Generalized Eruptive Keratoacanthoma (GEKA) after Pfizer mRNABNT162b2 (Comirnaty®) COVID-19 Vaccination Successfully Treated with Cemiplimab
Abstract
:1. Introduction
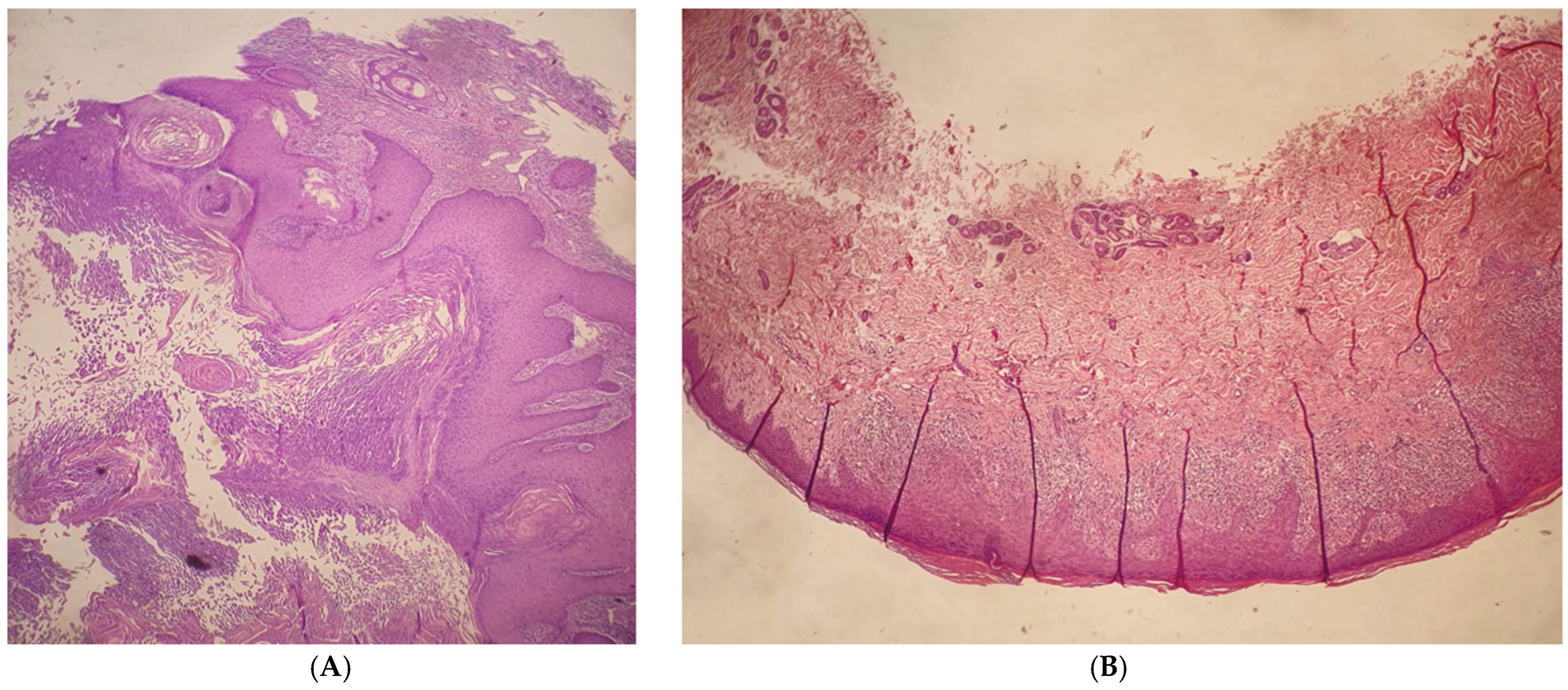
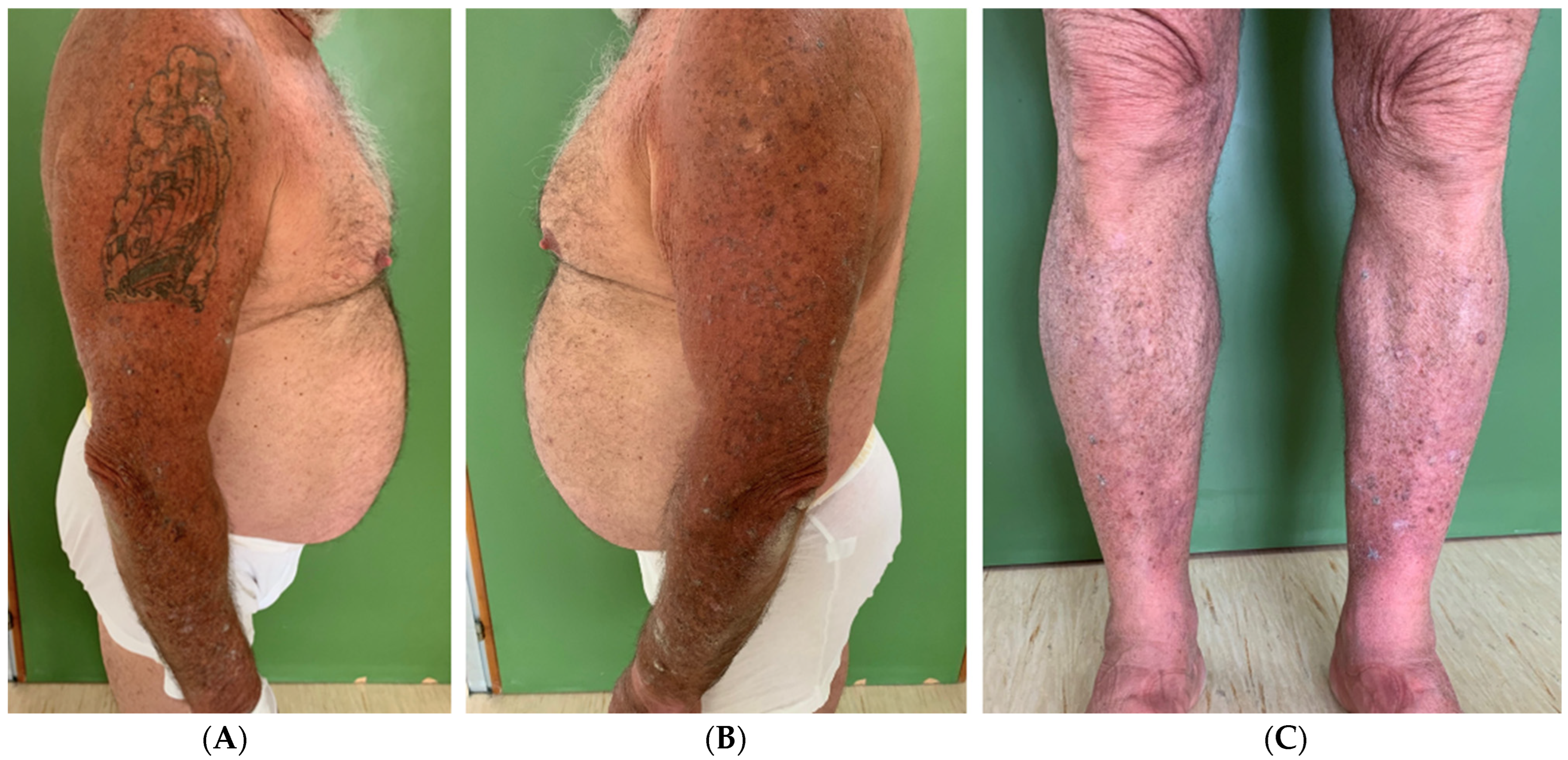
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.europeanbiosafetynetwork.eu/covid-19-vaccination-programme/ (accessed on 4 August 2024).
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Freeman, E.E.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Memon, N.; Haque, A. Multiple Keratoacanthomas Following Moderna Messenger RNA-1273 COVID-19 Vaccination Resolved With 5-Fluorouracil Treatment: Case Report. JMIR Dermatol. 2022, 5, e41739. [Google Scholar] [CrossRef] [PubMed]
- Consigli, J.E.; González, M.E.; Morsino, R.; Guidi, A.; Chappuis, J.M.; Papa, M.; Maldonado, S. Generalized eruptive keratoacanthoma (Grzybowski variant). Br. J. Dermatol. 2000, 142, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.H.; Hale, E.K.; Robins, P. Generalized eruptive keratoacanthomas. Dermatol. Online J. 2003, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Kostopoulou, C.; Toli, O.; Marinos, L.; Papadimitriou, G.; Roussaki Schulze, A.V.; Zafiriou, E. Multiple Keratoacanthoma-like Syndromes: Case Report and Literature Review. Medicina 2024, 60, 371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwartz, R.A. Keratoacanthoma: A clinico-pathologic enigma. Dermatol. Surg. 2004, 30, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Grine, R.C.; Hendrix, J.D.; Greer, K.E. Generalized eruptive keratoacanthoma of Grzybowski: Response to cyclophosphamide. J. Am. Acad. Dermatol. 1997, 36, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Davies, J.H. A widespread, itchy papular eruption. Clin. Exp. Dermatol. 2010, 35, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Barrios, D.M.; Do, M.H.; Phillips, G.S.; Postow, M.A.; Akaike, T.; Nghiem, P.; Lacouture, M.E. Immune checkpoint inhibitors to treat cutaneous malignancies. J. Am. Acad. Dermatol. 2020, 83, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous cell carcinoma of the skin: Epidemiology, classifcation, management, and novel trends. Int. J. Dermatol. 2015, 54, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Liu, C.; Li, W.; Li, W.; Fang, F.; Qian, Q.; Zhang, X. Programmed death-1 ligands 1 and 2 expression in cutaneous squamous cell carcinoma and their relationship with tumour-infltrating dendritic cells. Clin. Exp. Immunol. 2017, 188, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Papadopoulos, K.P.; Johnson, M.L. Phase I study of cemiplimab, a human monoclonal anti-PD-1, in patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC): Longer follow-up efcacy and safety data [abstract no 71P plus poster]. Ann. Oncol. 2018, 29 (Suppl. S10), x25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilaria, P.; Nevena, S.; Ersilia, T.; Federica, T.; Felice, F.; Agnieszka Ewa, D.; Francesco, F.; Concetta, P. Generalized Eruptive Keratoacanthoma (GEKA) after Pfizer mRNABNT162b2 (Comirnaty®) COVID-19 Vaccination Successfully Treated with Cemiplimab. Viruses 2024, 16, 1260. https://doi.org/10.3390/v16081260
Ilaria P, Nevena S, Ersilia T, Federica T, Felice F, Agnieszka Ewa D, Francesco F, Concetta P. Generalized Eruptive Keratoacanthoma (GEKA) after Pfizer mRNABNT162b2 (Comirnaty®) COVID-19 Vaccination Successfully Treated with Cemiplimab. Viruses. 2024; 16(8):1260. https://doi.org/10.3390/v16081260
Chicago/Turabian StyleIlaria, Proietti, Skroza Nevena, Tolino Ersilia, Trovato Federica, Forte Felice, Dybala Agnieszka Ewa, Fiorentino Francesco, and Potenza Concetta. 2024. "Generalized Eruptive Keratoacanthoma (GEKA) after Pfizer mRNABNT162b2 (Comirnaty®) COVID-19 Vaccination Successfully Treated with Cemiplimab" Viruses 16, no. 8: 1260. https://doi.org/10.3390/v16081260




