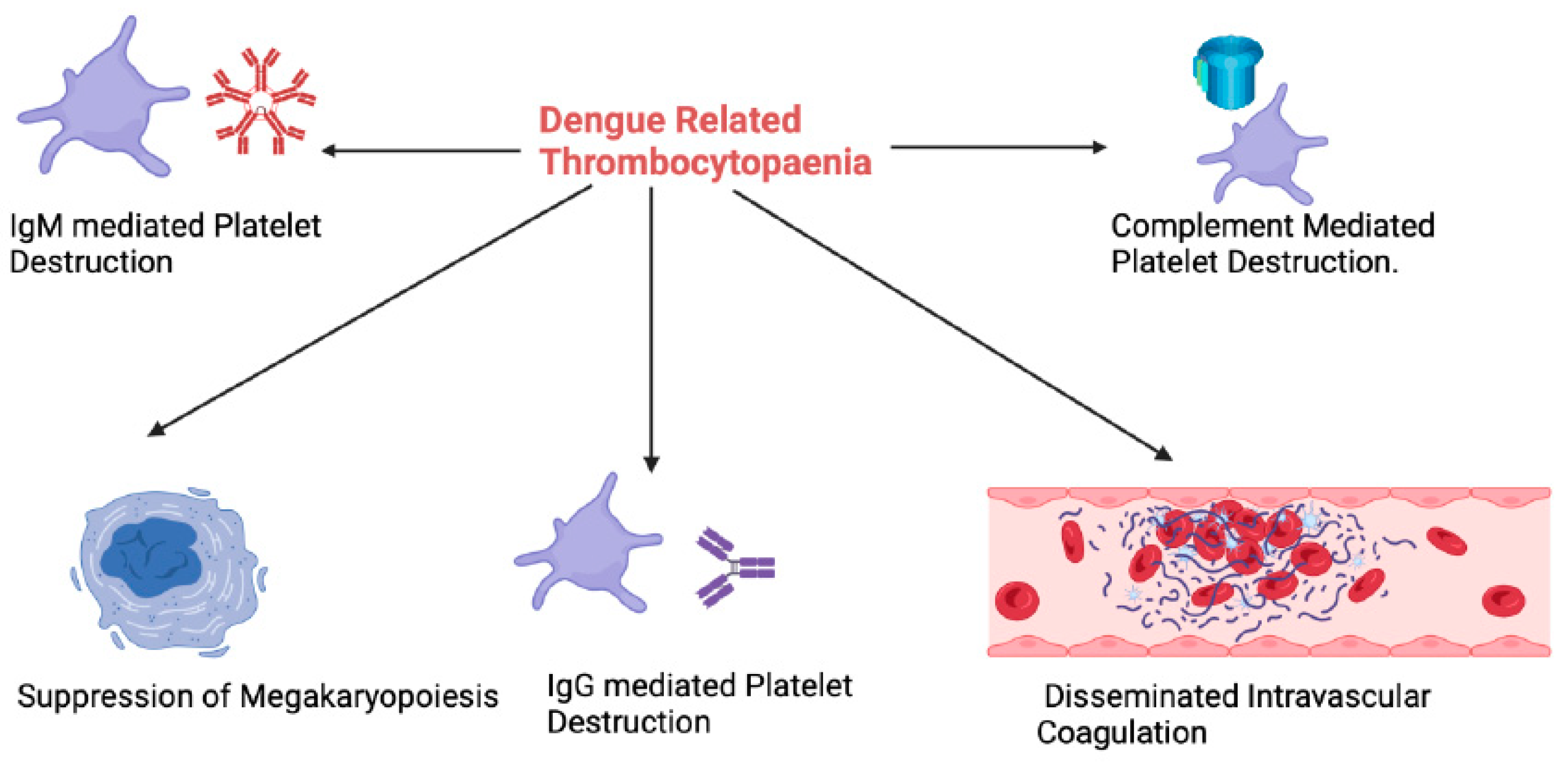
3. Thrombocytopaenia
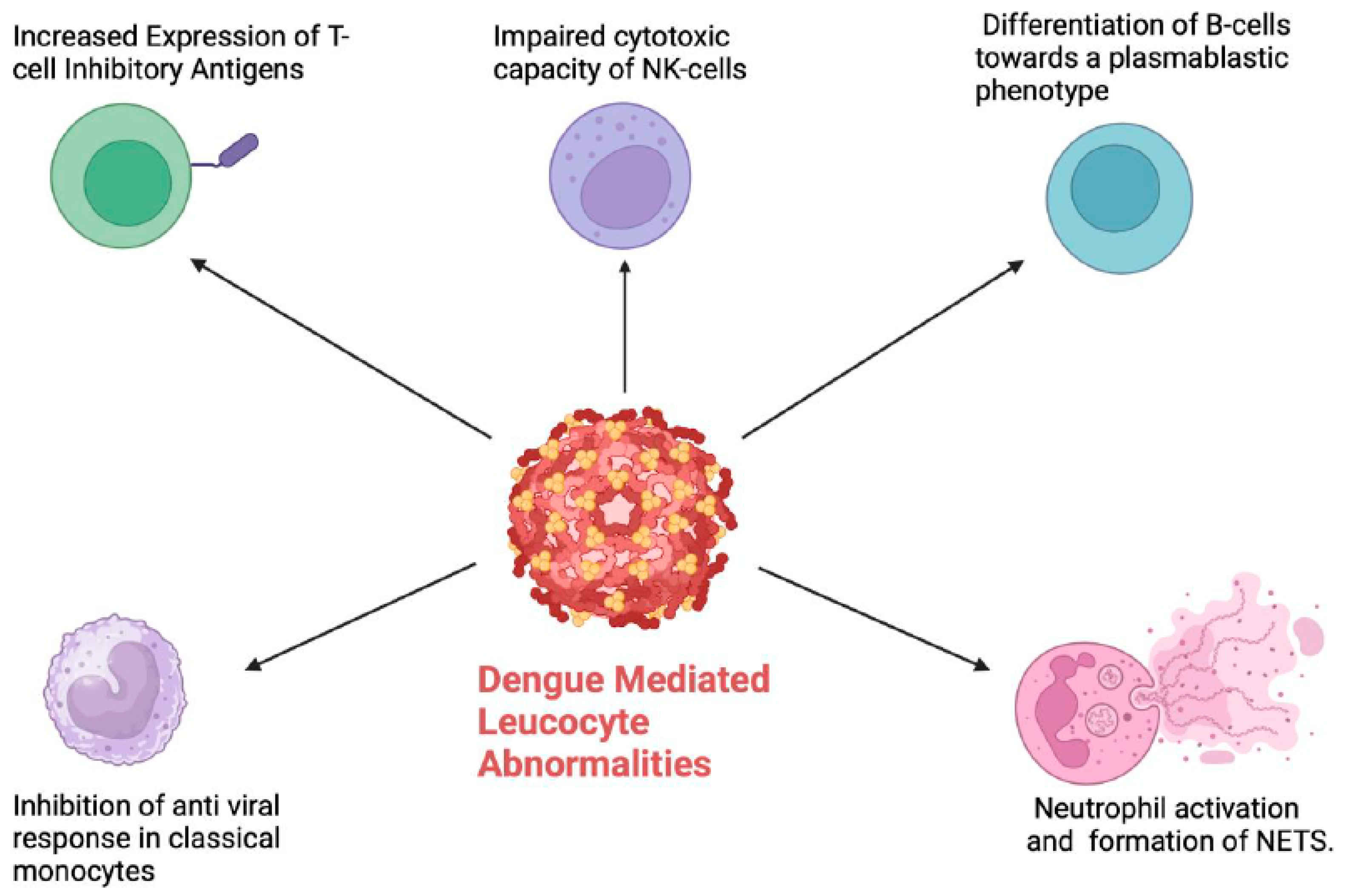
4. Leucocyte Abnormalities
4.1. Monocytes and Macrophages
4.2. Lymphocytes
4.3. Neutrophils
4.4. Mast Cells
4.5. Eosinophils and Basophils
5. Implications for Prognosis and Risk Stratification
6. Implications for Management
7. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Manoharan, M. Dengue Virus. In Emerging and Reemerging Viral Pathogens; Academic Press: Cambridge, MA, USA, 2020; pp. 281–359. [Google Scholar] [CrossRef]
- Roy, S.K.; Bhattacharjee, S. Dengue virus: Epidemiology, biology, and disease aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.B.; Budhathoki, P.; Gurung, B.; Subedi, S.; Aryal, S.; Basukala, A.; Aryal, B.; Adhikari, A.; Poudel, A.; Yadav, G.K.; et al. Epidemiology of dengue in SAARC territory: A systematic review and meta-analysis. Parasites Vectors 2022, 15, 389. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Dalugama, C. Dengue infection: Global importance, immunopathology, and management. Clin. Med. 2022, 22, 9–13. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; Google Books; World Health Organization: Geneva, Switzerland, 2009; Available online: https://books.google.com.sg/books?hl=en&lr=&id=dlc0YSIyGYwC&oi=fnd&pg=PP2&dq=World+Health+Organization+Dengue:+Guidelines+for+Diagnosis (accessed on 30 December 2023).
- Pal, S.; Dauner, A.L.; Mitra, I.; Forshey, B.M.; Garcia, P.; Morrison, A.C.; Halsey, E.S.; Kochel, T.J.; Wu, S.-J.L. Evaluation of Dengue NS1 Antigen Rapid Tests and ELISA Kits Using Clinical Samples. PLoS ONE 2014, 9, e113411. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Lum, L.C.S.; Kroeger, A. Classifying dengue: A review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Trop. Med. Int. Health 2006, 11, 1238–1255. [Google Scholar] [CrossRef]
- Tayal, A.; Kabra, S.K.; Lodha, R. Management of Dengue: An Updated Review. Indian J. Pediatr. 2022, 90, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Salles, T.S.; da Encarnação Sá-Guimarães, T.; de Alvarenga, E.; Guimarães-Ribeiro, V.; de Meneses, M.; de Castro-Salles, P.F.; dos Santos, C.R.; Melo, A.C.D.A.; Soares, M.R.; Ferreira, D.F.; et al. History, epidemiology and diagnostics of dengue in the American and Brazilian contexts: A review. Parasites Vectors 2018, 11, 264. [Google Scholar] [CrossRef]
- Khanam, A.; Gutiérrez-Barbosa, H.; Lyke, K.E.; Chua, J.V. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses 2022, 14, 2575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malavige, G.N.; Jeewandara, C.; Ogg, G.S. Dysfunctional Innate Immune Responses and Severe Dengue. Front. Cell. Infect. Microbiol. 2020, 10, 590004. [Google Scholar] [CrossRef]
- Adane, T.; Getawa, S. Coagulation abnormalities in Dengue fever infection: A systematic review and meta-analysis. PLOS Neglected Trop. Dis. 2021, 15, e0009666. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.R.; Thanvi, I.; Nadeem, M.; Kanwaria, R. Coagulation abnormalities and their relationship with bleeding manifestations in patients with dengue-A single center observational study. Asian Pac. J. Trop. Med. 2023, 16, 65–71. [Google Scholar] [CrossRef]
- John, K.J.; Gunasekaran, K.; Prasad, J.D.; Mathew, D.; Das, S.; Sultan, N.; Abraham, A.M.; Iyyadurai, R. Predictors of Major Bleeding and Mortality in Dengue Infection: A Retrospective Observational Study in a Tertiary Care Centre in South India. Interdiscip. Perspect. Infect. Dis. 2019, 2019, 4823791. [Google Scholar] [CrossRef] [PubMed]
- Ruttmann, T. Coagulation for the clinician. S. Afr. J. Surg. 2006, 44, 22, 24–26, 28–30, passim. Available online: https://pubmed.ncbi.nlm.nih.gov/16619987/ (accessed on 1 June 2024). [PubMed]
- Lin, S.W.; Chuang, Y.C.; Lin, Y.S.; Lei, H.Y.; Liu, H.S.; Yeh, T.M. Dengue virus nonstructural protein NS1 binds to prothrombin/thrombin and inhibits prothrombin activation. J. Infect. 2012, 64, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Isarangkura, P.B.; Pongpanich, B.; Pintadit, P.; Phanichyakarn, P.; Valyasevi, A. Hemostatic derangement in dengue haemorrhagic fever. Southeast Asian J. Trop. Med. Public Health 1987, 18, 331–339. [Google Scholar] [PubMed]
- Chuang, Y.C.; Lin, J.; Lin, Y.S.; Wang, S.; Yeh, T.M. Dengue Virus Nonstructural Protein 1-Induced Antibodies Cross-React with Human Plasminogen and Enhance Its Activation. J. Immunol. 2016, 196, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Teerasarntipan, T.; Chaiteerakij, R.; Komolmit, P.; Tangkijvanich, P.; Treeprasertsuk, S. Acute liver failure and death predictors in patients with dengue-induced severe hepatitis. World J. Gastroenterol. 2020, 26, 4983–4995. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, V.; Simna, L.; Kailas, L. The coagulation profile of children admitted with dengue fever and correlation with clinical severity. Int. J. Contemp. Pediatr. 2017, 4, 5. [Google Scholar] [CrossRef]
- De Azeredo, E.L.; Monteiro, R.Q.; de-Oliveira Pinto, L.M. Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediat. Inflamm. 2015, 2015, 313842. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lin, Y.-S.; Liu, C.-C.; Liu, H.-S.; Liao, S.-H.; Shi, M.-D.; Lei, H.-Y.; Yeh, T.-M. Factors contributing to the disturbance of coagulation and fibrinolysis in dengue virus infection. J. Formos. Med. Assoc. 2013, 112, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Riswari, S.F.; Tunjungputri, R.N.; Kullaya, V.; Garishah, F.M.; Utari, G.S.; Farhanah, N.; Overheul, G.J.; Alisjahbana, B.; Gasem, M.H.; Urbanus, R.T.; et al. Desialylation of platelets induced by Von Willebrand Factor is a novel mechanism of platelet clearance in dengue. PLOS Pathog. 2019, 15, e1007500. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Lei, H.-Y.; Liu, H.-S.; Lin, Y.-S.; Chen, S.-H.; Liu, C.-C.; Yeh, T.-M. Tissue plasminogen activator induced by dengue virus infection of human endothelial cells. J. Med. Virol. 2003, 70, 610–616. [Google Scholar] [CrossRef]
- Sosothikul, D.; Seksarn, P.; Pongsewalak, S.; Thisyakorn, U.; Lusher, J. Activation of endothelial cells, coagulation, and fibrinolysis in children with Dengue virus infection. Thromb. Haemost. 2007, 97, 627–634. Available online: https://pubmed.ncbi.nlm.nih.gov/17393026/ (accessed on 29 December 2023). [CrossRef]
- Renckens, R.; Roelofs, J.J.; de Waard, V.; Florquin, S.; Lijnen, H.R.; Carmeliet, P.; van der Poll, T. The role of plasminogen activator inhibitor type 1 in the inflammatory response to local tissue injury. J. Thromb. Haemost. 2005, 3, 1018–1025. [Google Scholar] [CrossRef]
- Chao, C.H.; Wu, W.C.; Lai, Y.C.; Tsai, P.J.; Perng, G.C.; Lin, Y.S.; Yeh, T.M. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019, 15, e1007625. [Google Scholar] [CrossRef] [PubMed]
- Nurnaningsih; Sunbanu, S.E.; Rusmawatiningtyas, D.; Arguni, E.; Makrufardi, F.; Kumara, I.F. Disseminated intravascular coagulation initial score as a predictor of mortality in children with dengue shock syndrome: A retrospective cohort study. Ann. Med. Surg. 2022, 79, 103890. [Google Scholar] [CrossRef] [PubMed]
- Castilho, B.M.; Silva, M.T.; Freitas, A.R.R.; Fulone, I.; Lopes, L.C. Factors associated with thrombocytopenia in patients with dengue fever: A retrospective cohort study. BMJ Open 2020, 10, e035120. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Nandi, D.; Batra, H.; Singhal, R.; Annarapu, G.K.; Bhattacharyya, S.; Seth, T.; Dar, L.; Medigeshi, G.R.; Vrati, S.; et al. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci. Rep. 2017, 7, 41697. [Google Scholar] [CrossRef]
- Lam, P.K.; Ngoc, T.V.; Thu Thuy, T.T.; Hong Van, N.T.; Nhu Thuy, T.T.; Hoai Tam, D.T.; Dung, N.M.; Hanh Tien, N.T.; Thanh Kieu, N.T.; Simmons, C.; et al. The value of daily platelet counts for predicting dengue shock syndrome: Results from a prospective observational study of 2301 Vietnamese children with dengue. PLOS Neglected Trop. Dis. 2017, 11, e0005498. [Google Scholar] [CrossRef]
- Saito, M.; Oishi, K.; Inoue, S.; Dimaano, E.M.; Alera, M.T.P.; Robles, A.M.P.; Estrella, B.D.; Kumatori, A.; Moji, K.; Alonzo, M.T.; et al. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin. Exp. Immunol. 2004, 138, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Wills, B.A.; Oragui, E.E.; Stephens, A.C.; Daramola, O.A.; Dung, N.M.; Loan, H.T.; Chau, N.V.; Chambers, M.; Stepniewska, K.; Farrar, J.J.; et al. Coagulation Abnormalities in Dengue Hemorrhagic Fever: Serial Investigations in 167 Vietnamese Children with Dengue Shock Syndrome. Clin. Infect. Dis. 2002, 35, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Zhang, Q.; Liu, W.; Wang, W.; Yu, Z.; Lao, Z.; Zhang, W.; Shen, M.; Wan, P.; Xiao, F.; et al. Dengue Virus Infection Activates Interleukin-1β to Induce Tissue Injury and Vascular Leakage. Front. Microbiol. 2019, 10, 2637. [Google Scholar] [CrossRef]
- Gomes de Azevedo-Quintanilha, I.; Campos, M.M.; Teixeira Monteiro, A.P.; Dantas do Nascimento, A.; Calheiros, A.S.; Oliveira, D.M.; Dias, S.S.G.; Soares, V.C.; Santos, J.D.C.; Tavares, I.; et al. Increased platelet activation and platelet-inflammasome engagement during chikungunya infection. Front. Immunol. 2022, 13, 958820. [Google Scholar] [CrossRef]
- La Russa, V.F.; Innis, B.L. Mechanisms of dengue virus-induced bone marrow suppression. Baillière’s Clin. Haematol. 1995, 8, 249–270. [Google Scholar] [CrossRef]
- Rai, A.; Sgrrimhs, D.; Azad, S.; Nautiyal, S.; Acharya, S. Correlation between hematological and serological parameters in dengue patients- an analysis of 2022 cases. Trop. J. Pathol. Microbiol. 2019, 5, 547–554. [Google Scholar] [CrossRef]
- Abeysuriya, V.; Seneviratne, S.L.; de Mel, P.; Clarice, C.S.H.; de Mel, C.; Chandrasena, L.; Yip, C.; Yap, E.S.; de Mel, S. The immature platelet fraction, a predictive tool for early recovery from dengue-related thrombocytopenia: A prospective study. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 424–432. [Google Scholar] [CrossRef]
- de Mel, S.; Thilakawardana, B.U.; de Mel, P.; Clarice, C.S.H.; Shalindi, M.; de Mel, C.; Chandrasena, L.; Yip, C.; Yap, E.-S.; Seneviratne, S.L.; et al. Triple positivity for nonstructural antigen 1, immunoglobulin M and immunoglobulin G is predictive of severe thrombocytopaenia related to dengue infection. J. Clin. Virol. 2020, 129, 104509. [Google Scholar] [CrossRef]
- Abeysuriya, V.; Choong, C.S.H.; Thilakawardana, B.U.; de Mel, P.; Shalindi, M.; de Mel, C.; Chandrasena, L.; Seneviratne, S.L.; Yip, C.; Yap, E.-S.; et al. The atypical lymphocyte count: A novel predictive factor for severe thrombocytopenia related to dengue. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 424–432. [Google Scholar] [CrossRef]
- Donaldson, C.D.; De Mel, S.; Clarice, C.S.H.; Thilakawardana, B.U.; De Mel, P.; Shalindi, M.; Samarasinghe, U.; de Mel, C.; Chandrasena, L.; Wijesinha, R.S.; et al. Admission ultrasonography as a predictive tool for thrombocytopenia and disease severity in dengue infection. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1396–1402. [Google Scholar] [CrossRef]
- Sun, P.; Bauza, K.; Pal, S.; Liang, Z.; Wu, S.J.; Beckett, C.; Burgess, T.; Porter, K. Infection and activation of human peripheral blood monocytes by dengue viruses through the mechanism of antibody-dependent enhancement. Virology 2011, 421, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.W.; Wu-Hsieh, B.A.; Lin, Y.S.; Chen, W.Y.; Huang, Y.; Anderson, R. The monocyte-macrophage-mast cell axis in dengue pathogenesis. J. Biomed. Sci. 2018, 25, 77. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Vargas, M.J.; Wanionek, K.; Hammond, S.N.; Gordon, A.; Rocha, C.; Balmaseda, A.; Harris, E. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology 2008, 376, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kwissa, M.; Nakaya, H.I.; Onlamoon, N.; Wrammert, J.; Villinger, F.; Perng, G.C.; Yoksan, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Ahmed, R.; et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe 2014, 16, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Singla, M.; Kar, M.; Sethi, T.; Kabra, S.K.; Lodha, R.; Chandele, A.; Medigeshi, G.R. Immune Response to Dengue Virus Infection in Pediatric Patients in New Delhi, India—Association of Viremia, Inflammatory Mediators and Monocytes with Disease Severity. PLoS Neglected Trop. Dis. 2016, 10, e0004497. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Chuang, Y.-J.; Lin, Y.-S.; Chang, C.-P.; Wan, S.-W.; Lin, S.-H.; Chen, C.-L.; Lin, C.-F. Antibody-Dependent Enhancement Infection Facilitates Dengue Virus-Regulated Signaling of IL-10 Production in Monocytes. PLoS Neglected Trop. Dis. 2014, 8, e3320. [Google Scholar] [CrossRef] [PubMed]
- Jessie, K.; Fong, M.Y.; Devi, S.; Lam, S.K.; Wong, K.T. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 2004, 189, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hofman, F.M.; Kung, J.T.; Lin, Y.D.; Wu-Hsieh, B.A. Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J. Virol. 2007, 81, 5518–5526. [Google Scholar] [CrossRef]
- Blackley, S.; Kou, Z.; Chen, H.; Quinn, M.; Rose, R.C.; Schlesinger, J.J.; Coppage, M.; Jin, X. Primary Human Splenic Macrophages, but Not T or B Cells, Are the Principal Target Cells for Dengue Virus Infection In Vitro. J. Virol. 2007, 81, 13325–13334. [Google Scholar] [CrossRef]
- Kou, Z.; Quinn, M.; Chen, H.; Rodrigo WS, I.; Rose, R.C.; Schlesinger, J.J.; Jin, X. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 2008, 80, 134–146. [Google Scholar] [CrossRef]
- Kan, F.K.; Tan, C.C.; von Bahr Greenwood, T.; Khalid, K.E.; Supramaniam, P.; Myrberg, I.H.; Tan, L.H.; Henter, J.-I. Dengue Infection Complicated by Hemophagocytic Lymphohistiocytosis: Experiences from 180 Patients with Severe Dengue. Clin. Infect. Dis. 2020, 70, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Ab-Rahman, H.A.; Wong, P.-F.; Rahim, H.; Abd-Jamil, J.; Tan, K.K.; Sulaiman, S.; Lum, C.-S.; Syed-Omar, S.-F.; AbuBakar, S. Dengue death with evidence of hemophagocytic syndrome and dengue virus infection in the bone marrow. SpringerPlus 2015, 4, 665. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Suzilah, I.; Wan Najdah, W.M.A.; Topek, O.; Mustafakamal, I.; Lee, H.L. Factors determining dengue outbreak in Malaysia. PLoS ONE 2018, 13, e0193326. [Google Scholar] [CrossRef]
- Kurane, I.; Innis, B.L.; Nimmannitya, S.; Nisalak, A.; Meager, A.; Janus, J.; Ennis, F.A. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J. Clin. Investig. 1991, 88, 1473–1480. [Google Scholar] [CrossRef]
- Salvatory Kalabamu, F.; Maliki, S. Use of Haematological Changes as a Predictor of Dengue Infection among Suspected Cases at Kairuki Hospital in Dar Es Salaam, Tanzania: A Retrospective Cross Sectional Study. East Afr. Health Res. J. 2021, 5, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.A.; Raaju, R.U.; Gosavi, S.; Menon, S. Dengue Fever: Prognostic Insights from a Complete Blood Count. Cureus. 2020, 12, e11594. [Google Scholar] [CrossRef]
- Tsai, J.-J.; Liu, L.-T.; Chang, K.; Wang, S.-H.; Hsiao, H.-M.; Clark, K.B.; Perng, G.C. The importance of hematopoietic progenitor cells in dengue. Ther. Adv. Hematol. 2011, 3, 59–71. [Google Scholar] [CrossRef]
- Thisyakorn, U.; Nimmannitya, S.; Ningsanond, V.; Soogarun, S. Atypical lymphocyte in dengue hemorrhagic fever: Its value in diagnosis. Southeast Asian J. Trop. Med. Public Health 1984, 15, 32–36. Available online: https://pubmed.ncbi.nlm.nih.gov/6740378/ (accessed on 29 December 2023).
- Clarice, C.S.H.; Abeysuriya, V.; de Mel, S.; Uvindu Thilakawardana, B.; de Mel, P.; de Mel, C.; Chandrasena, L.; Seneviratne, S.L.; Yip, C.; Yap, E.S. Atypical lymphocyte count correlates with the severity of dengue infection. PLoS ONE 2019, 14, e0215061. [Google Scholar] [CrossRef]
- Chaloemwong, J.; Tantiworawit, A.; Rattanathammethee, T.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Norasetthada, L. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: A retrospective study. BMC Hematol. 2018, 18, 20. [Google Scholar] [CrossRef]
- Azeredo, E.L.; Zagne, S.M.; Alvarenga, A.R.; Nogueira, R.M.; Kubelka, C.F.; de Oliveira-Pinto, L.M. Activated peripheral lymphocytes with increased expression of cell adhesion molecules and cytotoxic markers are associated with dengue fever disease. Memórias Inst. Oswaldo Cruz 2006, 101, 437–449. [Google Scholar] [CrossRef]
- Adikari, T.N.; Kamaladasa, A.; Fernando, R.H.; Fernando, S.M.; Perera, T.M.K.; Gomes, L.; Jayaratne, S.; Ogg, G.S.; Malavige, G.N. High CTLA-4 expression in T cells in patients with acute dengue infection. Int. J. Infect. Dis. 2014, 21, 330. [Google Scholar] [CrossRef]
- Jayaratne, H.E.; Wijeratne, D.; Fernando, S.; Kamaladasa, A.; Gomes, L.; Wijewickrama, A.; Ogg, G.S.; Malavige, G.N. Regulatory T-cells in acute dengue viral infection. Immunology 2017, 154, 89–97. [Google Scholar] [CrossRef]
- Arora, J.K.; Opasawatchai, A.; Poonpanichakul, T.; Jiravejchakul, N.; Sungnak, W.; Sakuntabhai, A.; Singhasivanon, P.; Suraamornkul, S.; Yingtaweesak, T.; Manopwisedjaroen, K.; et al. Single-cell temporal analysis of natural dengue infection reveals skin-homing lymphocyte expansion one day before defervescence. iScience 2022, 25, 104034. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.R.V.; Berbel, A.C.E.R.; Papa, M.P.; de Morais, A.T.S.; Peçanha, L.M.T.; de Arruda, L.B. Dengue Virus Directly Stimulates Polyclonal B Cell Activation. PLoS ONE 2015, 10, e0143391. [Google Scholar] [CrossRef]
- Zanini, F.; Robinson, M.L.; Croote, D.; Sahoo, M.K.; Sanz, A.M.; Ortiz-Lasso, E.; Albornoz, L.L.; Rosso, F.; Montoya, J.G.; Goo, L.; et al. Virus-inclusive single-cell RNA sequencing reveals the molecular signature of progression to severe dengue. Proc. Natl. Acad. Sci. USA 2018, 115, E12363–E12369. [Google Scholar] [CrossRef]
- Carnec, X.; Meertens, L.; Dejarnac, O.; Perera-Lecoin, M.; Hafirassou, M.L.; Kitaura, J.; Ramdasi, R.; Schwartz, O.; Amara, A. The Phosphatidylserine and Phosphatidylethanolamine Receptor CD300a Binds Dengue Virus and Enhances Infection. J. Virol. 2015, 90, 92–102. [Google Scholar] [CrossRef]
- Upasani, V.; Thi, H.; Auerswald, H.; Laurent, D.; Heng, S.; Duong, V.; Rodenhuis-Zybert, I.A.; Dussart, P.; Cantaert, T. Direct Infection of B Cells by Dengue Virus Modulates B Cell Responses in a Cambodian Pediatric Cohort. Front. Immunol. 2021, 11, 594813. [Google Scholar] [CrossRef] [PubMed]
- Upasani, V.; Vo, H.T.M.; Ung, S.; Heng, S.; Laurent, D.; Choeung, R.; Duong, V.; Sorn, S.; Ly, S.; Rodenhuis-Zybert, I.A.; et al. Impaired Antibody-Independent Immune Response of B Cells in Patients with Acute Dengue Infection. Front. Immunol. 2019, 10, 2500. [Google Scholar] [CrossRef]
- Baclig, M.O.; Gervacio, L.T.S.; Suarez, L.-A.C.; Buerano, C.C.; Matias, R.R.; Kumatori, A.; Inoue, S.; Morita, K.; Natividad, F.F.; Hasebe, F. Flow cytometric analysis of dengue virus-infected cells in peripheral blood. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1352–1358. Available online: https://pubmed.ncbi.nlm.nih.gov/21329310/ (accessed on 20 June 2024).
- Guzman, M.G.; Vazquez, S. The complexity of antibody-dependent enhancement of dengue virus infection. Viruses 2010, 2, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A. Defining the role of NK cells during dengue virus infection. Immunology 2018, 154, 557–562. [Google Scholar] [CrossRef]
- Shabrish, S.; Karnik, N.D.; Gupta, V.; Bhate, P.; Madkaikar, M. Impaired NK cell activation during acute dengue virus infection: A contributing factor to disease severity. Heliyon 2020, 6, e04320. [Google Scholar] [CrossRef]
- McKechnie, J.L.; Beltrán, D.; Ferreira, A.-M.M.; Vergara, R.; Saenz, L.; Vergara, O.; Estripeaut, D.; Araúz, A.B.; Simpson, L.J.; Holmes, S.; et al. Mass Cytometry Analysis of the NK Cell Receptor–Ligand Repertoire Reveals Unique Differences between Dengue-Infected Children and Adults. ImmunoHorizons 2020, 4, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, A.; Reid, S.P. Complex Roles of Neutrophils during Arboviral Infections. Cells 2021, 10, 1324. [Google Scholar] [CrossRef]
- Thein, T.-L.; Wong, J.G.X.; Lye, D.C.; Hao, Y.; Wilder-Smith, A.; Leo, Y.-S. Severe Neutropenia in Dengue Patients: Prevalence and Significance. Am. J. Trop. Med. Hyg. 2014, 90, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.Y.; Chng, W.J.; Liu, H.; de Mel, S. Tumor-Associated Macrophages and Related Myelomonocytic Cells in the Tumor Microenvironment of Multiple Myeloma. Cancers 2022, 14, 5654. [Google Scholar] [CrossRef]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef]
- Jenne, C.N.; Wong, C.H.Y.; Zemp, F.J.; McDonald, B.; Rahman, M.M.; Forsyth, P.A.; McFadden, G.; Kubes, P. Neutrophils Recruited to Sites of Infection Protect from Virus Challenge by Releasing Neutrophil Extracellular Traps. Cell Host Microbe 2013, 13, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-S.; Huang, T.-F.; Hsieh, S.-L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; Mantri, C.K.; Aman, S.A.B.; Syenina, A.; Ooi, J.; Jagaraj, C.J.; Goh, C.C.; Tissera, H.; Wilder-Smith, A.; Ng, L.G.; et al. Dengue virus–elicited tryptase induces endothelial permeability and shock. J. Clin. Investig. 2019, 129, 4180–4193. [Google Scholar] [CrossRef] [PubMed]
- Sherif, N.A.; Zayan, A.H.; Elkady, A.H.; Ghozy, S.; Ahmed, A.R.; Omran, E.S.; Taha, E.A.; Eldesoky, E.A.; Ebied, A.; Tieu, T.; et al. Mast cell mediators in relation to dengue severity: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 30, e2084. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.E.; Koifman, S. Clinical and laboratory characteristics of patients with dengue hemorrhagic fever manifestations and their transfusion profile. Rev. Bras. Hematol. Hemoter. 2014, 36, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.E.; Chou, Y.J.; Shen, Y.J.; Tsai, T.; Huang, N. A Population-Based Cohort Study on Chronic Comorbidity Risk Factors for Adverse Dengue Outcomes. Am. J. Trop. Med. Hyg. 2021, 105, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Moallemi, S.; Lloyd, A.R.; Rodrigo, C. Early biomarkers for prediction of severe manifestations of dengue fever: A systematic review and a meta-analysis. Sci. Rep. 2023, 13, 17485. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A. Plasma leakage in dengue haemorrhagic fever. Thromb. Haemost. 2009, 102, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Anandnathan, K.; Shivbalan, S.; Datta, M.; Amalraj, E. Cut-off Hematocrit Value for Hemoconcentration in Dengue Hemorrhagic Fever. J. Trop. Pediatr. 2004, 50, 123–124. [Google Scholar] [CrossRef]
- Looi, K.W.; Matsui, Y.; Kono, M.; Samudi, C.; Kojima, N.; Ong, J.X.; Tan, C.A.; Ang, C.S.; Tan, P.H.Y.; Shamnugam, H.; et al. Evaluation of immature platelet fraction as a marker of dengue fever progression. Int. J. Infect. Dis. 2021, 110, 187–194. [Google Scholar] [CrossRef]
- Chuansumrit, A.; Apiwattanakul, N.; Sirachainan, N.; Paisooksantivatana, K.; Athipongarporn, A.; Tangbubpha, N.; Kadegasem, P.; Tangnararatchakit, K.; Yoksan, S. The use of immature platelet fraction to predict time to platelet recovery in patients with dengue infection. Paediatr. Int. Child Health 2019, 40, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Lye, D.C.; Archuleta, S.; Syed-Omar, S.F.; Low, J.G.; Oh, H.M.; Wei, Y.; Fisher, D.; Ponnampalavanar, S.S.L.; Wijaya, L.; Lee, L.K.; et al. Prophylactic platelet transfusion plus supportive care versus supportive care alone in adults with dengue and thrombocytopenia: A multicentre, open label, randomised, superiority trial. Lancet 2017, 389, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Assir, M.Z.K.; Kamran, U.; Ahmad, H.I.; Bashir, S.; Mansoor, H.; Anees, S.B.; Akram, J. Effectiveness of Platelet Transfusion in Dengue Fever: A Randomized Controlled Trial. Transfus. Med. Hemotherapy 2013, 40, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.M.; Kruskall, M.S.; Kaye, J.A.; Robinson, S.H. Efficacy of platelet transfusions in immune thrombocytopenia. Am. J. Med. 1986, 80, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- de Mel, S.; Thilakawardana, B.U.; de Mel, P.; de Silva, A.P.; de Mel, C.; Chandrasena, L.; Seneviratne, S.L.; Abeysuriya, V. The impact of empirical hydrocortisone therapy on clinical outcomes in dengue fever: A retrospective chart review. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.T.H.; Ngoc, T.V.; Tien, N.T.H.; Kieu, N.T.T.; Thuy, T.T.T.; Thanh, L.T.C.; Tam, C.T.; Truong, N.T.; Dung, N.T.; Qui, P.T.; et al. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: A randomized, placebo-controlled trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Shashidhara, K.C.; Murthy, K.A.S.; Gowdappa, H.B.; Bhograj, A. Effect of High Dose of Steroid on Platelet count in Acute Stage of Dengue Fever with Thrombocytopenia. J. Clin. Diagn. Res. 2013, 7, 1400. [Google Scholar] [CrossRef]
- Kularatne, S.A.M.; Walathara, C.; Mahindawansa, S.I.; Wijesinghe, S.; Pathirage, M.M.K.; Kumarasiri, P.V.R.; Dissanayake, A.M.S.D.M. Efficacy of low dose dexamethasone in severe thrombocytopenia caused by dengue fever: A placebo-controlled study. Postgrad. Med. J. 2009, 85, 525–529. [Google Scholar] [CrossRef]
- Panpanich, R.; Sornchai, P.; Kanjanaratanakorn, K. Corticosteroids for treating dengue shock syndrome. Cochrane Database Syst. Rev. 2006, 3, CD003488. [Google Scholar] [CrossRef]
- Low, J.G.; Sung, C.; Wijaya, L.; Wei, Y.; Rathore, A.P.S.; Watanabe, S.; Tan, B.H.; Toh, L.; Chua, L.T.; Hou, Y.; et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): A phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Palanichamy Kala, M.; St. John, A.L.; Rathore, A.P.S. Dengue: Update on Clinically Relevant Therapeutic Strategies and Vaccines. Curr. Treat. Options Infect. Dis. 2023, 15, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Suputtamongkol, Y.; Avirutnan, P.; Mairiang, D.; Angkasekwinai, N.; Niwattayakul, K.; Yamasmith, E.; Saleh-Arong, F.A.-H.; Songjaeng, A.; Prommool, T.; Tangthawornchaikul, N.; et al. Ivermectin Accelerates circulating nonstructural protein 1 (NS1) clearance in adult dengue patients: A combined phase 2/3 randomized double-blinded placebo controlled trial. Clin. Infect. Dis. 2021, 72, e586–e593. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Mohamed, Z.; Paydar, M.; Rahman, N.A.; Yusof, R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol. 2014, 159, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Lee, L.T.; Wang, Q.Y.; Xie, X.; Lu, S.; Yau, Y.H.; Yuan, Z.; Shochat, S.G.; Kang, C.; Lescar, J.; et al. Mapping the interactions between the NS4B and NS3 proteins of dengue virus. J. Virol. 2015, 89, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, S.J.F.; Goethals, O.; Kiemel, D.; Marchand, A.; Kesteleyn, B.; Bonfanti, J.F.; Bardiot, D.; Stoops, B.; Jonckers, T.H.M.; Dallmeier, K.; et al. A pan-serotype dengue virus inhibitor targeting the NS3-NS4B interaction. Nature 2021, 598, 504–509. [Google Scholar] [CrossRef]
- Wu-Chuang, A.; Rojas, A.; Bernal, C.; Cardozo, F.; Valenzuela, A.; Romero, C.; Mateos-Hernández, L.; Cabezas-Cruz, A. Influence of microbiota-driven natural antibodies on dengue transmission. Front. Immunol. 2024, 15, 1368599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherie, T.J.J.; Choong, C.S.H.; Abid, M.B.; Weber, M.W.; Yap, E.S.; Seneviratne, S.L.; Abeysuriya, V.; de Mel, S. Immuno-Haematologic Aspects of Dengue Infection: Biologic Insights and Clinical Implications. Viruses 2024, 16, 1090. https://doi.org/10.3390/v16071090
Cherie TJJ, Choong CSH, Abid MB, Weber MW, Yap ES, Seneviratne SL, Abeysuriya V, de Mel S. Immuno-Haematologic Aspects of Dengue Infection: Biologic Insights and Clinical Implications. Viruses. 2024; 16(7):1090. https://doi.org/10.3390/v16071090
Chicago/Turabian StyleCherie, Tan Jiao Jie, Clarice Shi Hui Choong, Muhammad Bilal Abid, Matthew W. Weber, Eng Soo Yap, Suranjith L. Seneviratne, Visula Abeysuriya, and Sanjay de Mel. 2024. "Immuno-Haematologic Aspects of Dengue Infection: Biologic Insights and Clinical Implications" Viruses 16, no. 7: 1090. https://doi.org/10.3390/v16071090
APA StyleCherie, T. J. J., Choong, C. S. H., Abid, M. B., Weber, M. W., Yap, E. S., Seneviratne, S. L., Abeysuriya, V., & de Mel, S. (2024). Immuno-Haematologic Aspects of Dengue Infection: Biologic Insights and Clinical Implications. Viruses, 16(7), 1090. https://doi.org/10.3390/v16071090





