Characterization of Human Immortalized Keratinocyte Cells Infected by Monkeypox Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of MPXV
2.3. Genome Sequencing and Assembly
2.4. MPXV Phylogenetic Analysis
2.5. DNA Extraction and Quantitative PCR
2.6. Transmission Electron Microscope
2.7. RNA Sequencing
2.8. Statistical Analysis
3. Results
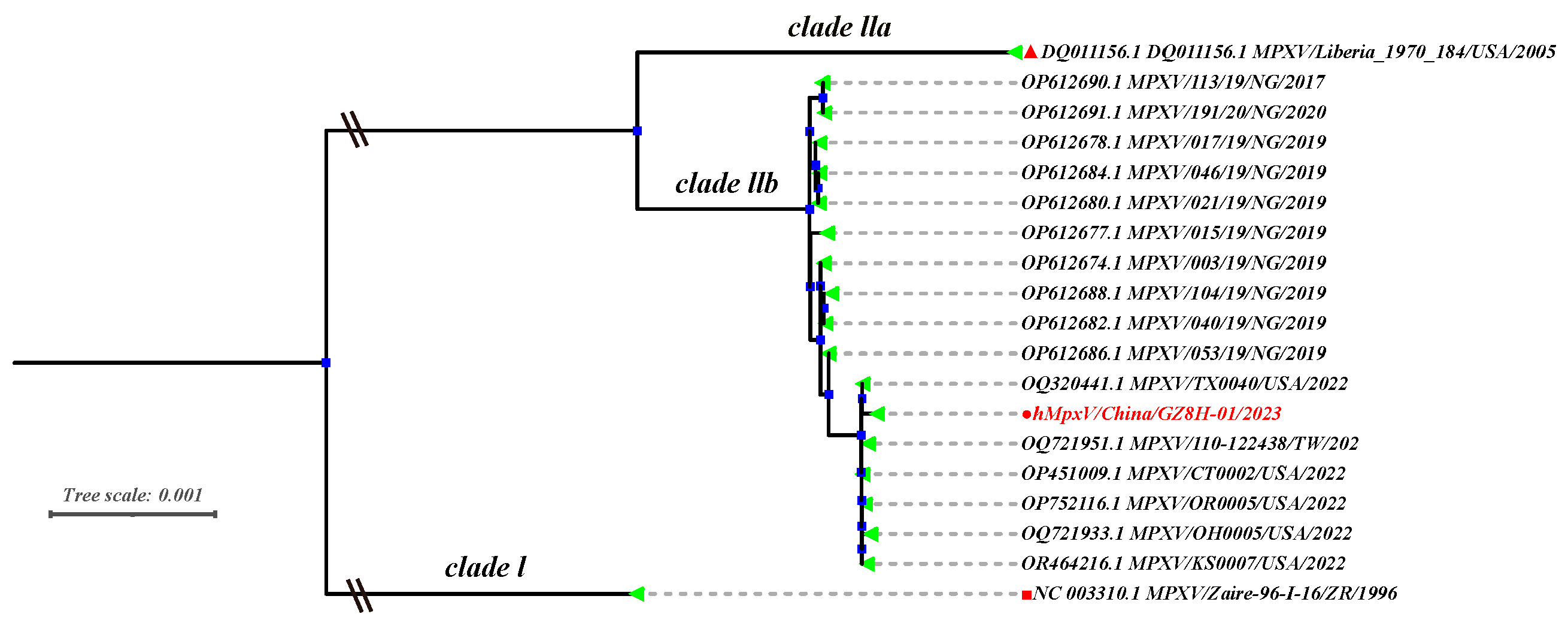
3.1. Phylogenetic Analysis of MPVX Strains
3.2. Growth Kinetics
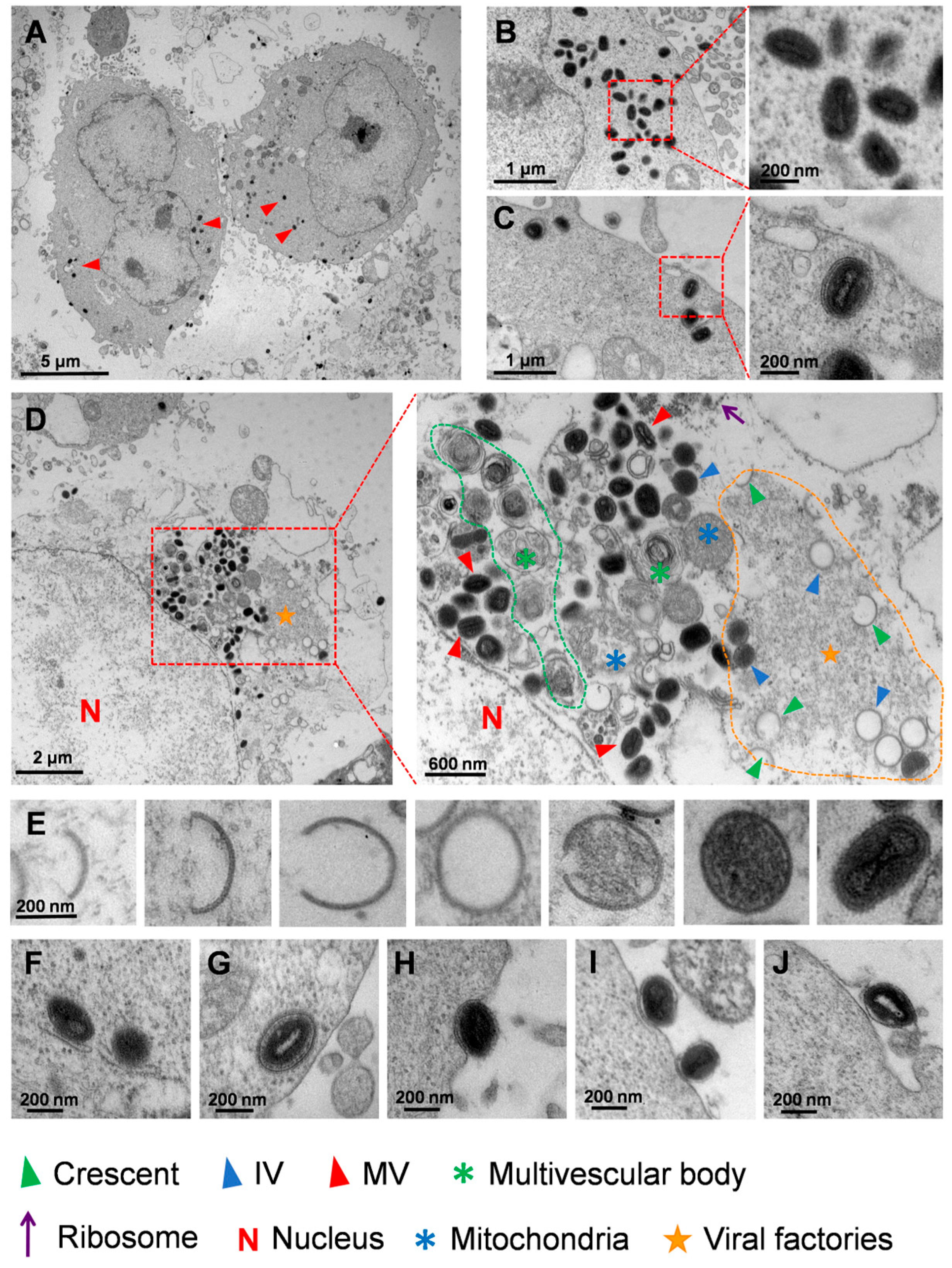
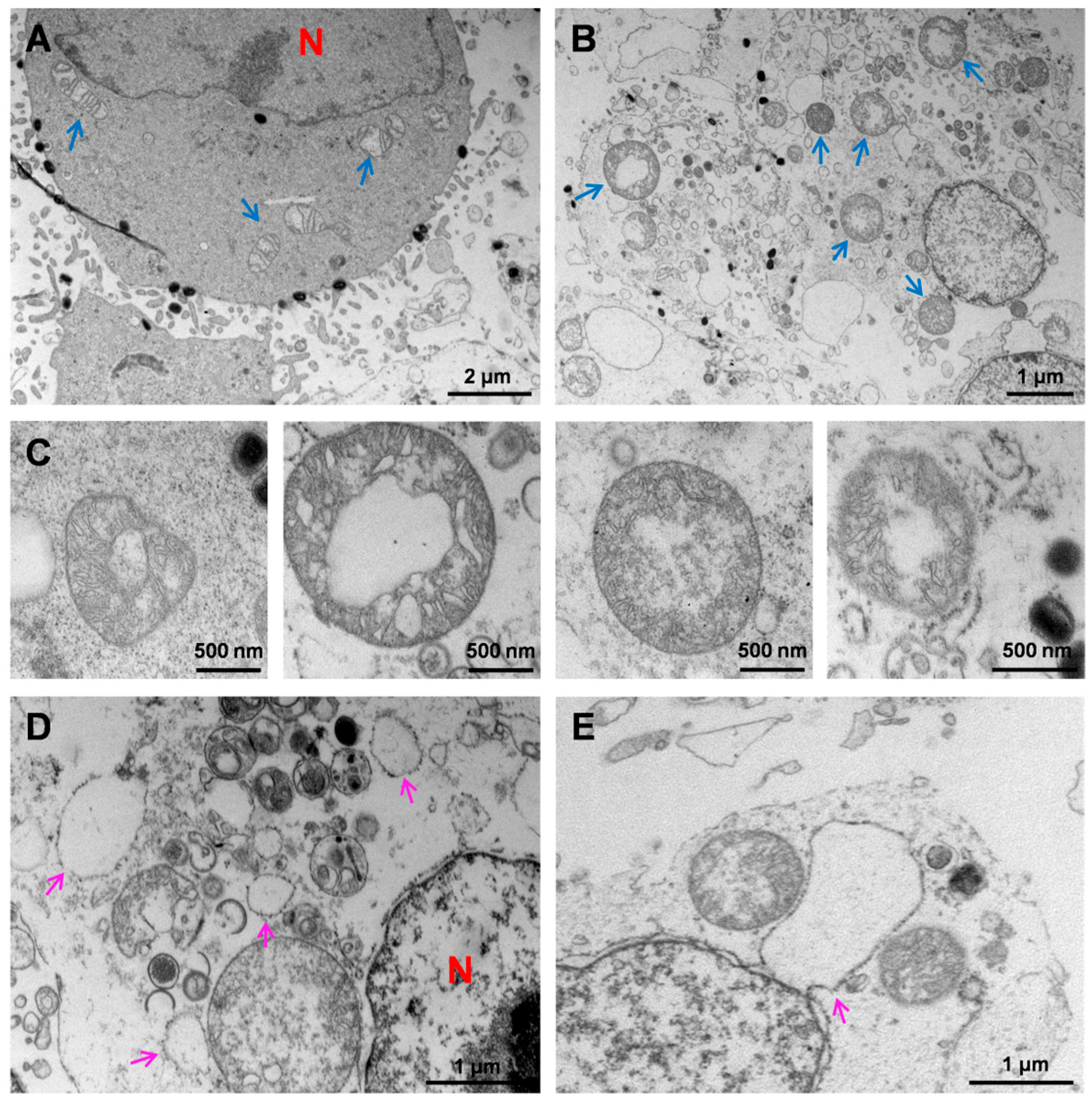
3.3. Ultrastructural Analysis of MPXV in HaCaT Cells
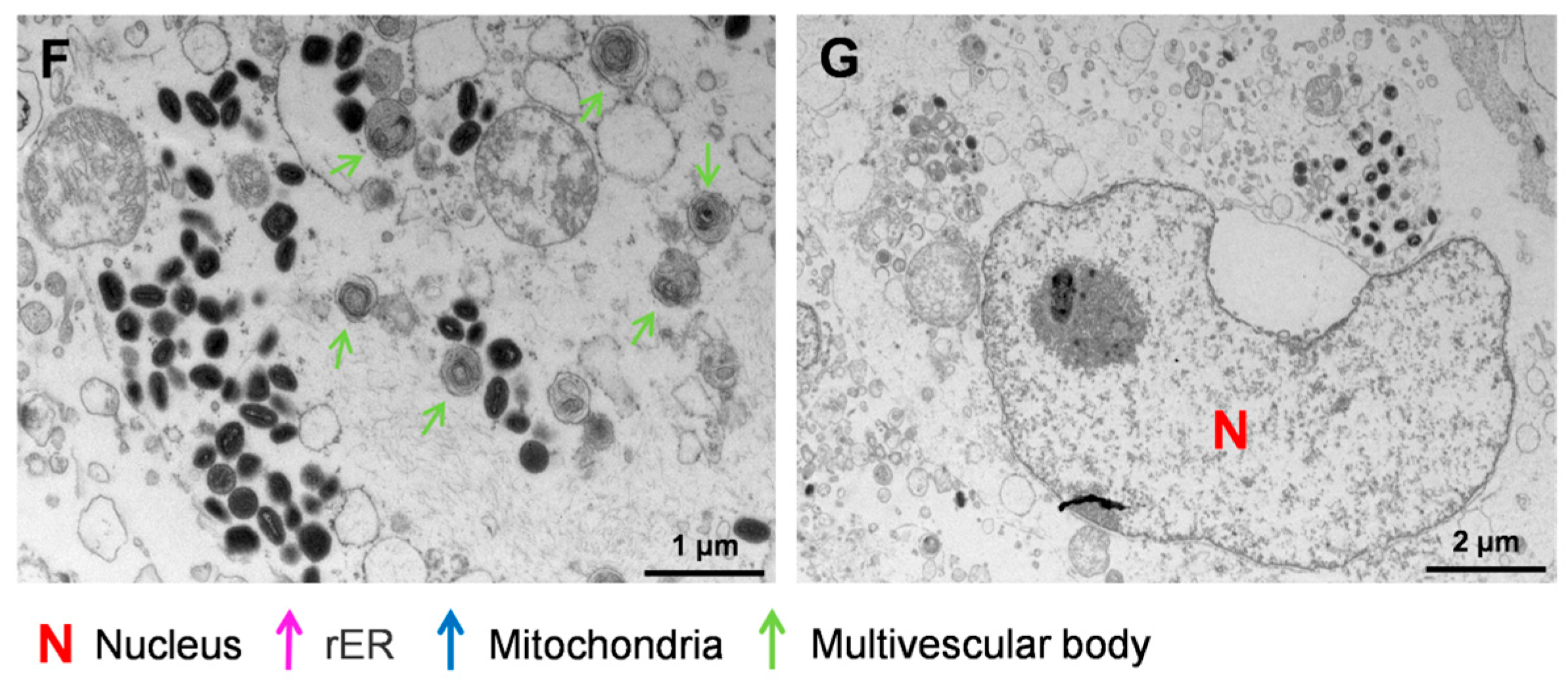
3.4. Ultrastructural Analysis of HaCaT Cells Infected with MPXV
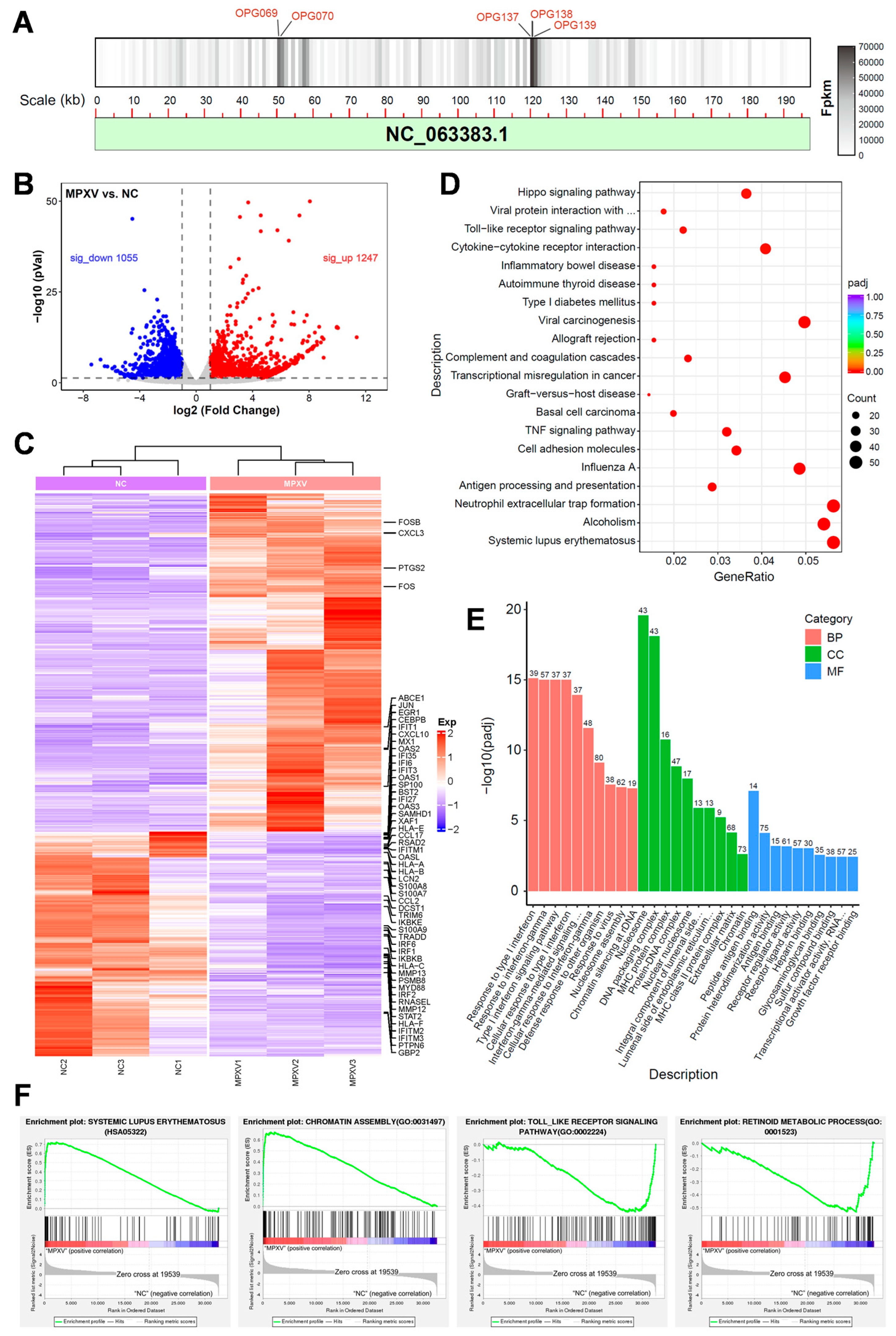
3.5. Transcriptomic Analysis of HaCaT Cells Infected with MPXV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, S.O.; Brink, E.W.; Hutchins, D.L.; Pifer, J.M.; Lourie, B.; Moser, C.R.; Cummings, E.C.; Kuteyi, O.E.; Eke, R.E.; Titus, J.B.; et al. Human monkeypox. Bull. World Health Organ. 1972, 46, 569–576. [Google Scholar] [PubMed]
- Del Rio, C.; Malani, P.N. Update on the Monkeypox Outbreak. JAMA 2022, 328, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Li, F.; Ren, Z.; Zhang, D.; Li, J.; Li, D.; Sun, Y.; Jin, H.; Li, R.; Li, W.; et al. Clinical, epidemiological, and virological features of Mpox in Beijing, China—May 31–June 21, 2023. Emerg. Microbes Infect. 2023, 12, 2254407. [Google Scholar] [CrossRef]
- Rosa, R.B.; Ferreira de Castro, E.; Vieira da Silva, M.; Paiva Ferreira, D.C.; Jardim, A.C.G.; Santos, I.A.; Marinho, M.d.S.; Ferreira França, F.B.; Pena, L.J. In vitro and in vivo models for monkeypox. iScience 2023, 26, 105702. [Google Scholar] [CrossRef] [PubMed]
- Beeson, A.; Styczynski, A.; Hutson, C.L.; Whitehill, F.; Angelo, K.M.; Minhaj, F.S.; Morgan, C.; Ciampaglio, K.; Reynolds, M.G.; McCollum, A.M.; et al. Mpox respiratory transmission: The state of the evidence. Lancet Microbe 2023, 4, e277–e283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mucker, E.M.; Chapman, J.L.; Babka, A.M.; Gordon, J.M.; Bryan, A.V.; Raymond, J.L.W.; Bell, T.M.; Facemire, P.R.; Goff, A.J.; et al. Retrospective detection of monkeypox virus in the testes of nonhuman primate survivors. Nat. Microbiol. 2022, 7, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.; Abdelaal, A.; Brakat, A.M.; Lashin, B.I.; Abouelkheir, M.; Abdelazeem, B.; Rodriguez-Morales, A.J.; Sah, R. Monkeypox viral detection in semen specimens of confirmed cases: A systematic review and meta-analysis. J. Med. Virol. 2022, 95, e28250. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, C.; Zhang, Q.; Zhu, H.; Cui, F.; Zhao, Z.; Song, M.; Zhou, B.; Zhang, Y.; Hu, P.; et al. The first detection of mpox virus DNA from wastewater in China. Sci. Total Environ. 2024, 932, 172742. [Google Scholar] [CrossRef] [PubMed]
- Sanchiz, Á.; Martín, R.; Del Val, M.; Corell, A.; Alcamí, A. MPXV and SARS-CoV-2 in the air of nightclubs in Spain. Lancet Microbe 2023, 4, e389. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, K.; Wong, J.C.C.; Lim, P.L.; Octavia, S.; Huan, X.; Ng, Y.K.; Yang, J.J.; Sutjipto, S.; Linn, K.Z.; Setoh, Y.X.; et al. Viable mpox virus in the environment of a patient room. Int. J. Infect. Dis. 2023, 131, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, S.; Zeng, Y.; Chen, Z.; Xia, F.; Zhang, H.; Dan, D.; Hu, C.; Tang, Y.; Yang, Q.; et al. Evaluation of Stability, Inactivation, and Disinfection Effectiveness of Mpox Virus. Viruses 2024, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Brüggemann, Y.; Todt, D.; Tao, R.; Müller, L.; Steinmann, J.; Steinmann, J.; Timm, J.; Drexler, I.; Steinmann, E. Stability and Inactivation of Monkeypox Virus on Inanimate Surfaces. J. Infect. Dis. 2023, 228, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shen, X.; Zhang, H.; Jin, H.; Zhang, L.; Lv, B.; Li, W.; Liang, Z.; Zhang, X.; Zhang, D.; et al. Stability of mpox virus on different commonly contacted surfaces. J. Med. Virol. 2023, 95, e29296. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, D.; Bettini, A.; Meschi, S.; Notari, S.; Francalancia, M.; Tartaglia, E.; Lapa, D.; Specchiarello, E.; Girardi, E.; Matusali, G.; et al. Effect of chemical and physical agents on monkeypox virus infectivity and downstream research applications. Virology 2024, 592, 109993. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Tao, R.; Brüggemann, Y.; Todt, D.; Steinmann, J.; Timm, J.; Drexler, I.; Steinmann, E. Efficient Inactivation of Monkeypox Virus by World Health Organization—Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 2023, 29, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, G.; Di Biagio, A.; Bruzzone, B.; Guadagno, A.; Taramasso, L.; Oddenino, G.; Brucci, G.; Labate, L.; De Pace, V.; Mastrolonardo, M.; et al. Monkeypox outbreak in Genoa, Italy: Clinical, laboratory, histopathologic features, management, and outcome of the infected patients. J. Med. Virol. 2023, 95, e28560. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Port, J.R.; Riopelle, J.C.; Smith, S.G.; Myers, L.; Kaiser, F.K.; Lewis, M.C.; Gallogly, S.; Okumura, A.; Bushmaker, T.; Schulz, J.E.; et al. Infection with mpox virus via the genital mucosae increases shedding and transmission in the multimammate rat (Mastomys natalensis). Nat. Microbiol. 2024, 9, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yan, B.; Fang, Y.; Yang, X.; Jia, H.; Zhang, M.; Li, S.; Zhang, Y.; Wang, W.; Guo, C.; et al. Cases of Monkeypox show highly-overlapping co-infection with HIV and syphilis. Front. Public Health 2024, 11, 1276821. [Google Scholar] [CrossRef]
- O’Shea, J.; Zucker, J.; Stampfer, S.; Cash-Goldwasser, S.; Minhaj, F.S.; Dretler, A.; Cheeley, J.; Chaudhuri, S.; Gallitano, S.M.; Gunaratne, S.; et al. Prolonged Mpox Disease in People With Advanced HIV: Characterization of Mpox Skin Lesions. J. Infect. Dis. 2024, 229 (Suppl. S2), S243–S248. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Ding, K.; Wang, X.-H.; Sun, G.-Y.; Liu, Z.-X.; Luo, Y. The evolving epidemiology of monkeypox virus. Cytokine Growth Factor Rev. 2022, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Park, S.; Kim, H.J.; Lee, M.; Choi, Y.S.; Yeo, S.G.; Lee, J.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Clinical characteristics and outcomes of patients with mpox during the 2022 mpox outbreak compared with those before the outbreak: A systematic review and meta-analysis. Rev. Med. Virol. 2024, 34, e2508. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Safronov, P.F.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Petrov, N.A.; Babkin, I.V.; Uvarova, E.A.; Sandakhchiev, L.S.; et al. Analysis of the monkeypox virus genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef]
- Hatmal, M.m.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Ahmad, S.; Hasan, H.; Ahmad Suhaimi, N.A.; Albakri, K.A.; Abedalbaset Alzyoud, A.; Kadir, R.; Mohamud, R. Comprehensive literature review of monkeypox. Emerg. Microbes Infect. 2022, 11, 2600–2631. [Google Scholar] [CrossRef] [PubMed]
- Ulaeto, D.; Agafonov, A.; Burchfield, J.; Carter, L.; Happi, C.; Jakob, R.; Krpelanova, E.; Kuppalli, K.; Lefkowitz, E.J.; Mauldin, M.R.; et al. New nomenclature for mpox (monkeypox) and monkeypox virus clades. Lancet Infect. Dis. 2023, 23, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Smith, G.L. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008, 16, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Law, M. The exit of Vaccinia virus from infected cells. Virus Res. 2004, 106, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pickup, D.J. Extracellular Virions: The Advance Guard of Poxvirus Infections. PLoS Pathog. 2015, 11, e1004904. [Google Scholar] [CrossRef]
- Sivan, G.; Weisberg, A.S.; Americo, J.L.; Moss, B. Retrograde Transport from Early Endosomes to the trans-Golgi Network Enables Membrane Wrapping and Egress of Vaccinia Virus Virions. J. Virol. 2016, 90, 8891–8905. [Google Scholar] [CrossRef] [PubMed]
- Witt, A.S.A.; Trindade, G.d.S.; Souza, F.G.d.; Serafim, M.S.M.; da Costa, A.V.B.; Silva, M.V.F.; de Melo Iani, F.C.; Rodrigues, R.A.L.; Kroon, E.G.; Abrahão, J.S. Ultrastructural analysis of monkeypox virus replication in Vero cells. J. Med. Virol. 2023, 95, e28536. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Kimura, I.; Hashimoto, R.; Sakamoto, A.; Yasuhara, N.; Yamamoto, T.; Sato, K.; Takayama, K. Virological characterization of the 2022 outbreak-causing monkeypox virus using human keratinocytes and colon organoids. J. Med. Virol. 2023, 95, e28827. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Reidy, J.; Pagani, N.; Lednicky, J.A.; McGrail, J.P.; Kasminskaya, Y.; Patino, L.H.; Garcia-Sastre, A.; Palacios, G.; Gonzalez-Reiche, A.S.; et al. Genomic and ultrastructural analysis of monkeypox virus in skin lesions and in human/animal infected cells reveals further morphofunctional insights into viral pathogenicity. J. Med. Virol. 2023, 95, e28878. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gu, C.; Lian, P.; Wazir, J.; Lu, R.; Ruan, B.; Wei, L.; Li, L.; Pu, W.; Peng, Z.; et al. Sulforaphane alleviates psoriasis by enhancing antioxidant defense through KEAP1-NRF2 Pathway activation and attenuating inflammatory signaling. Cell Death Dis. 2023, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Dash, P.K.; Yadav, R.G.; Shrivastava, A.; Menon, R.; Kumar, J.S.; Sharma, S.; Dhankher, S.; Dhiman, S.; Kumari, D.; et al. Isolation and characterization of emerging Mpox virus from India. J. Med. Virol. 2023, 95, e28911. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, L.; Wang, M.; Peng, Y.; Wang, H.; Yang, X.; Zhao, J.; Zhang, M.; Wang, F.; Zhang, Z. Isolation and characterization of mpox virus from the first mpox case in Shenzhen, China. Virol. Sin. 2024, 39, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Pachis, S.T.; Xu, G.; Schraauwen, R.; Incitti, R.; de Vries, A.C.; Bruno, M.J.; Peppelenbosch, M.P.; Alam, I.; Raymond, K.; et al. Mpox virus infection and drug treatment modelled in human skin organoids. Nat. Microbiol. 2023, 8, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, J.; Valade, M.; Lebideau, M.; Bretelle, F.; Mège, J.L.; Wurtz, N.; Mezouar, S.; La Scola, B.; Baudoin, J.P. Pan-microscopic examination of monkeypox virus in trophoblasts cells reveals new insights into virions release through filopodia-like projections. J. Med. Virol. 2024, 96, e29620. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, M.; Rodger, G.; Van Eijl, H.; Law, M.; Hollinshead, R.; Vaux, D.J.; Smith, G.L. Vaccinia virus utilizes microtubules for movement to the cell surface. J. Cell Biol. 2001, 154, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Blasco, R.; Moss, B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 1992, 66, 4170–4179. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Yu, J.; Wu, Z.; Jiang, J.; Gong, L.; Wang, B.; Guo, H.; Zhao, H.; Lu, B.; Engelender, S.; et al. Hypoxia-reprogramed megamitochondrion contacts and engulfs lysosome to mediate mitochondrial self-digestion. Nat. Commun. 2023, 14, 4105. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, C.; Huang, Z.; Sun, Y.; Shi, S.; Li, X.; Li, N.; Liu, Y.; Guo, Z.; Jin, N.; Zhao, Z.; et al. Characterization of Human Immortalized Keratinocyte Cells Infected by Monkeypox Virus. Viruses 2024, 16, 1206. https://doi.org/10.3390/v16081206
Gu C, Huang Z, Sun Y, Shi S, Li X, Li N, Liu Y, Guo Z, Jin N, Zhao Z, et al. Characterization of Human Immortalized Keratinocyte Cells Infected by Monkeypox Virus. Viruses. 2024; 16(8):1206. https://doi.org/10.3390/v16081206
Chicago/Turabian StyleGu, Chaode, Zhiqiang Huang, Yongyang Sun, Shaowen Shi, Xiubo Li, Nan Li, Yang Liu, Zhendong Guo, Ningyi Jin, Zongzheng Zhao, and et al. 2024. "Characterization of Human Immortalized Keratinocyte Cells Infected by Monkeypox Virus" Viruses 16, no. 8: 1206. https://doi.org/10.3390/v16081206





