Chronic, Low-Dose Methamphetamine Reveals Sexual Dimorphism of Memory Performance, Histopathology, and Gene Expression Affected by HIV-1 Tat Protein in a Transgenic Model of NeuroHIV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Drug Treatment
2.2. Behavioral Testing
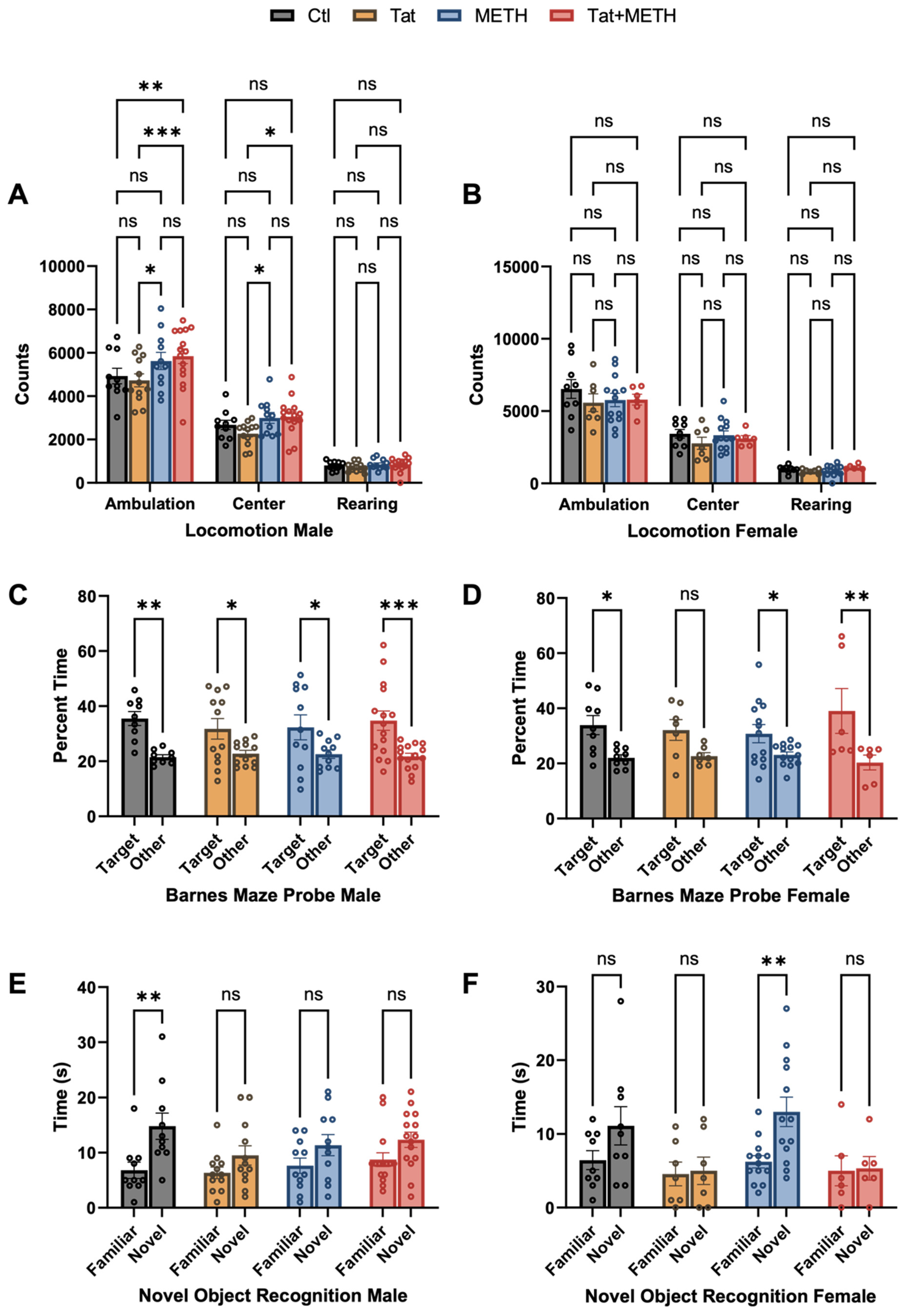
2.2.1. Locomotor Activity Test
2.2.2. Optomotor Vision Test
2.2.3. Light/Dark Transfer Test
2.2.4. Barnes Maze Test
2.2.5. Novel Object Recognition Test
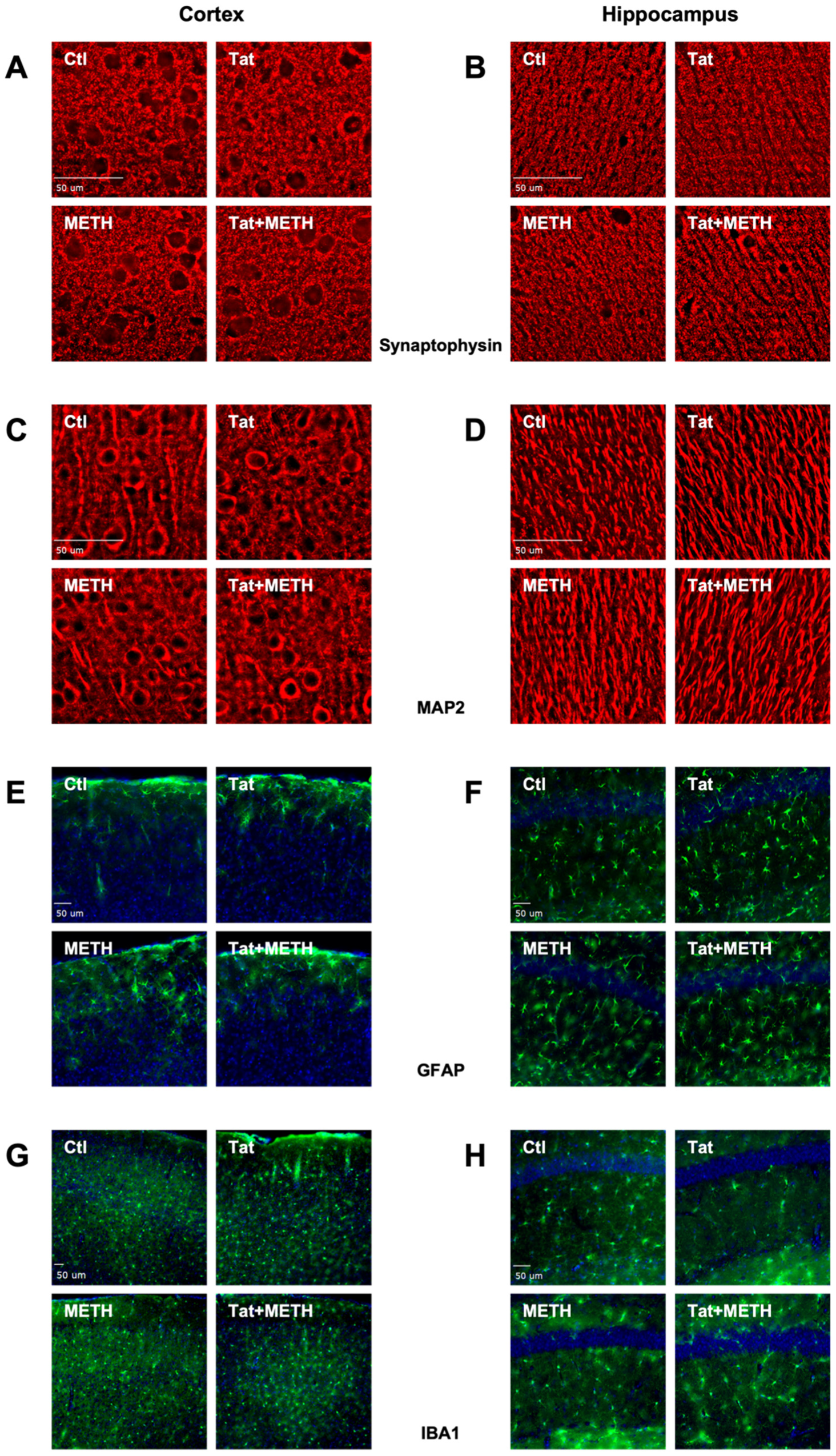
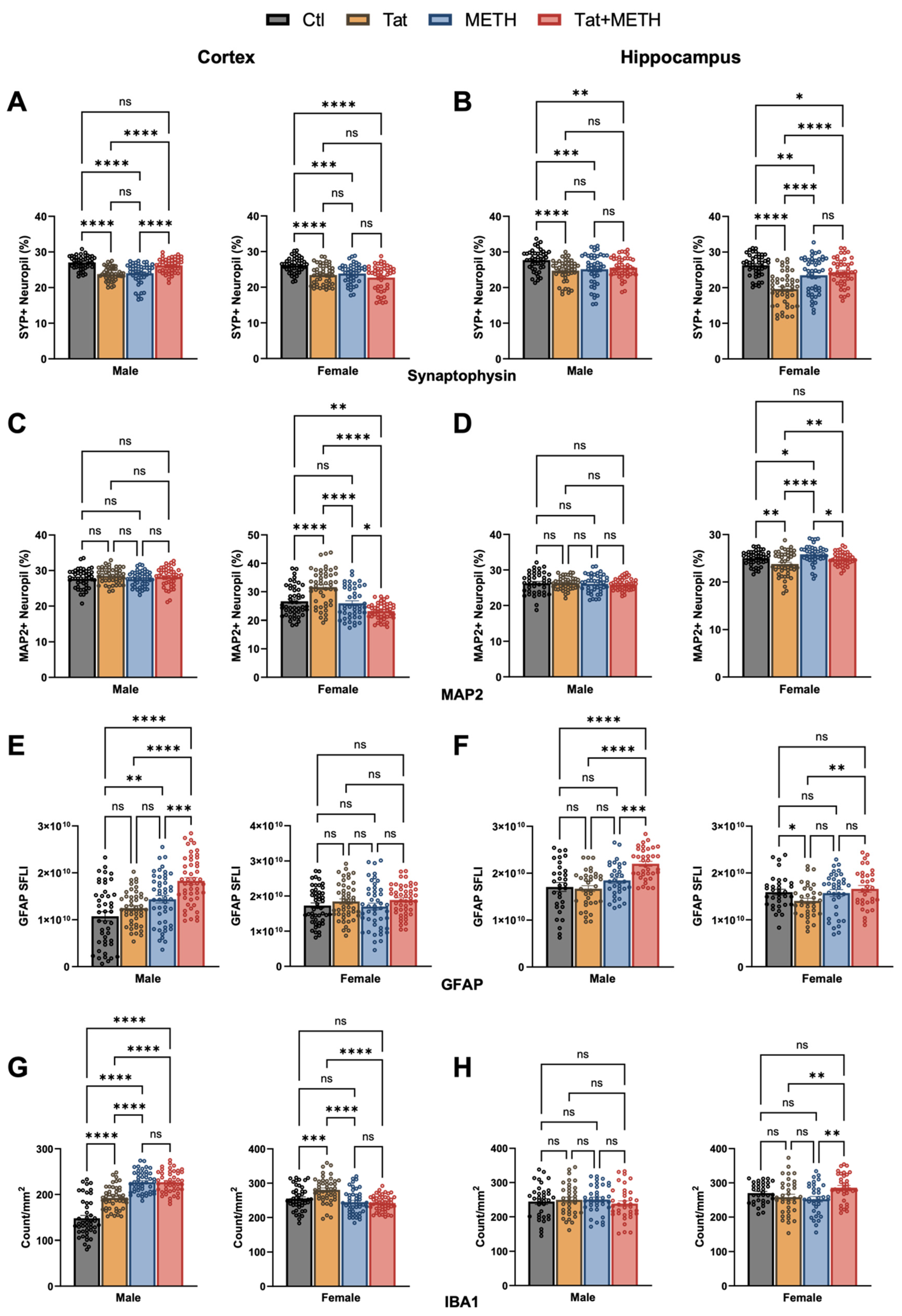
2.3. Immunohistology
2.4. Imaging and Analysis
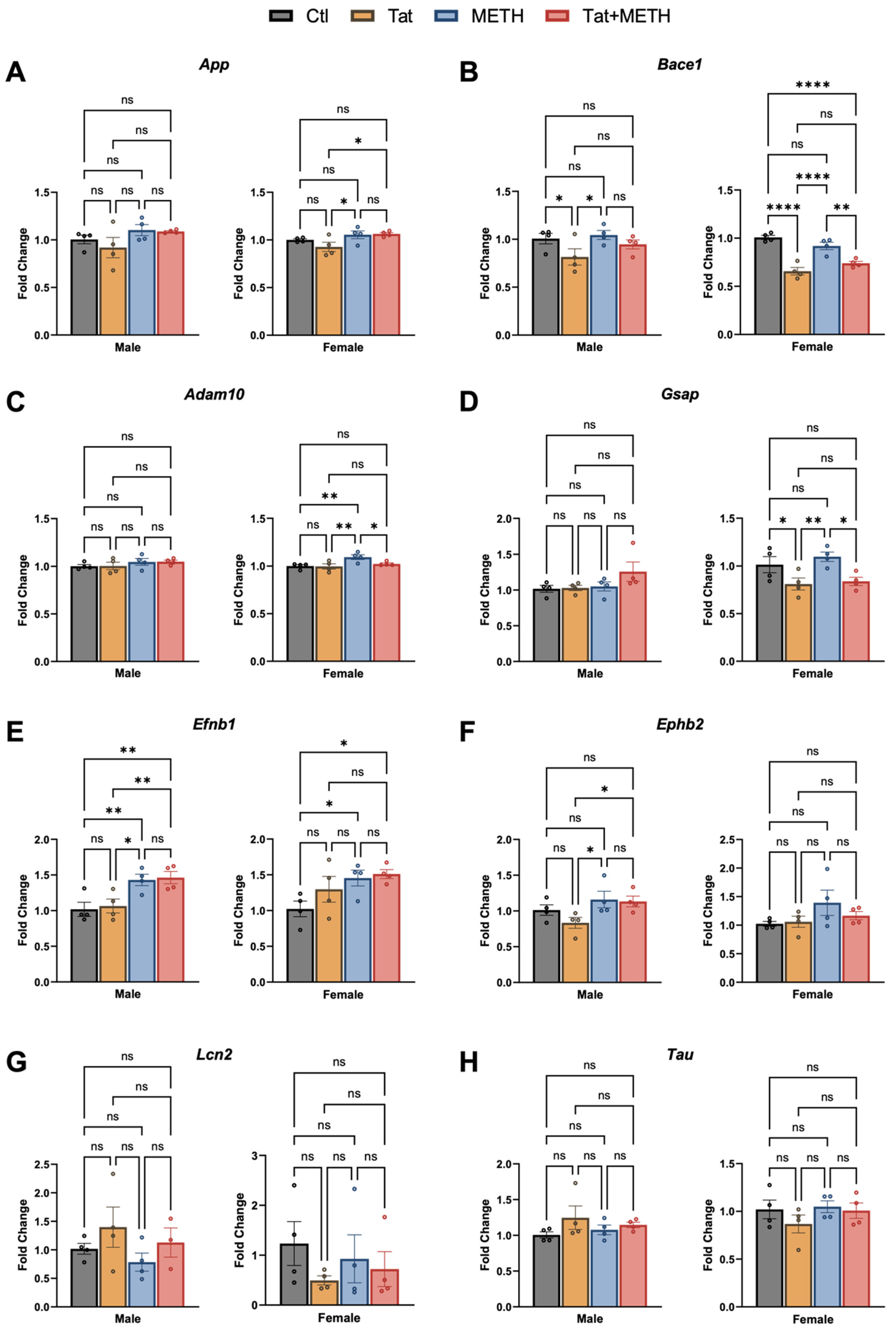
2.5. Isolation of mRNA and Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. RNA-Seq and Differential Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Behavioral Changes Due to Tat Expression and METH Treatment
3.2. Neuronal Injury Associated with Tat Expression and METH Treatment
3.3. Activation of Astrocytes and Microglia Due to Tat Expression and METH Treatment
3.4. Modulation of RNA Expression Associated with Tat and METH Exposure
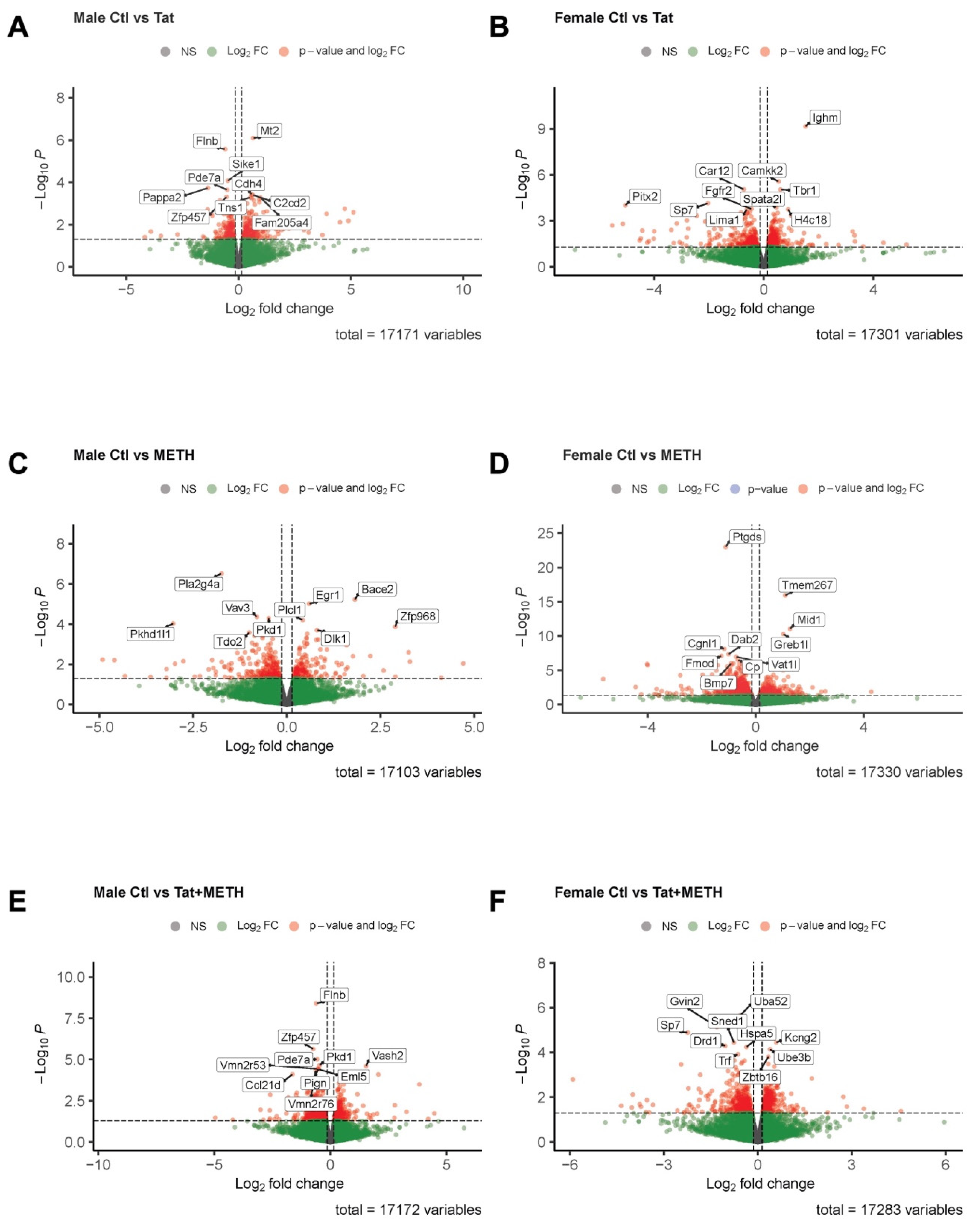
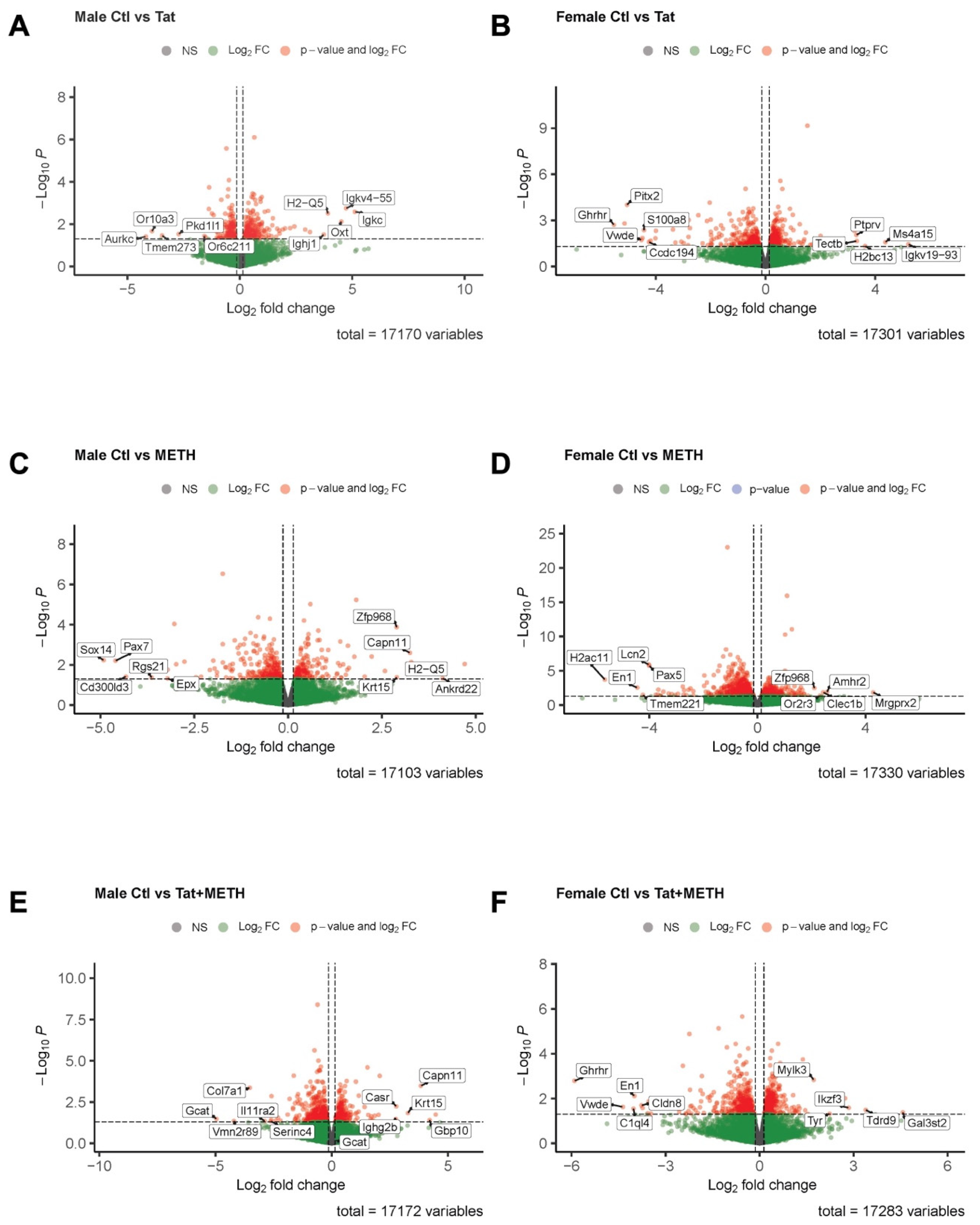
3.5. Analysis of RNA-Sequencing Data Reveals That Gene Modulation Associated with Tat Expression and METH Treatment Is Sex-Dependent
3.6. Analysis of RNA-Sequencing Data Using IPA Confirmed Sex-Dependent and Sex-Independent Responses
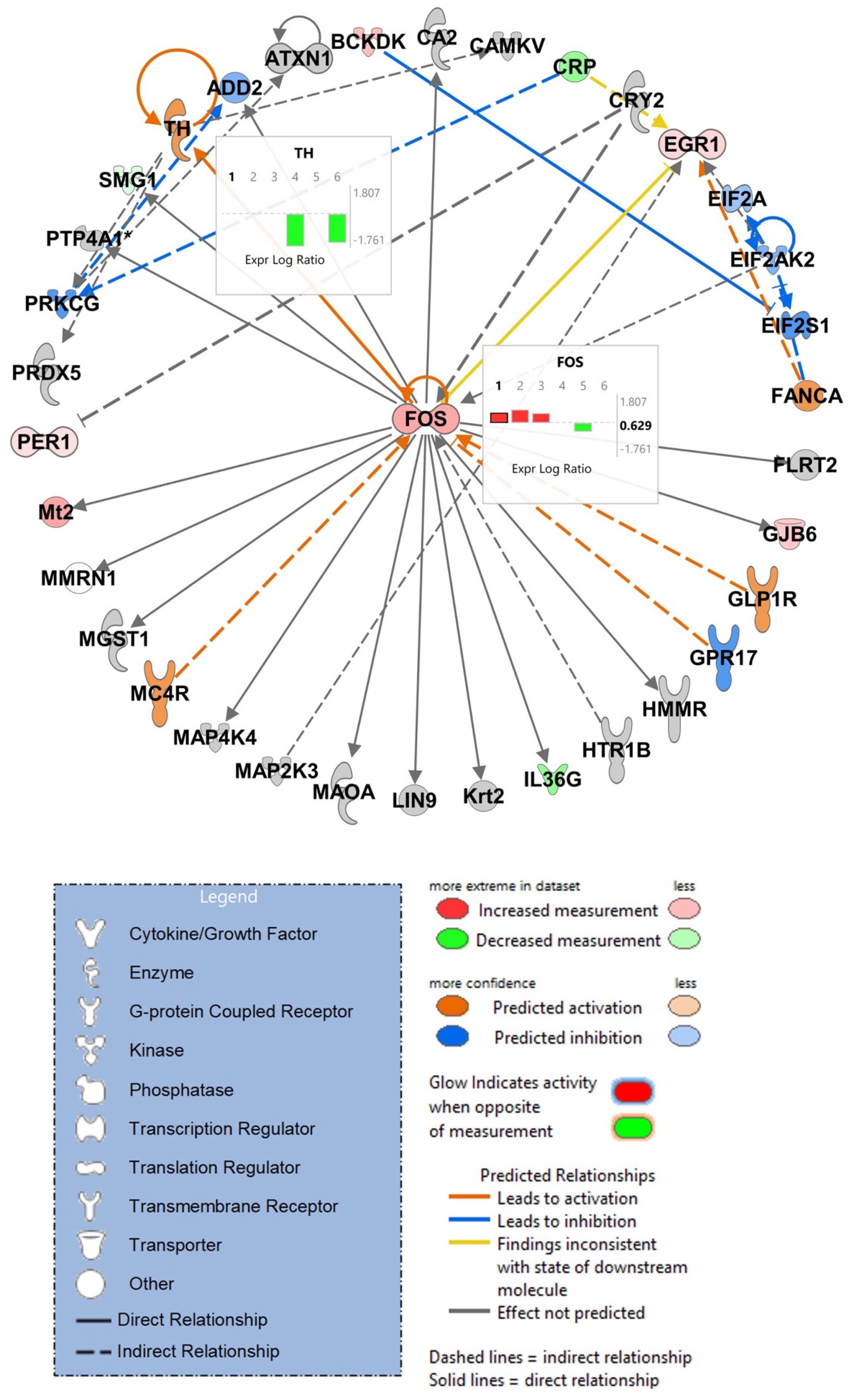
3.7. IPA Analysis of RNA-Sequencing Data Discloses Top Scoring Networks, Highlighting the Role of Tyrosine Hydroxylase and Fos
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cART | Combined antiretroviral therapy |
| PWH | People with HIV |
| NCI | Neurocognitive impairment |
| HAND | HIV-associated neurocognitive disorders |
| neuroHIV | Neuropathological features of HIV infection of the brain |
| HIVE | HIV encephalitis |
| METH | Methamphetamine |
| ADHD | Attention deficit hyperactivity disorder |
| Tat | HIV-1 Transactivator of transcription |
| BM | Barnes maze |
| NOR | Novel object recognition |
| APP | Amyloid precursor protein |
| TBI | Traumatic brain injury |
| AD | Alzheimer’s disease |
| Dox | Doxycycline |
| GFAP | Glial fibrillary acidic protein |
| Tet | Tetracycline |
| IACUC | Institutional animal care and use committees |
| TSRI | The Scripps Research Institute |
| LDT | Light/dark transfer |
| LM | Locomotor |
| OM | Optomotor |
| IF | Immunofluorescence |
| MAP2 | Microtubule-associated protein 2 |
| Iba1 | Ionized calcium-binding adaptor molecule 1 |
| LIII | Layer 3 |
| CA1 | Cornu ammonis 1 |
| SYP | Synaptophysin |
| IPA | Ingenuity pathway analysis |
| Th | Tyrosine hydroxylase |
References
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; LeBlanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P.; et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.D.; Fedor, H.; Wesselingh, S.L.; McArthur, J.C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: Correlations with dementia. Ann. Neurol. 1995, 38, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Wesselingh, S.L.; Takahashi, K.; Glass, J.D.; McArthur, J.C.; Griffin, J.W.; Griffin, D.E. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J. Neuroimmunol. 1997, 74, 1–8. [Google Scholar] [CrossRef]
- Masliah, E.; Heaton, R.K.; Marcotte, T.D.; Ellis, R.J.; Wiley, C.A.; Mallory, M.; Achim, C.L.; McCutchan, J.A.; Nelson, J.A.; Atkinson, J.H.; et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 1997, 42, 963–972. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Morris, S.R.; Kent, C.K.; Stansell, J.; Klausner, J.D. Methamphetamine use and sexual activity among HIV-infected patients in care—San Francisco, 2004. AIDS Patient Care STDS 2006, 20, 502–510. [Google Scholar] [CrossRef]
- Quinton, M.S.; Yamamoto, B.K. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006, 8, E337–E347. [Google Scholar] [CrossRef]
- Ellis, R.J.; Childers, M.E.; Cherner, M.; Lazzaretto, D.; Letendre, S.; Grant, I.; HIV Neurobehavioral Research Center Group. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J. Infect. Dis. 2003, 188, 1820–1826. [Google Scholar] [CrossRef]
- Langford, D.; Adame, A.; Grigorian, A.; Grant, I.; McCutchan, J.A.; Ellis, R.J.; Marcotte, T.D.; Masliah, E.; HIV Neurobehavioral Research Center Group. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J. Acquir. Immune Defic. Syndr. 2003, 34, 467–474. [Google Scholar] [CrossRef]
- Moratalla, R.; Khairnar, A.; Simola, N.; Granado, N.; Garcia-Montes, J.R.; Porceddu, P.F.; Tizabi, Y.; Costa, G.; Morelli, M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog. Neurobiol. 2017, 155, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Vincent, B. The multi-faceted impact of methamphetamine on Alzheimer’s disease: From a triggering role to a possible therapeutic use. Ageing Res. Rev. 2020, 60, 101062. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.F.; Kothiwal, A.S.; Rova, A.R.; Brooks, D.M.; Poulsen, D.J. Treatment with low-dose methamphetamine improves behavioral and cognitive function after severe traumatic brain injury. J. Trauma Acute Care Surg. 2012, 73, S165–S172. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; Richardson, K.; Dacey, J.; Glynn, S.; Domier, C.P.; Rawson, R.A.; Ling, W. A comparison of patterns of methamphetamine and cocaine use. J. Addict. Dis. 2002, 21, 35–44. [Google Scholar] [CrossRef]
- Ellis, R.; Langford, D.; Masliah, E. HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat. Rev. Neurosci. 2007, 8, 33–44. [Google Scholar] [CrossRef]
- Kaul, M.; Garden, G.A.; Lipton, S.A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001, 410, 988–994. [Google Scholar] [CrossRef]
- Ellis, R.J.; Marquine, M.J.; Kaul, M.; Fields, J.A.; Schlachetzki, J.C.M. Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management. Nat. Rev. Neurol. 2023, 19, 668–687. [Google Scholar] [CrossRef]
- Toggas, S.M.; Masliah, E.; Rockenstein, E.M.; Rall, G.F.; Abraham, C.R.; Mucke, L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 1994, 367, 188–193. [Google Scholar] [CrossRef]
- Hoefer, M.M.; Sanchez, A.B.; Maung, R.; de Rozieres, C.M.; Catalan, I.C.; Dowling, C.C.; Thaney, V.E.; Pina-Crespo, J.; Zhang, D.; Roberts, A.J.; et al. Combination of methamphetamine and HIV-1 gp120 causes distinct long-term alterations of behavior, gene expression, and injury in the central nervous system. Exp. Neurol. 2015, 263, 221–234. [Google Scholar] [CrossRef]
- Kim, B.O.; Liu, Y.; Ruan, Y.; Xu, Z.C.; Schantz, L.; He, J.J. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003, 162, 1693–1707. [Google Scholar] [CrossRef]
- Carey, A.N.; Sypek, E.I.; Singh, H.D.; Kaufman, M.J.; McLaughlin, J.P. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav. Brain Res. 2012, 229, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Russo, G.; Passiatore, G.; Del Valle, L.; Giordano, A.; Khalili, K.; Gualco, E.; Peruzzi, F. Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. J. Cell. Physiol. 2008, 216, 764–770. [Google Scholar] [CrossRef]
- Rozzi, S.J.; Avdoshina, V.; Fields, J.A.; Mocchetti, I. Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Kistner, A.; Gossen, M.; Zimmermann, F.; Jerecic, J.; Ullmer, C.; Lübbert, H.; Bujard, H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA 1996, 93, 10933–10938. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.J.; Johnson, T.P.; Smith, B.R.; Reoma, L.B.; Santamaria, U.A.; Bachani, M.; Demarino, C.; Barclay, R.A.; Snow, J.; Sacktor, N.; et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 2019, 33 (Suppl. S2), S145–S157. [Google Scholar] [CrossRef]
- Ojeda-Juarez, D.; Shah, R.; Fields, J.A.; Harahap-Carrillo, I.S.; Koury, J.; Maung, R.; Gelman, B.B.; Baaten, B.J.; Roberts, A.J.; Kaul, M. Lipocalin-2 mediates HIV-1 induced neuronal injury and behavioral deficits by overriding CCR5-dependent protection. Brain Behav. Immun. 2020, 89, 184–199. [Google Scholar] [CrossRef]
- Singh, H.; Koury, J.; Maung, R.; Roberts, A.J.; Kaul, M. Interferon-beta deficiency alters brain response to chronic HIV-1 envelope protein exposure in a transgenic model of NeuroHIV. Brain Behav. Immun. 2024, 118, 1–21. [Google Scholar] [CrossRef]
- Yuan, N.Y.; Medders, K.E.; Sanchez, A.B.; Shah, R.; de Rozieres, C.M.; Ojeda-Juarez, D.; Maung, R.; Williams, R.; Gelman, B.B.; Baaten, B.J.; et al. A critical role for Macrophage-derived Cysteinyl-Leukotrienes in HIV-1 induced neuronal injury. Brain Behav. Immun. 2024, 118, 149–166. [Google Scholar] [CrossRef]
- Maung, R.; Hoefer, M.M.; Sanchez, A.B.; Sejbuk, N.E.; Medders, K.E.; Desai, M.K.; Catalan, I.C.; Dowling, C.C.; de Rozieres, C.M.; Garden, G.A.; et al. CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J. Immunol. 2014, 193, 1895–1910. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Community, T.G. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res. 2024, 52, W83–W94. [Google Scholar] [CrossRef]
- Fitting, S.; Ignatowska-Jankowska, B.M.; Bull, C.; Skoff, R.P.; Lichtman, A.H.; Wise, L.E.; Fox, M.A.; Su, J.; Medina, A.E.; Krahe, T.E.; et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 tat transgenic mice. Biol. Psychiatry 2013, 73, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L.; Masliah, E.; Yu, G.Q.; Mallory, M.; Rockenstein, E.M.; Tatsuno, G.; Hu, K.; Kholodenko, D.; Johnson-Wood, K.; McConlogue, L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J. Neurosci. 2000, 20, 4050–4058. [Google Scholar] [CrossRef] [PubMed]
- Daiwile, A.P.; Jayanthi, S.; Cadet, J.L. Sex differences in methamphetamine use disorder perused from pre-clinical and clinical studies: Potential therapeutic impacts. Neurosci. Biobehav. Rev. 2022, 137, 104674. [Google Scholar] [CrossRef]
- Hensleigh, E.; Pritchard, L.M. The effect of early environmental manipulation on locomotor sensitivity and methamphetamine conditioned place preference reward. Behav. Brain Res. 2014, 268, 66–71. [Google Scholar] [CrossRef]
- Schindler, C.W.; Bross, J.G.; Thorndike, E.B. Gender differences in the behavioral effects of methamphetamine. Eur. J. Pharmacol. 2002, 442, 231–235. [Google Scholar] [CrossRef]
- Yang, H.; Tao, L.; Li, L. Long-Term Systemic Treatment With Methamphetamine Causes Retinal Damage in CD1 Mice. Int. J. Toxicol. 2018, 37, 448–456. [Google Scholar] [CrossRef]
- Lee, M.; Leskova, W.; Eshaq, R.S.; Harris, N.R. Acute changes in the retina and central retinal artery with methamphetamine. Exp. Eye Res. 2020, 193, 107964. [Google Scholar] [CrossRef]
- Belblidia, H.; Leger, M.; Abdelmalek, A.; Quiedeville, A.; Calocer, F.; Boulouard, M.; Jozet-Alves, C.; Freret, T.; Schumann-Bard, P. Characterizing age-related decline of recognition memory and brain activation profile in mice. Exp. Gerontol. 2018, 106, 222–231. [Google Scholar] [CrossRef]
- Pitts, M.W. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio. Protoc. 2018, 8, e2744. [Google Scholar] [CrossRef]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 141, 58593. [Google Scholar] [CrossRef]
- Tanimizu, T.; Kono, K.; Kida, S. Brain networks activated to form object recognition memory. Brain Res. Bull. 2018, 141, 27–34. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Johnson, J.L.; Knierim, J.J. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: Comparison with lateral entorhinal cortex. Hippocampus 2012, 22, 2045–2058. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Schroder, E.; Schmoll, H.; Walker, L.C.; Kessler, C. Upregulation of MAP1B and MAP2 in the rat brain after middle cerebral artery occlusion: Effect of age. J. Cereb. Blood Flow Metab. 1999, 19, 425–434. [Google Scholar] [CrossRef]
- Shukla, M.; Maitra, S.; Hernandez, J.F.; Govitrapong, P.; Vincent, B. Methamphetamine regulates βAPP processing in human neuroblastoma cells. Neurosci. Lett. 2019, 701, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bhargavan, B.; Woollard, S.M.; McMillan, J.E.; Kanmogne, G.D. CCR5 antagonist reduces HIV-induced amyloidogenesis, tau pathology, neurodegeneration, and blood-brain barrier alterations in HIV-infected hu-PBL-NSG mice. Mol. Neurodegener. 2021, 16, 78. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W. The role of APP and BACE1 trafficking in APP processing and amyloid-beta generation. Alzheimers Res. Ther. 2013, 5, 46. [Google Scholar] [CrossRef]
- Ernst, A.S.; Bohler, L.I.; Hagenston, A.M.; Hoffmann, A.; Heiland, S.; Sticht, C.; Bendszus, M.; Hecker, M.; Bading, H.; Marti, H.H.; et al. EphB2-dependent signaling promotes neuronal excitotoxicity and inflammation in the acute phase of ischemic stroke. Acta Neuropathol. Commun. 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Melega, W.P.; Laćan, G.; Harvey, D.C.; Way, B.M. Methamphetamine increases basal ganglia iron to levels observed in aging. Neuroreport 2007, 18, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.J.; Ren, Z.X.; Shi, Z.F.; Wan, C.L.; Han, C.J.; Zhu, L.S.; Li, N.N.; Waddington, J.L.; Zhen, X.C. Dysregulation of iron homeostasis and methamphetamine reward behaviors in Clk1-deficient mice. Acta Pharmacol. Sin. 2022, 43, 1686–1698. [Google Scholar] [CrossRef]
- Deschepper, C.F. Regulatory effects of the Uty/Ddx3y locus on neighboring chromosome Y genes and autosomal mRNA transcripts in adult mouse non-reproductive cells. Sci. Rep. 2020, 10, 14900. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Wahlestedt, C.; Nordqvist, K. Isolation of sex-specific cDNAs from fetal mouse brain using mRNA differential display and representational difference analysis. Brain Res. Mol. Brain Res. 1999, 74, 91–97. [Google Scholar] [CrossRef]
- Mizukami, H.; Kim, J.D.; Tabara, S.; Lu, W.; Kwon, C.; Nakashima, M.; Fukamizu, A. KDM5D-mediated H3K4 demethylation is required for sexually dimorphic gene expression in mouse embryonic fibroblasts. J. Biochem. 2019, 165, 335–342. [Google Scholar] [CrossRef]
- Duc Nguyen, H.; Pal Yu, B.; Hoang, N.H.M.; Jo, W.H.; Young Chung, H.; Kim, M.S. Prolactin and Its Altered Action in Alzheimer’s Disease and Parkinson’s Disease. Neuroendocrinology 2022, 112, 427–445. [Google Scholar] [CrossRef]
- Holubova, M.; Hruba, L.; Popelova, A.; Bencze, M.; Prazienkova, V.; Gengler, S.; Kratochvilova, H.; Haluzik, M.; Zelezna, B.; Kunes, J.; et al. Liraglutide and a lipidized analog of prolactin-releasing peptide show neuroprotective effects in a mouse model of beta-amyloid pathology. Neuropharmacology 2019, 144, 377–387. [Google Scholar] [CrossRef]
- Yousefvand, S.; Hadjzadeh, M.A.; Vafaee, F.; Dolatshad, H. The protective effects of prolactin on brain injury. Life Sci. 2020, 263, 118547. [Google Scholar] [CrossRef]
- Molina-Salinas, G.; Rivero-Segura, N.A.; Cabrera-Reyes, E.A.; Rodríguez-Chávez, V.; Langley, E.; Cerbon, M. Decoding signaling pathways involved in prolactin-induced neuroprotection: A review. Front. Neuroendocrinol. 2021, 61, 100913. [Google Scholar] [CrossRef]
- Ma, F.Y.; Grattan, D.R.; Goffin, V.; Bunn, S.J. Prolactin-regulated tyrosine hydroxylase activity and messenger ribonucleic acid expression in mediobasal hypothalamic cultures: The differential role of specific protein kinases. Endocrinology 2005, 146, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mellado, S.; Moreno-Ruiz, B.; Exposito, S.; Fernandez, M.; Martin, E.D. Prolactin Reduces Hippocampal Parvalbumin and GABAA Receptor Expression in Female Mice. Neuroendocrinology 2022, 112, 796–806. [Google Scholar] [CrossRef]
- Vergara-Castañeda, E.; Grattan, D.R.; Pasantes-Morales, H.; Pérez-Domínguez, M.; Cabrera-Reyes, E.A.; Morales, T.; Cerbón, M. Prolactin mediates neuroprotection against excitotoxicity in primary cell cultures of hippocampal neurons via its receptor. Brain Res. 2016, 1636, 193–199. [Google Scholar] [CrossRef]
- Zauli, G.; Secchiero, P.; Rodella, L.; Gibellini, D.; Mirandola, P.; Mazzoni, M.; Milani, D.; Dowd, D.R.; Capitani, S.; Vitale, M. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J. Biol. Chem. 2000, 275, 4159–4165. [Google Scholar] [CrossRef] [PubMed]
- Goulding, D.R.; Kraft, A.; Mouton, P.R.; McPherson, C.A.; Avdoshina, V.; Mocchetti, I.; Harry, G.J. Age-Related Decrease in Tyrosine Hydroxylase Immunoreactivity in the Substantia Nigra and Region-Specific Changes in Microglia Morphology in HIV-1 Tg Rats. Neurotox. Res. 2019, 36, 563–582. [Google Scholar] [CrossRef]
- Zigmond, R.E.; Schwarzschild, M.A.; Rittenhouse, A.R. Acute Regulation of Tyrosine Hydroxylase by Nerve Activity and by Neurotransmitters via Phosphorylation. Annu. Rev. Neurosci. 1989, 12, 415–461. [Google Scholar] [CrossRef] [PubMed]
- Branch, S.Y.; Beckstead, M.J. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J. Neurophysiol. 2012, 108, 802–809. [Google Scholar] [CrossRef]
- Jedynak, J.P.; Cameron, C.M.; Robinson, T.E. Repeated methamphetamine administration differentially alters fos expression in caudate-putamen patch and matrix compartments and nucleus accumbens. PLoS ONE 2012, 7, e34227. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Jiao, H.; Zhang, Q.; Zhang, D.; Lou, D.; Katz, J.L.; Xu, M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J. Neurosci. 2006, 26, 13287–13296. [Google Scholar] [CrossRef]
- Tripathi, K.; Hazra, S.; Hazra, J.D.; Mandel, S.; Anunu, R.; Kriebel, M.; Volkmer, H.; Richter-Levin, G. Selective knockdown of GABAA-α2 subunit in the dorsal dentate gyrus in adulthood induces anxiety, learning and memory deficits and impairs synaptic plasticity. Eur. J. Neurosci. 2024, 60, 4393–4408. [Google Scholar] [CrossRef]
- Lim, C.S.; Kim, H.; Yu, N.K.; Kang, S.J.; Kim, T.; Ko, H.G.; Lee, J.; Yang, J.E.; Ryu, H.H.; Park, T.; et al. Enhancing inhibitory synaptic function reverses spatial memory deficits in Shank2 mutant mice. Neuropharmacology 2017, 112, 104–112. [Google Scholar] [CrossRef] [PubMed]
- FDA, U.S. Desoxyn® (Methamphetamine Hydrochloride Tablets, USP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/005378s034lbl.pdf (accessed on 6 August 2024).
- Kesby, J.P.; Markou, A.; Semenova, S.; Group, T. Effects of HIV/TAT protein expression and chronic selegiline treatment on spatial memory, reversal learning and neurotransmitter levels in mice. Behav. Brain Res. 2016, 311, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Chang, A.; Markou, A.; Semenova, S. Modeling human methamphetamine use patterns in mice: Chronic and binge methamphetamine exposure, reward function and neurochemistry. Addict. Biol. 2018, 23, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Fields, J.A.; Chang, A.; Coban, H.; Achim, C.L.; Semenova, S.; Group, T. Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav. Brain Res. 2018, 349, 73–79. [Google Scholar] [CrossRef]
- Nookala, A.R.; Schwartz, D.C.; Chaudhari, N.S.; Glazyrin, A.; Stephens, E.B.; Berman, N.E.J.; Kumar, A. Methamphetamine augment HIV-1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors. Brain Behav. Immun. 2018, 71, 37–51. [Google Scholar] [CrossRef]
- Jacobs, I.R.; Xu, C.; Hermes, D.J.; League, A.F.; Xu, C.; Nath, B.; Jiang, W.; Niphakis, M.J.; Cravatt, B.F.; Mackie, K.; et al. Inhibitory Control Deficits Associated with Upregulation of CB(1)R in the HIV-1 Tat Transgenic Mouse Model of Hand. J. Neuroimmune Pharmacol. 2019, 14, 661–678. [Google Scholar] [CrossRef]
- Cirino, T.J.; Harden, S.W.; McLaughlin, J.P.; Frazier, C.J. Region-specific effects of HIV-1 Tat on intrinsic electrophysiological properties of pyramidal neurons in mouse prefrontal cortex and hippocampus. J. Neurophysiol. 2020, 123, 1332–1341. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| App | (Fwd) GGC CCT CGA GAA TTA CAT CA (Rev) GTT CAT GCG CTC GTA GAT CA |
| Adam10 | (Fwd) ATG GTG TTG CCG ACA GTG TTA (Rev) GTT TGG CAC GCT GGT GTT TTT |
| Bace1 | (Fwd) ATG TGG AGA TGA CCG TAG GC (Rev) TAC ACA CCC TTT CGG AGG TC |
| Gsap | (Fwd) ATG GCT GCA CTG CAC TGT AT (Rev) AGC TCA GAC ACC ATC AAG CC |
| Efnb1 | (Fwd) ACC CTA AGT TCC TAA GTG GGA (Rev) CTT GTA GTA CTC GTA GGG C |
| Ephb2 | (Fwd) TAC ATC CCC CAT CAG GGT GG (Rev) GCC GGA TGA ATT TGG TCC GC |
| Lcn2 | (Fwd) CCA GTT CGC CAT GGT ATT TT (Rev) GGT GGG GAC AGA GAA GAT GA |
| Tau | (Fwd) TGA GGG ACT AGG GCA GCT AA (Rev) CTG CCT TCC TCA CCT CTG TC |
| Gapdh | (Fwd) AGG TCG GTG TGA ACG GAT TTG (Rev) TGT AGA CCA TGT AGT TGA GGT CA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harahap-Carrillo, I.S.; Fok, D.; Wong, F.; Malik, G.; Maung, R.; Qiu, X.; Ojeda-Juárez, D.; Thaney, V.E.; Sanchez, A.B.; Godzik, A.; et al. Chronic, Low-Dose Methamphetamine Reveals Sexual Dimorphism of Memory Performance, Histopathology, and Gene Expression Affected by HIV-1 Tat Protein in a Transgenic Model of NeuroHIV. Viruses 2025, 17, 361. https://doi.org/10.3390/v17030361
Harahap-Carrillo IS, Fok D, Wong F, Malik G, Maung R, Qiu X, Ojeda-Juárez D, Thaney VE, Sanchez AB, Godzik A, et al. Chronic, Low-Dose Methamphetamine Reveals Sexual Dimorphism of Memory Performance, Histopathology, and Gene Expression Affected by HIV-1 Tat Protein in a Transgenic Model of NeuroHIV. Viruses. 2025; 17(3):361. https://doi.org/10.3390/v17030361
Chicago/Turabian StyleHarahap-Carrillo, Indira S., Dominic Fok, Frances Wong, Gabriel Malik, Ricky Maung, Xinru Qiu, Daniel Ojeda-Juárez, Victoria E. Thaney, Ana B. Sanchez, Adam Godzik, and et al. 2025. "Chronic, Low-Dose Methamphetamine Reveals Sexual Dimorphism of Memory Performance, Histopathology, and Gene Expression Affected by HIV-1 Tat Protein in a Transgenic Model of NeuroHIV" Viruses 17, no. 3: 361. https://doi.org/10.3390/v17030361
APA StyleHarahap-Carrillo, I. S., Fok, D., Wong, F., Malik, G., Maung, R., Qiu, X., Ojeda-Juárez, D., Thaney, V. E., Sanchez, A. B., Godzik, A., Roberts, A. J., & Kaul, M. (2025). Chronic, Low-Dose Methamphetamine Reveals Sexual Dimorphism of Memory Performance, Histopathology, and Gene Expression Affected by HIV-1 Tat Protein in a Transgenic Model of NeuroHIV. Viruses, 17(3), 361. https://doi.org/10.3390/v17030361






