Parainfluenza Virus 5 V Protein Blocks Interferon Gamma-Mediated Upregulation of NK Cell Inhibitory Ligands and Improves NK Cell Killing of Neuroblastoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Viruses, and Infections
2.2. Construction of Lentiviral V Protein and Transduction of Stable Cell Lines
2.3. PM21-NK Cell Preparation and Cryopreservation
2.4. Cytotoxicity and Cell Killing Assays
2.5. Flow Cytometry
2.6. Western Blotting
2.7. Statistics
3. Results
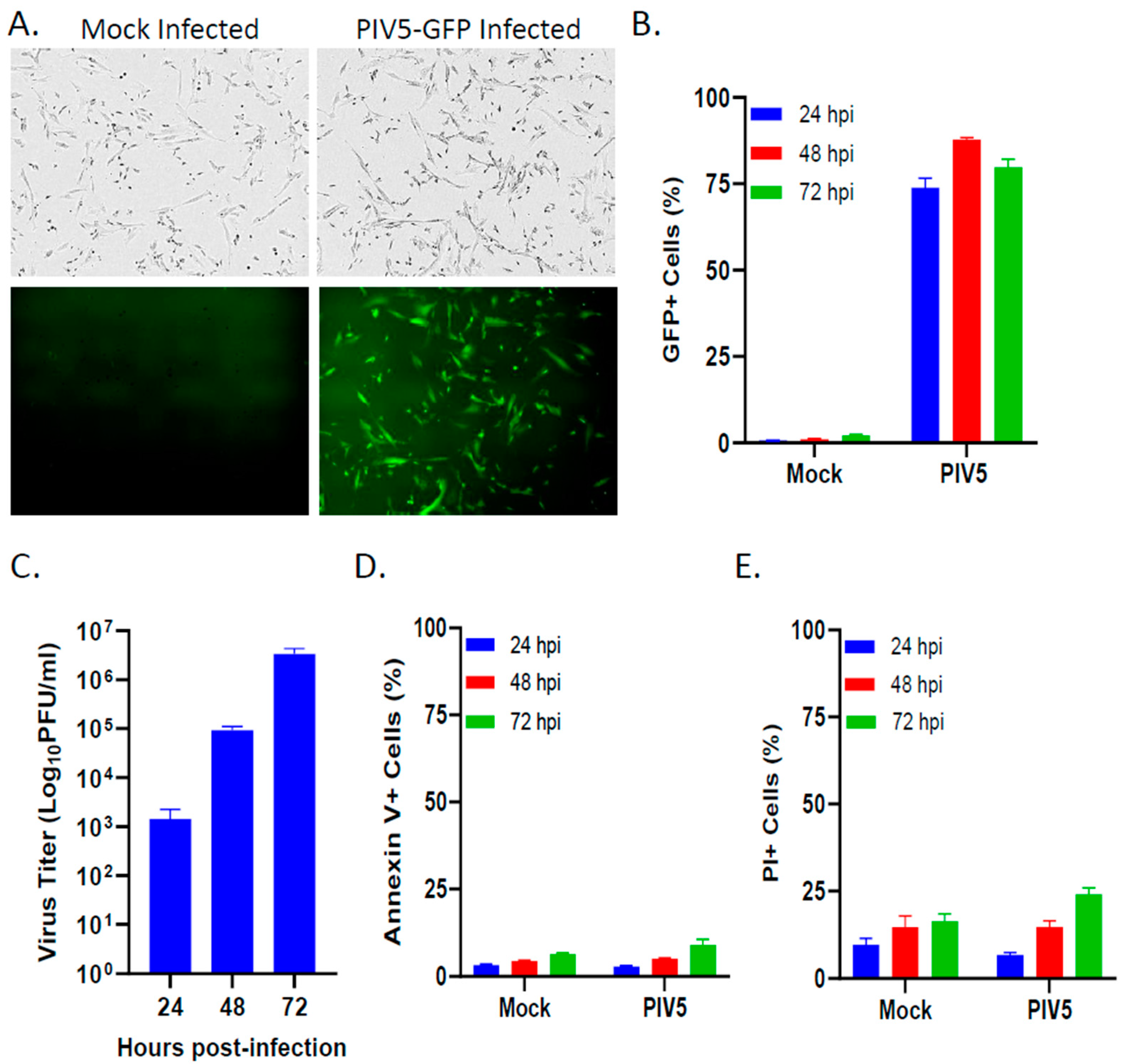
3.1. PIV5 Established a Productive Infection of Pediatric SK-N-SH Neuroblastoma Cells with Minimal Cytopathic Effects
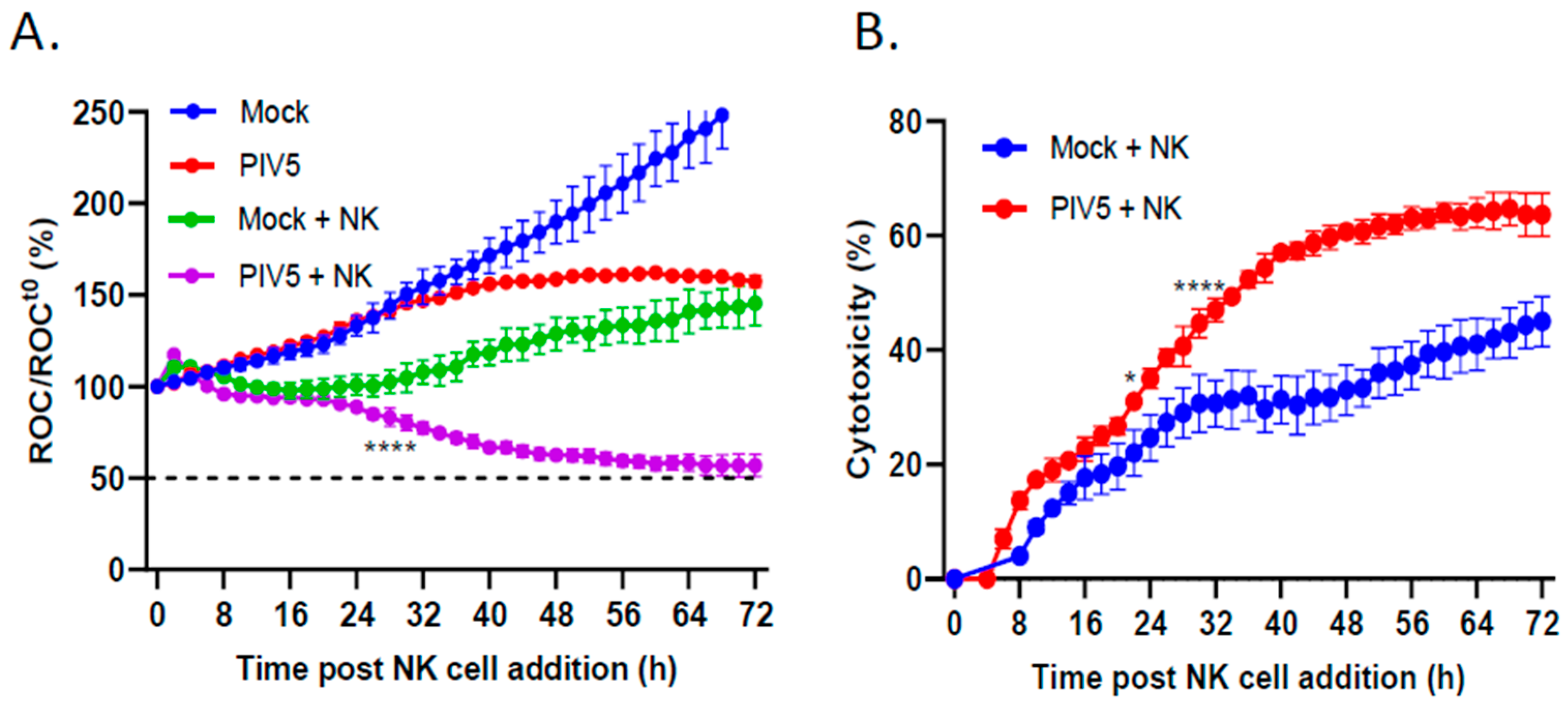
3.2. PIV5 Infection Enhanced PM21-NK Cell-Mediated Killing of Pediatric Neuroblastoma Cells
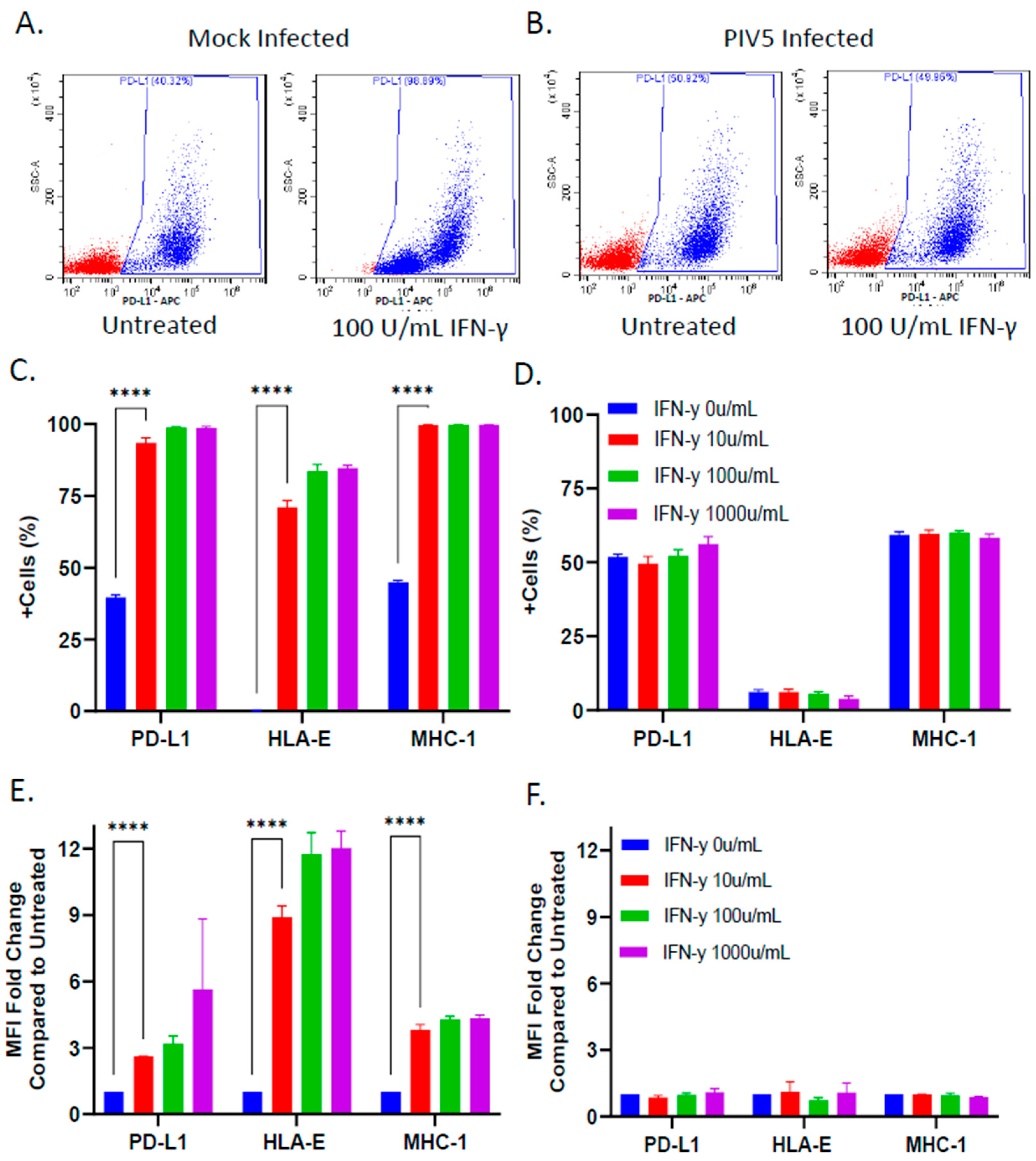
3.3. PIV5 Infection Inhibited IFN-γ Mediated Upregulation of NK Cell Inhibitory Ligands on the Surface of SK-N-SH Tumor Cells
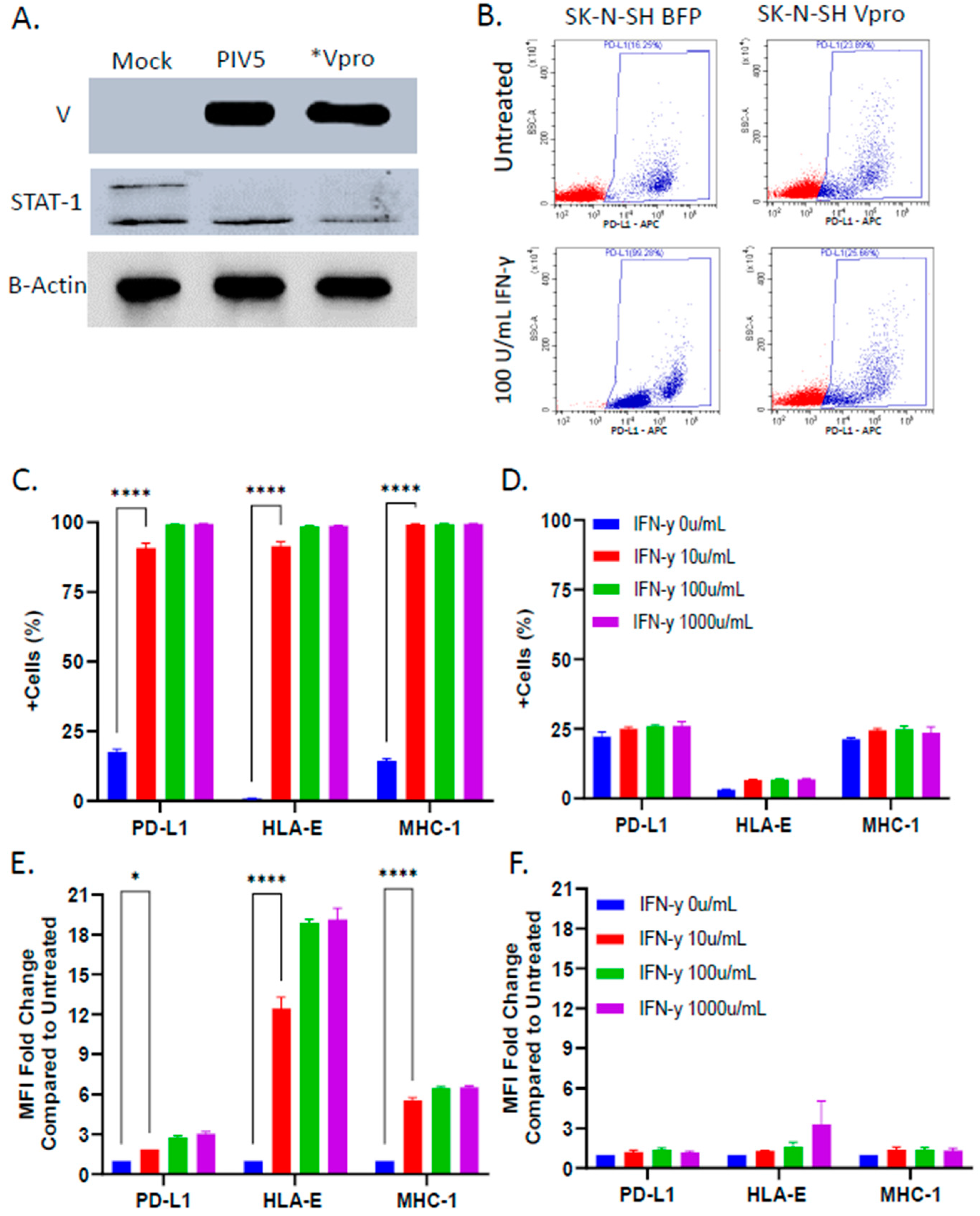
3.4. PIV5 V Protein Alone Was Sufficient to Block IFN-γ-Mediated Upregulation of NK Cell Inhibitory Ligands
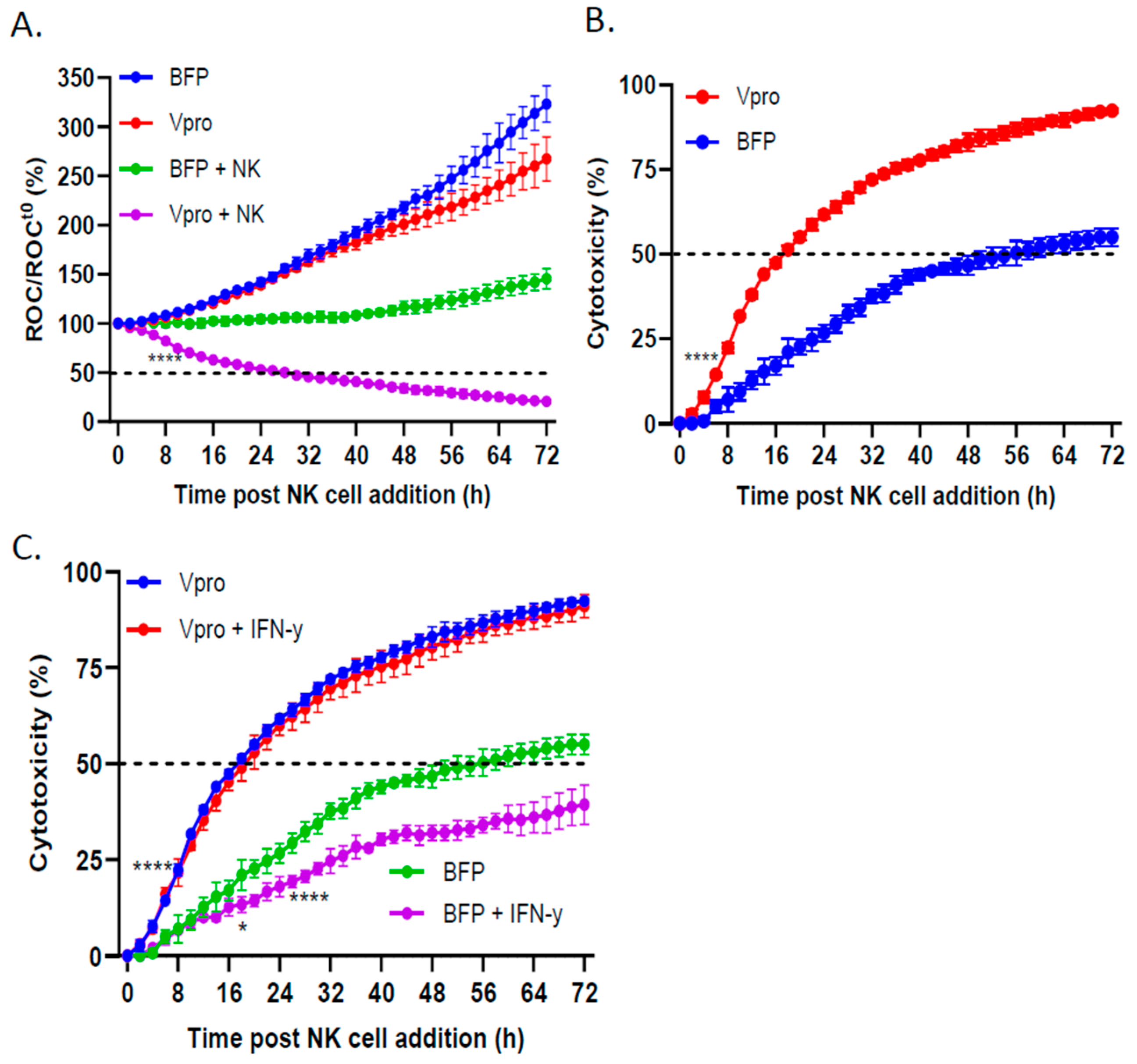
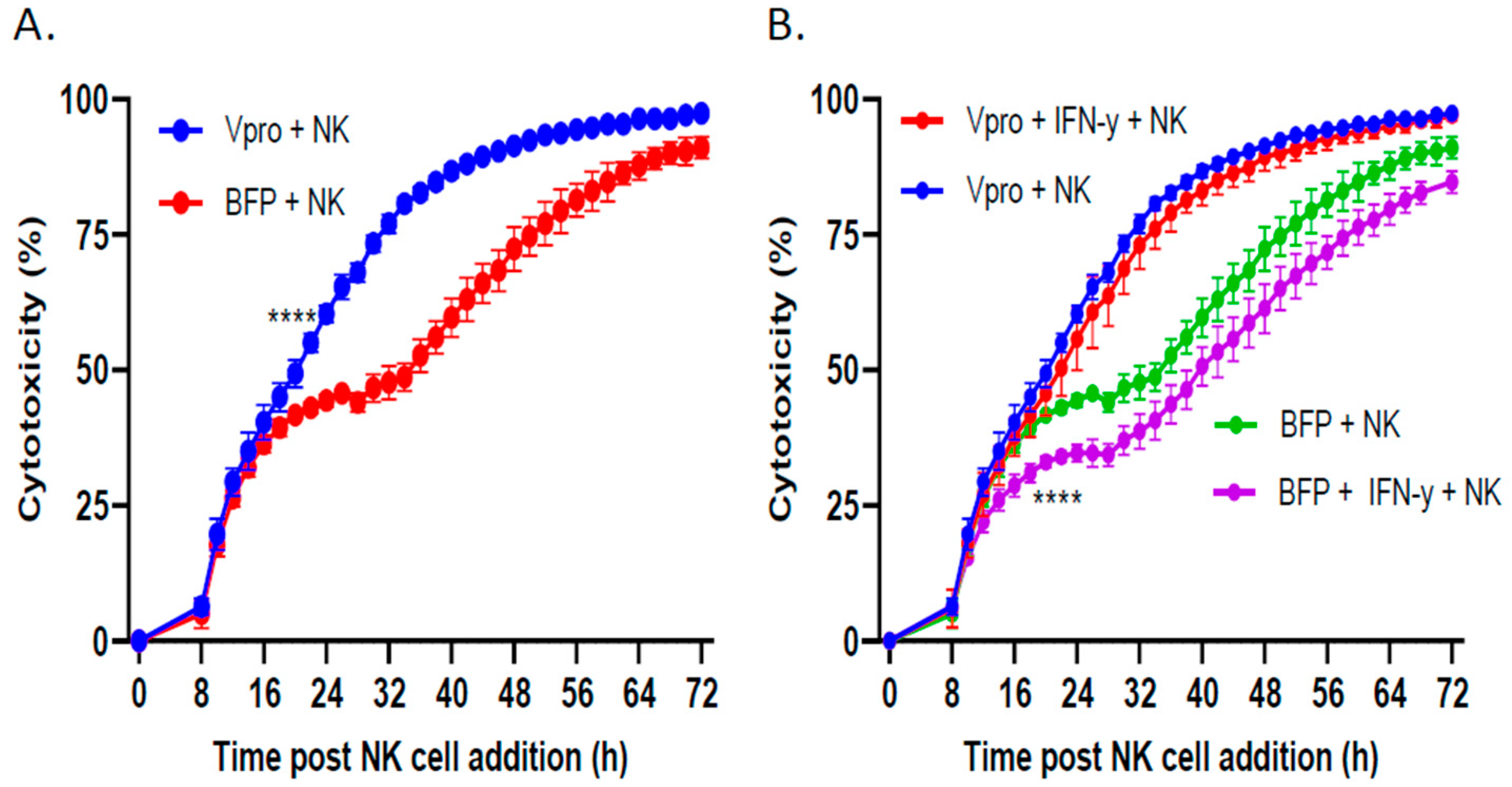
3.5. PIV5 V Protein Alone Was Sufficient to Enhance NK Cell-Mediated Killing and Overcome IFN-γ-Induced Reduction in NK Killing
3.6. Lentiviral Delivery of V Protein Enhanced NK Cell-Mediated Killing of Tumor Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, J.; Gao, F.; Yan, M.; Zhao, S.; Yan, Z.; Shi, B.; Liu, Y. Natural killer cells: A promising immunotherapy for cancer. J. Transl. Med. 2022, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Guo, F.; Wang, Y.; Cui, J. NK Cell Therapy: A Rising Star in Cancer Treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef] [PubMed]
- Shaver, K.A.; Croom-Perez, T.J.; Copik, A.J. Natural Killer Cells: The Linchpin for Successful Cancer Immunotherapy. Front. Immunol. 2021, 12, 679117. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Oyer, J.L.; Igarashi, R.Y.; Kulikowski, A.R.; Colosimo, D.A.; Solh, M.M.; Zakari, A.; Khaled, Y.A.; Altomare, D.A.; Copik, A.J. Generation of highly cytotoxic natural killer cells for treatment of acute myelogenous leukemia using a feeder-free, particle-based approach. Biol. Blood Marrow Transplant. 2015, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Oyer, J.L.; Pandey, V.; Igarashi, R.Y.; Somanchi, S.S.; Zakari, A.; Solh, M.; Lee, D.A.; Altomare, D.A.; Copik, A.J. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: Clinical implications for cancer treatment. Cytotherapy 2016, 18, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Oyer, J.L.; Croom-Perez, T.J.; Dieffenthaller, T.A.; Robles-Carillo, L.D.; Gitto, S.B.; Altomare, D.A.; Copik, A.J. Cryopreserved PM21-Particle-Expanded Natural Killer Cells Maintain Cytotoxicity and Effector Functions In Vitro and In Vivo. Front. Immunol. 2022, 13, 861681. [Google Scholar] [CrossRef] [PubMed]
- Oyer, J.L.; Gitto, S.B.; Altomare, D.A.; Copik, A.J. PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology 2018, 7, e1509819. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef] [PubMed]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e1713. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Cui, J. Burgeoning Exploration of the Role of Natural Killer Cells in Anti-PD-1/PD-L1 Therapy. Front. Immunol. 2022, 13, 886931. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, H.E.; Thielens, A.; Marabelle, A.; Sagiv-Barfi, I.; Sola, C.; Chanuc, F.; Fuseri, N.; Bonnafous, C.; Czerwinski, D.; Rajapaksa, A.; et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014, 123, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Wang, W.; Arakawa, T.; Ohtake, S. Developments and Challenges for mAb-Based Therapeutics. Antibodies 2013, 2, 452–500. [Google Scholar] [CrossRef]
- Chamoto, K.; Hatae, R.; Honjo, T. Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int. J. Clin. Oncol. 2020, 25, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.D.; He, W.; Kotenko, S.; Pestka, S. Modulation of the activation of Stat1 by the interferon-gamma receptor complex. Cell Res. 2006, 16, 113–123. [Google Scholar] [CrossRef]
- Zheng, H.; Guan, X.; Meng, X.; Tong, Y.; Wang, Y.; Xie, S.; Guo, L.; Lu, R. IFN-gamma in ovarian tumor microenvironment upregulates HLA-E expression and predicts a poor prognosis. J. Ovarian Res. 2023, 16, 229. [Google Scholar] [CrossRef]
- Aquino-Lopez, A.; Senyukov, V.V.; Vlasic, Z.; Kleinerman, E.S.; Lee, D.A. Interferon Gamma Induces Changes in Natural Killer (NK) Cell Ligand Expression and Alters NK Cell-Mediated Lysis of Pediatric Cancer Cell Lines. Front. Immunol. 2017, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, R.; Miest, T.; Shashkova, E.V.; Barry, M.A. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat. Rev. Microbiol. 2008, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Shobana, R.; Samal, S.K.; Elankumaran, S. Prostate-specific antigen-retargeted recombinant newcastle disease virus for prostate cancer virotherapy. J. Virol. 2013, 87, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Solaymani-Mohammadi, F.; Miri, S.M.; Ghaemi, A. Oncolytic paramyxoviruses-induced autophagy; a prudent weapon for cancer therapy. J. Biomed. Sci. 2019, 26, 48. [Google Scholar]
- Ammayappan, A.; Russell, S.J.; Federspiel, M.J. Recombinant mumps virus as a cancer therapeutic agent. Mol. Ther. Oncolytics 2016, 3, 16019. [Google Scholar] [CrossRef] [PubMed]
- Varudkar, N.; Oyer, J.L.; Copik, A.; Parks, G.D. Oncolytic parainfluenza virus combines with NK cells to mediate killing of infected and non-infected lung cancer cells within 3D spheroids: Role of type I and type III interferon signaling. J. Immunother. Cancer 2021, 9, e002373. [Google Scholar] [CrossRef] [PubMed]
- Didcock, L.; Young, D.F.; Goodbourn, S.; Randall, R.E. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 1999, 73, 9928–9933. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.Y.; Lamb, R.A. The paramyxovirus simian virus 5 V protein slows progression of the cell cycle. J. Virol. 2000, 74, 9152–9166. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef]
- Wansley, E.K.; Parks, G.D. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 2002, 76, 10109–10121. [Google Scholar] [CrossRef]
- Vogler, M.; Shanmugalingam, S.; Sarchen, V.; Reindl, L.M.; Greze, V.; Buchinger, L.; Kuhn, M.; Ullrich, E. Unleashing the power of NK cells in anticancer immunotherapy. J. Mol. Med. 2022, 100, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Pal, P.; Lal, G. Key Activating and Inhibitory Ligands Involved in the Mobilization of Natural Killer Cells for Cancer Immunotherapies. Immunotargets Ther. 2021, 10, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Della Chiesa, M.; Carlomagno, S.; Quatrini, L.; Munari, E.; Vacca, P.; Tumino, N.; Mariotti, F.R.; Mingari, M.C.; Pende, D.; et al. Inhibitory Receptors and Checkpoints in Human NK Cells, Implications for the Immunotherapy of Cancer. Front. Immunol. 2020, 11, 2156. [Google Scholar] [CrossRef]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; Lopez-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef]
- Thielens, A.; Vivier, E.; Romagne, F. NK cell MHC class I specific receptors (KIR): From biology to clinical intervention. Curr. Opin. Immunol. 2012, 24, 239–245. [Google Scholar] [CrossRef]
- Nguyen, S.; Beziat, V.; Dhedin, N.; Kuentz, M.; Vernant, J.P.; Debre, P.; Vieillard, V. HLA-E upregulation on IFN-gamma-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant. 2009, 43, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Teng, W.; Tian, Y.; Li, S.; Xia, N.; Huang, C. Immune landscape and response to oncolytic virus-based immunotherapy. Front. Med. 2024, 18, 411–429. [Google Scholar] [CrossRef]
- Marotel, M.; Hasim, M.S.; Hagerman, A.; Ardolino, M. The two-faces of NK cells in oncolytic virotherapy. Cytokine Growth Factor. Rev. 2020, 56, 59–68. [Google Scholar] [CrossRef]
- He, B.; Paterson, R.G.; Stock, N.; Durbin, J.E.; Durbin, R.K.; Goodbourn, S.; Randall, R.E.; Lamb, R.A. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: The multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 2002, 303, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P.J.; Wansley, E.K.; Young, V.A.; Alexander-Miller, M.A.; Parks, G.D. Exchange of P/V genes between two non-cytopathic simian virus 5 variants results in a recombinant virus that kills cells through death pathways that are sensitive to caspase inhibitors. J. Gen. Virol. 2006, 87, 3643–3648. [Google Scholar] [CrossRef]
- Andrejeva, J.; Childs, K.S.; Young, D.F.; Carlos, T.S.; Stock, N.; Goodbourn, S.; Randall, R.E. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 2004, 101, 17264–17269. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lin, G.Y.; Durbin, J.E.; Durbin, R.K.; Lamb, R.A. The SH integral membrane protein of the paramyxovirus simian virus 5 is required to block apoptosis in MDBK cells. J. Virol. 2001, 75, 4068–4079. [Google Scholar] [CrossRef]
- Sun, M.; Rothermel, T.A.; Shuman, L.; Aligo, J.A.; Xu, S.; Lin, Y.; Lamb, R.A.; He, B. Conserved cysteine-rich domain of paramyxovirus simian virus 5 V protein plays an important role in blocking apoptosis. J. Virol. 2004, 78, 5068–5078. [Google Scholar] [CrossRef]
- Mandelboim, O.; Lieberman, N.; Lev, M.; Paul, L.; Arnon, T.I.; Bushkin, Y.; Davis, D.M.; Strominger, J.L.; Yewdell, J.W.; Porgador, A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 2001, 409, 1055–1060. [Google Scholar] [CrossRef]
- Precious, B.L.; Carlos, T.S.; Goodbourn, S.; Randall, R.E. Catalytic turnover of STAT1 allows PIV5 to dismantle the interferon-induced anti-viral state of cells. Virology 2007, 368, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.T.; Tofani, L.B.; Araujo, V.H.S.; Di Filippo, L.D.; Duarte, J.L.; Marchetti, J.M.; Chorilli, M. Gene Therapy Based on Lipid Nanoparticles as Non-viral Vectors for Glioma Treatment. Curr. Gene Ther. 2021, 21, 452–463. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, S.; Hu, Y.; Zhao, X.; Lee, R.J. Application of lipid-based nanoparticles in cancer immunotherapy. Front. Immunol. 2022, 13, 967505. [Google Scholar] [CrossRef]
- Hallermalm, K.; Seki, K.; Wei, C.; Castelli, C.; Rivoltini, L.; Kiessling, R.; Levitskaya, J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood 2001, 98, 1108–1115. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-alpha up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, X.; Meng, W.; Guo, W.; Duan, C.; Cao, J.; Kang, L.; Guo, N.; Lin, Q.; Lv, P.; et al. TNF-alpha-dependent lung inflammation upregulates PD-L1 in monocyte-derived macrophages to contribute to lung tumorigenesis. FASEB J. 2022, 36, e22595. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Yamaguchi, M.; Zhou, M.; Nishio, M.; Itoh, M.; Gotoh, B. Human parainfluenza virus type 2 V protein inhibits TRAF6-mediated ubiquitination of IRF7 to prevent TLR7- and TLR9-dependent interferon induction. J. Virol. 2013, 87, 7966–7976. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.J.; Parisien, J.P.; Horvath, C.M. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 2002, 76, 11476–11483. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiffer, E.M.; Oyer, J.L.; Copik, A.J.; Parks, G.D. Parainfluenza Virus 5 V Protein Blocks Interferon Gamma-Mediated Upregulation of NK Cell Inhibitory Ligands and Improves NK Cell Killing of Neuroblastoma Cells. Viruses 2024, 16, 1270. https://doi.org/10.3390/v16081270
Shiffer EM, Oyer JL, Copik AJ, Parks GD. Parainfluenza Virus 5 V Protein Blocks Interferon Gamma-Mediated Upregulation of NK Cell Inhibitory Ligands and Improves NK Cell Killing of Neuroblastoma Cells. Viruses. 2024; 16(8):1270. https://doi.org/10.3390/v16081270
Chicago/Turabian StyleShiffer, Elisabeth M., Jeremiah L. Oyer, Alicja J. Copik, and Griffith D. Parks. 2024. "Parainfluenza Virus 5 V Protein Blocks Interferon Gamma-Mediated Upregulation of NK Cell Inhibitory Ligands and Improves NK Cell Killing of Neuroblastoma Cells" Viruses 16, no. 8: 1270. https://doi.org/10.3390/v16081270




