Detection of Enteric Viruses in Children under Five Years of Age before and after Rotavirus Vaccine Introduction in Manhiça District, Southern Mozambique, 2008–2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Study Design
2.3. Sample Collection
2.4. Laboratory Testing
2.4.1. Enzyme-Linked Immunosorbent Assay (ELISA) for Virus Detection
2.4.2. Multiplex Reverse Transcription Polymerase Chain Reaction (RT-PR) for Virus Detection
2.5. Ethical Approval
2.6. Data Management and Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Frequency of Enteric Viruses among MSD and LSD Cases and Controls before and after Rotavirus Vaccine Introduction
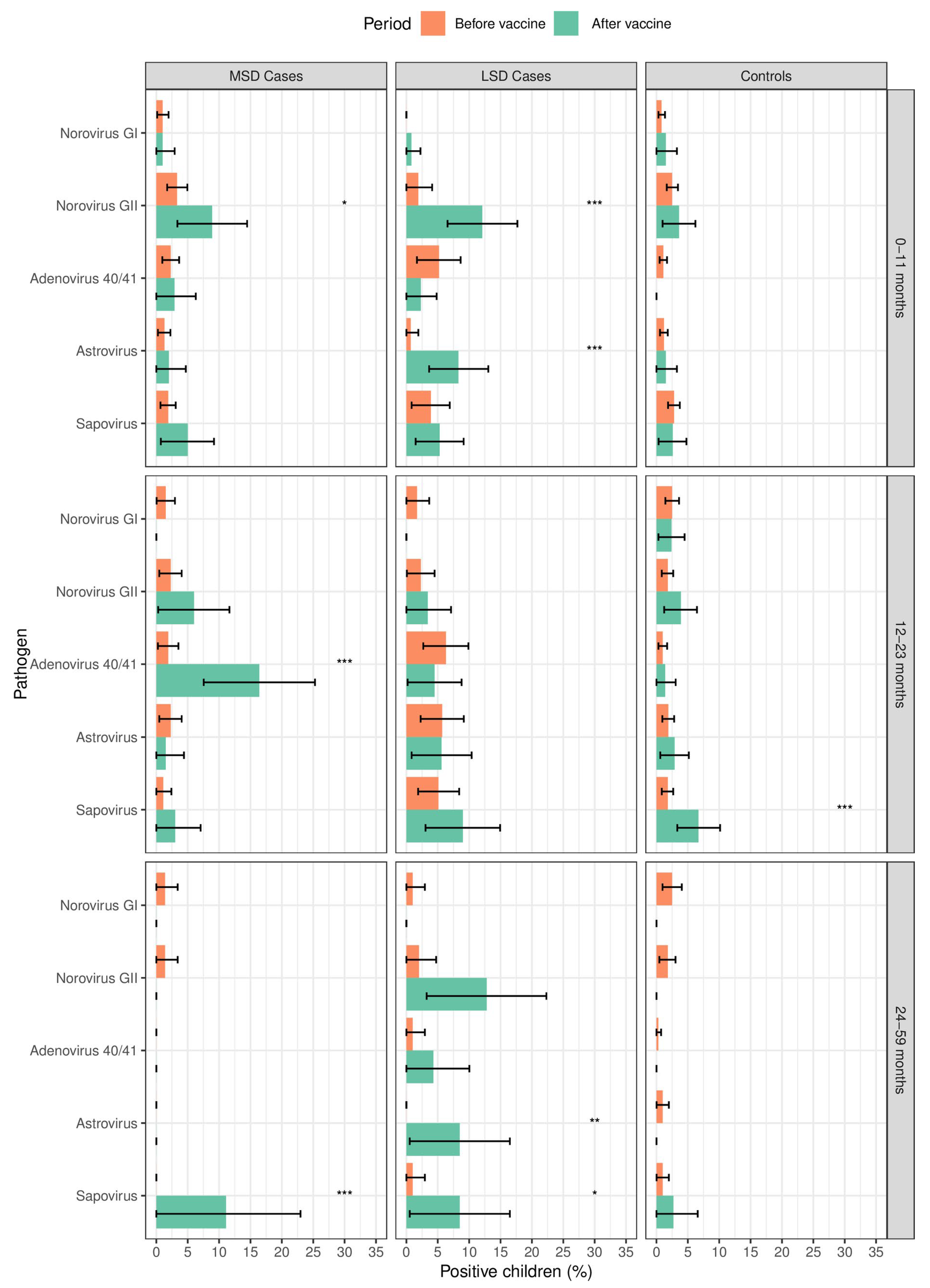
3.3. Frequency of Enteric Viruses among Cases and Controls According to the Age Strata before and after Rotavirus Vaccine Introduction
3.4. Seasonality of Enteric Viruses before and after Rotavirus Vaccine Introduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diarrhoea. World Health Organization. 2019. Available online: https://www.who.int/health-topics/diarrhoea#tab=tab_1 (accessed on 22 September 2023).
- Mousavi Nasab, S.D.; Zali, F.; Kaghazian, H.; Aghasadeghi, M.R.; Mardani, R.; Gachkar, L.; Vasmehjani, A.A.; Ahmadi, N.; Ghasemzadeh, A. Prevalence of astrovirus, adenovirus, and sapovirus infections among Iranian children with acute gastroenteritis. Gastroenterol. Hepatol. Bed Bench 2020, 13, S122–S127. [Google Scholar] [PubMed]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Yinda, C.K.; Conceição-Neto, N.; Zeller, M.; Heylen, E.; Maes, P.; Ghogomu, S.M.; Van Ranst, M.; Matthijnssens, J. Novel highly divergent sapoviruses detected by metagenomics analysis in straw-colored fruit bats in Cameroon: Divergent bat sapoviruses. Emerg. Microbes Infect. 2017, 6, e38. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, P.; Schultz-Cherry, S.; Burnham, A.; Cattoli, G. Astrovirus infections in humans and animals—Molecular biology, genetic diversity, and interspecies transmissions. Infect. Genet. Evol. 2011, 11, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Makimaa, H.; Ingle, H.; Baldridge, M.T. Enteric Viral Co-Infections: Pathogenesis and Perspective. Viruses 2020, 12, 904. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Nhampossa, T.; Mandomando, I.; Acacio, S.; Quintó, L.; Vubil, D.; Ruiz, J.; Nhalungo, D.; Sacoor, C.; Nhabanga, A.; Nhacolo, A.; et al. Diarrheal Disease in Rural Mozambique: Burden, Risk Factors and Etiology of Diarrheal Disease among Children Aged 0–59 Months Seeking Care at Health Facilities. PLoS ONE 2015, 10, e0119824. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.; Panchalingham, S.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob. Health 2019, 7, e568–e584. [Google Scholar] [CrossRef]
- WHO. Moçambique Introduz a Vacina Contra o Rotavírus. 2015. Available online: https://www.afro.who.int/pt/news/mocambique-introduz-vacina-contra-o-rotavirus (accessed on 20 February 2024).
- WHO. Meeting of the immunization Strategic Advisory Group of Experts, April 2009—Conclusions and recommendations. Wkly. Epidemiol. Rec. = Relev. Épidémiologique Hebd. 2009, 84, 220–236. [Google Scholar]
- Raboni, S.M.; Damasio, G.A.C.; Ferreira, C.E.; Pereira, L.A.; Nogueira, M.B.; Vidal, L.R.; Cruz, C.R.; Almeida, S.M. Acute gastroenteritis and enteric viruses in hospitalised children in southern Brazil: Aetiology, seasonality and clinical outcomes. Memórias Inst. Oswaldo Cruz 2014, 109, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Lambisia, A.W.; Onchaga, S.; Murunga, N.; Lewa, C.S.; Nyanjom, S.G.; Agoti, C.N. Epidemiological Trends of Five Common Diarrhea-Associated Enteric Viruses Pre- and Post-Rotavirus Vaccine Introduction in Coastal Kenya. Pathogens 2020, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Manjate, F.; Quintó, L.; Chirinda, P.; Acácio, S.; Garrine, M.; Vubil, D.; Nhampossa, T.; João, E.D.; Nhacolo, A.; Cossa, A.; et al. Impact of rotavirus vaccination on diarrheal hospitalizations in children younger than 5 years of age in a rural southern Mozambique. Vaccine 2022, 40, 6422–6430. [Google Scholar] [CrossRef]
- Sacoor, C.; Nhacolo, A.; Nhalungo, D.; Aponte, J.J.; Bassat, Q.; Augusto, O.; Mandomando, I.; Sacarlal, J.; Lauchande, N.; Sigaúque, B.; et al. Profile: Manhica Health Research Centre (Manhica HDSS). Int. J. Epidemiol. 2013, 42, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Nhacolo, A.; Jamisse, E.; Augusto, O.; Matsena, T.; Hunguana, A.; Mandomando, I.; Arnaldo, C.; Munguambe, K.; Macete, E.; Alonso, P.; et al. Cohort Profile Update: Manhiça Health and Demographic Surveillance System (HDSS) of the Manhiça Health Research Centre (CISM). Int. J. Epidemiol. 2021, 50, 395. [Google Scholar] [CrossRef]
- Manjate, F.; João, E.D.; Chirinda, P.; Garrine, M.; Vubil, D.; Nobela, N.; Kotloff, K.; Nataro, J.P.; Nhampossa, T.; Acácio, S.; et al. Molecular Epidemiology of Rotavirus Strains in Symptomatic and Asymptomatic Children in Manhiça District, Southern Mozambique 2008–2019. Viruses 2022, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Panchalingam, S.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, B.; Oundo, J.; Ramamurthy, T.; Tamboura, B.; Zaidi, A.K.M.; Petri, W.; et al. Diagnostic Microbiologic Methods in the GEMS-1 Case/Control Study. Clin. Infect. Dis. 2012, 55, S294–S302. [Google Scholar] [CrossRef] [PubMed]
- Agoti, C.N.; Curran, M.D.; Murunga, N.; Ngari, M.; Muthumbi, E.; Lambisia, A.W.; Frost, S.D.W.; Blacklaws, B.A.; Nokes, D.J.; Drumright, L.N. Differences in epidemiology of enteropathogens in children pre- and post-rotavirus vaccine introduction in Kilifi, coastal Kenya. Gut Pathog. 2022, 14, 32. [Google Scholar] [CrossRef]
- Olivares, A.I.O.; Leitão, G.A.A.; Pimenta, Y.C.; Cantelli, C.P.; Fumian, T.M.; Fialho, A.M.; Delgado, I.F.; Nordgren, J.; Svensson, L.; Miagostovich, M.P.; et al. Epidemiology of enteric virus infections in children living in the Amazon region. Int. J. Infect. Dis. 2021, 108, 494–502. [Google Scholar] [CrossRef]
- McAtee, C.L.; Webman, R.; Gilman, R.H.; Meija, C.; Bern, C.; Apaza, S.; Espetia, S.; Pajuelo, M.; Saito, M.; Challappa, R.; et al. Burden of Norovirus and Rotavirus in Children After Rotavirus Vaccine Introduction, Cochabamba, Bolivia. Am. J. Trop. Med. Hyg. 2016, 94, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Bucardo, F.; Reyes, Y.; Svensson, L.; Nordgren, J. Predominance of Norovirus and Sapovirus in Nicaragua after Implementation of Universal Rotavirus Vaccination. PLoS ONE 2014, 9, e98201. [Google Scholar] [CrossRef]
- Hassan, F.; Kanwar, N.; Harrison, C.J.; Halasa, N.B.; Chappell, J.D.; Englund, J.A.; Klein, E.J.; Weinberg, G.A.; Szilagyi, P.G.; Moffatt, M.E.; et al. Viral Etiology of Acute Gastroenteritis in <2-Year-Old US Children in the Post–Rotavirus Vaccine Era. J. Pediatr. Infect. Dis. Soc. 2019, 8, 414–421. [Google Scholar] [CrossRef]
- Cardemil, C.V.; Sherchand, J.B.; Shrestha, L.; Sharma, A.; Gary, H.E.; Estivariz, C.F.; Diez-Valcarce, M.; Ward, M.L.; Bowen, M.D.; Vinjé, J.; et al. Pathogen-Specific Burden of Outpatient Diarrhea in Infants in Nepal: A Multisite Prospective Case-Control Study. J. Pediatr. Infect. Dis. Soc. 2017, 6, e75–e85. [Google Scholar] [CrossRef] [PubMed]
- Keita, A.M.; Doh, S.; Sow, S.O.; Powell, H.; Omore, R.; Jahangir Hossain, M.; Ogwel, B.; Ochieng, J.B.; Jones, J.C.M.; Zaman, S.M.A.; et al. Prevalence, Clinical Severity, and Seasonality of Adenovirus 40/41, Astrovirus, Sapovirus, and Rotavirus Among Young Children with Moderate-to-Severe Diarrhea: Results From the Vaccine Impact on Diarrhea in Africa (VIDA) Study. Clin. Infect. Dis. 2023, 76, S123–S131. [Google Scholar] [CrossRef] [PubMed]
- Moser, L.A.; Schultz-Cherry, S. Pathogenesis of Astrovirus Infection. Viral Immunol. 2005, 18, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.B.; Lee, T.W.; Craig, J.W.; Reed, S.E. Astrovirus infection in volunteers. J. Med. Virol. 1979, 3, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Midthun, K.; Greenberg, H.B.; Kurtz, J.B.; Gary, G.W.; Lin, F.Y.; Kapikian, A.Z. Characterization and seroepidemiology of a type 5 astrovirus associated with an outbreak of gastroenteritis in Marin County, California. J. Clin. Microbiol. 1993, 31, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Hargest, V.; Cortez, V.; Meliopoulos, V.; Schultz-Cherry, S. Astrovirus Pathogenesis. Viruses 2017, 9, 22. [Google Scholar] [CrossRef]
- Chen, C.-J.; Wu, F.-T.; Huang, Y.-C.; Chang, W.-C.; Wu, H.-S.; Wu, C.-Y.; Lin, J.-S.; Huang, F.-C.; Hsiung, C.A. Clinical and Epidemiologic Features of Severe Viral Gastroenteritis in Children: A 3-Year Surveillance, Multicentered Study in Taiwan with Partial Rotavirus Immunization. Medicine 2015, 94, e1372. [Google Scholar] [CrossRef]
- Cao, R.-R.; Ma, X.-Z.; Li, W.-Y.; Wang, B.-N.; Yang, Y.; Wang, H.-R.; Kuang, Y.; You, J.-Z.; Zhao, Z.-Y.; Ren, M.; et al. Epidemiology of norovirus gastroenteritis in hospitalized children under five years old in western China, 2015–2019. J. Microbiol. Immunol. Infect. 2021, 54, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Goggins, W.B.; Chan, E.Y.Y. A time-series study of the association of rainfall, relative humidity and ambient temperature with hospitalizations for rotavirus and norovirus infection among children in Hong Kong. Sci. Total Environ. 2018, 643, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.S.; Ghosh, S.; Chawla-Sarkar, M.; Panchalingam, S.; Nataro, J.P.; Sur, D.; Manna, B.; Ramamurthy, T. Circulation of a Novel Pattern of Infections by Enteric Adenovirus Serotype 41 among Children below 5 Years of Age in Kolkata, India. J. Clin. Microbiol. 2011, 49, 500–505. [Google Scholar] [CrossRef] [PubMed]

| Cases N = 1779 | Controls N = 2855 | |||||
|---|---|---|---|---|---|---|
| MSD N = 1081 | LSD N = 698 | |||||
| Characteristics | Before Vaccine [N = 886] n (%) | After Vaccine [N = 195] n (%) | Before Vaccine [N = 430] n (%) | After Vaccine [N = 268] n (%) | Before Vaccine [N = 2380] n (%) | After Vaccine [N = 475] n (%) |
| Age strata | ||||||
| 0–11 months | 480 (54.2) | 101 (51.8) | 155 (30.0) | 132 (49.3) | 1184 (49.7) | 195 (41.0) |
| 12–23 months | 266 (30.0) | 67 (34.4) | 175 (40.7) | 89 (33.2) | 797 (33.5) | 208 (43.8) |
| 24–59 months | 140 (15.8) | 27 (13.8) | 100 (23.3) | 47 (17.5) | 399 (16.8) | 72 (15.2) |
| Sex | ||||||
| Male | 527 (59.5) | 115 (59.0) | 236 (54.9) | 147 (54.9) | 1427 (60.0) | 256 (53.9) |
| Female | 359 (40.5) | 80 (41.0) | 194 (45.1) | 121 (45.1) | 953 (40.0) | 219 (46.1) |
| Rotavirus vaccination status | N = 175 | N = 254 | N = 452 | |||
| Vaccinated * | NA | 137 (78) | NA | 220 (87) | NA | 364 (81) |
| Unvaccinated # | NA | 38 (22) | NA | 34 (13) | NA | 88 (19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirinda, P.; Manjate, F.; Garrine, M.; Messa, A., Jr.; Nobela, N.; Vubil, D.; Nhampossa, T.; Acácio, S.; Bassat, Q.; Kotloff, K.L.; et al. Detection of Enteric Viruses in Children under Five Years of Age before and after Rotavirus Vaccine Introduction in Manhiça District, Southern Mozambique, 2008–2019. Viruses 2024, 16, 1159. https://doi.org/10.3390/v16071159
Chirinda P, Manjate F, Garrine M, Messa A Jr., Nobela N, Vubil D, Nhampossa T, Acácio S, Bassat Q, Kotloff KL, et al. Detection of Enteric Viruses in Children under Five Years of Age before and after Rotavirus Vaccine Introduction in Manhiça District, Southern Mozambique, 2008–2019. Viruses. 2024; 16(7):1159. https://doi.org/10.3390/v16071159
Chicago/Turabian StyleChirinda, Percina, Filomena Manjate, Marcelino Garrine, Augusto Messa, Jr., Nélio Nobela, Delfino Vubil, Tacilta Nhampossa, Sozinho Acácio, Quique Bassat, Karen L. Kotloff, and et al. 2024. "Detection of Enteric Viruses in Children under Five Years of Age before and after Rotavirus Vaccine Introduction in Manhiça District, Southern Mozambique, 2008–2019" Viruses 16, no. 7: 1159. https://doi.org/10.3390/v16071159






