Environmental Suitability of Kazakhstan to Highly Pathogenic Avian Influenza Using Data on Eurasian Outbreaks, 2020–2024
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. HPAI Data
2.3. Study Design
2.4. Ecological Niche Models
2.5. Software
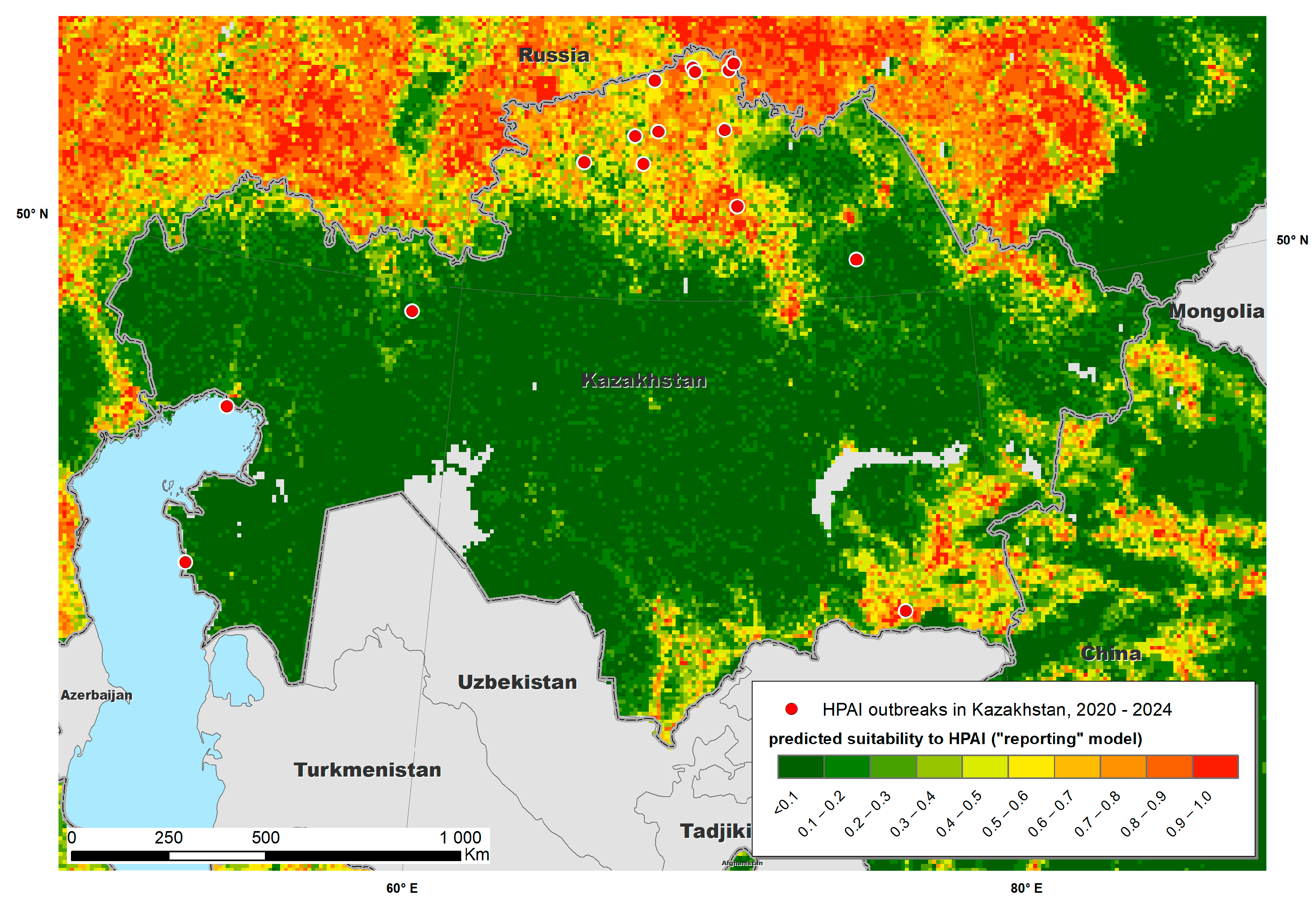
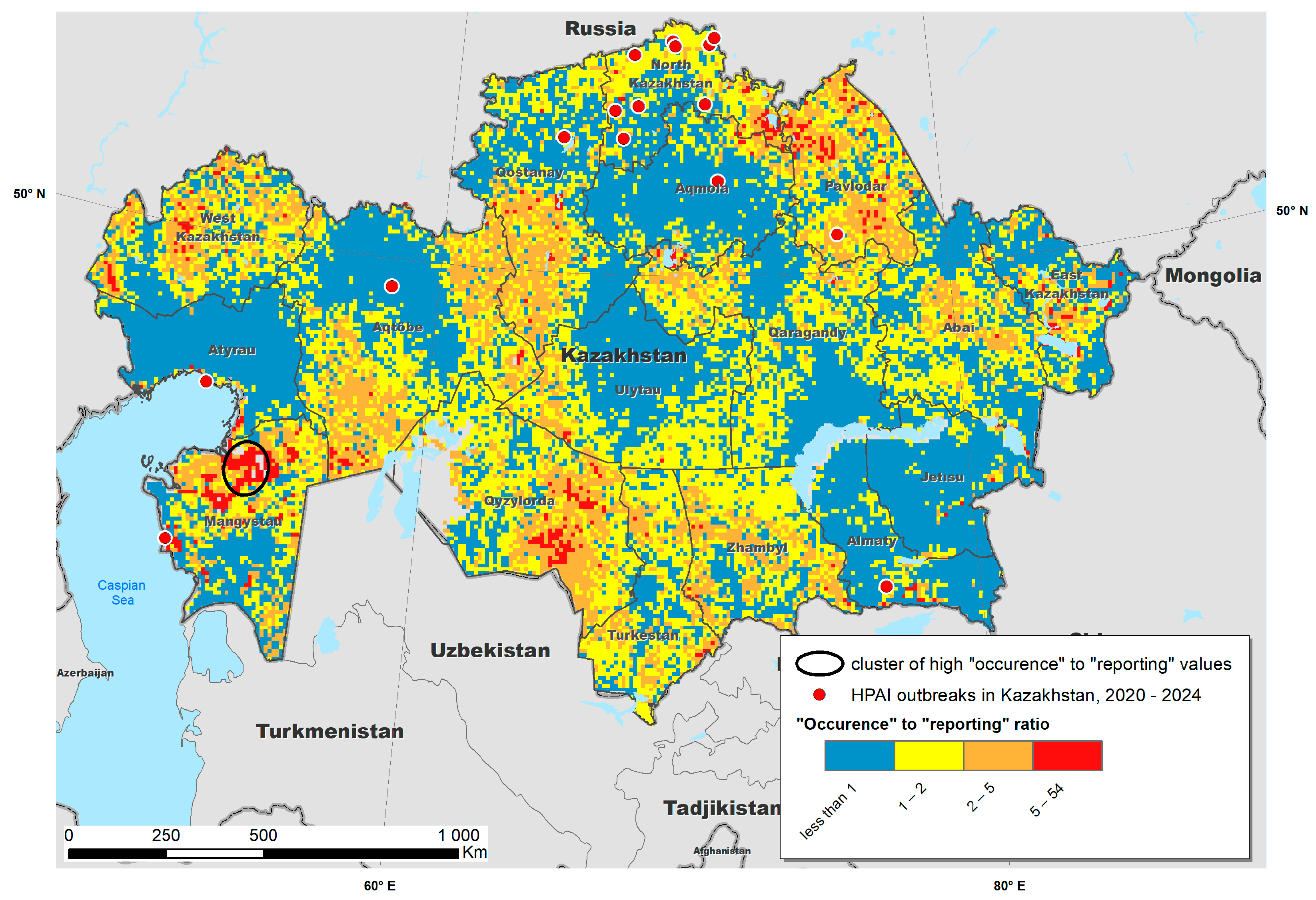
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capua, I.; Alexander, D.J. Avian Influenza and Newcastle Disease: A Field and Laboratory Manual; Springer: Milan, Italy, 2009; pp. 1–186. [Google Scholar] [CrossRef]
- The World Organization for Animal Health (WOAH). Avian Influenza. Available online: https://www.woah.org/en/disease/avian-influenza/ (accessed on 25 December 2024).
- WOAH—World Organisation for Animal Health. Terrestrial Manual Online Access. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 25 December 2024).
- World Health Organization. Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2024. Available online: https://cdn.who.int/media/docs/default-source/2021-dha-docs/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who--2003-2024.pdf (accessed on 11 March 2025).
- Assessment of Risk Associated with Recent Influenza A(H5N1) Clade 2.3.4.4b Viruses. Available online: https://www.who.int/publications/m/item/assessment-of-risk-associated-with-recent-influenza-a(h5n1)-clade-2.3.4.4b-viruses (accessed on 25 January 2025).
- Amirgazin, A.; Shevtsov, A.; Karibayev, T.; Berdikulov, M.; Kozhakhmetova, T.; Syzdykova, L.; Ramankulov, Y.; Shustov, A.V. Highly Pathogenic Avian Influenza Virus of the A/H5N8 Subtype, Clade 2.3.4.4b, Caused Outbreaks in Kazakhstan in 2020. PeerJ 2022, 10, e13038. [Google Scholar] [CrossRef] [PubMed]
- FUJIMOTO, Y.; HAGA, T. Association between Highly Pathogenic Avian Influenza Outbreaks and Weather Conditions in Japan. J. Vet. Med. Sci. 2024, 86, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hossain, M.E.; Amin, E.; Islam, S.; Islam, M.; Sayeed, M.A.; Hasan, M.M.; Miah, M.; Hassan, M.M.; Rahman, M.Z.; et al. Epidemiology and Phylodynamics of Multiple Clades of H5N1 Circulating in Domestic Duck Farms in Different Production Systems in Bangladesh. Front. Public Health 2023, 11, 1168613. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Ståhl, K.; Staubach, C.; Terregino, C.; Willgert, K.; et al. Avian Influenza Overview September–December 2023. EFSA J. 2023, 21, e8539. [Google Scholar] [CrossRef]
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic Spread of Highly Pathogenic Avian Influenza H5N1 by Wild Birds from Europe to North America in 2021. Sci. Rep. 2022, 12, 11729. [Google Scholar] [CrossRef]
- Lambert, S.; Bauzile, B.; Mugnier, A.; Durand, B.; Vergne, T.; Paul, M.C. A Systematic Review of Mechanistic Models Used to Study Avian Influenza Virus Transmission and Control. Vet. Res. 2023, 54, 96. [Google Scholar] [CrossRef]
- The World Organization for Animal Health (WOAH). Available online: https://wahis.woah.org/#/event-management (accessed on 27 December 2024).
- Xie, R.; Edwards, K.M.; Wille, M.; Wei, X.; Wong, S.S.; Zanin, M.; El-Shesheny, R.; Ducatez, M.; Poon, L.L.M.; Kayali, G.; et al. The Episodic Resurgence of Highly Pathogenic Avian Influenza H5 Virus. Nature 2023, 622, 810–817. [Google Scholar] [CrossRef]
- Dao, D.T.; Coleman, K.K.; Bui, V.N.; Bui, A.N.; Tran, L.H.; Nguyen, Q.D.; Than, S.; Pulscher, L.A.; Marushchak, L.V.; Robie, E.R.; et al. High Prevalence of Highly Pathogenic Avian Influenza: A Virus in Vietnam’s Live Bird Markets. Open Forum Infect. Dis. 2024, 11, ofae355. [Google Scholar] [CrossRef]
- Marchenko, V.Y.; Goncharova, N.I.; Gavrilova, E.V.; Maksyutov, R.A.; Ryzhikov, A.B. Overview of the Epizootiological Situation on Highly Pathogenic Avian Influenza in Russia in 2020. Probl. Part. Danger. Infect. 2021, 2, 33–40. [Google Scholar] [CrossRef]
- Zhiltsova, M.V.; Akimova, T.P.; Varkentin, A.V.; Mitrofanova, M.N.; Mazneva, A.V.; Semakina, V.P.; Vystavkina, E.S. Global Avian Influenza Situation (2019–2022). Host Range Expansion Asevidence of High Pathogenicity Avian Influenza Virus Evolution. Vet. Sci. Today 2023, 12, 293–302. [Google Scholar] [CrossRef]
- Ly, H. Recent Global Outbreaks of Highly Pathogenic and Low-Pathogenicity Avian Influenza A Virus Infections. Virulence 2024, 15, 2383478. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, S.; Flora, M.S.; Amin, E.; Woodard, K.; Webb, A.; Webster, R.G.; Webby, R.J.; Ducatez, M.F.; Hassan, M.M.; et al. Epidemiology and Molecular Characterization of Avian Influenza A Viruses H5N1 and H3N8 Subtypes in Poultry Farms and Live Bird Markets in Bangladesh. Sci. Rep. 2023, 13, 7912. [Google Scholar] [CrossRef] [PubMed]
- Burashev, Y.; Strochkov, V.; Sultankulova, K.; Orynbayev, M.; Kassenov, M.; Kozhabergenov, N.; Shorayeva, K.; Sadikaliyeva, S.; Issabek, A.; Almezhanova, M.; et al. Near-Complete Genome Sequence of an H5N1 Avian Influenza Virus Strain Isolated from a Swan in Southwest Kazakhstan in 2006. Microbiol. Resour. Announc. 2020, 9, e00016–e00020. [Google Scholar] [CrossRef] [PubMed]
- Issabek, A.; Burashev, Y.; Chervyakova, O.; Orynbayev, M.; Kydyrbayev, Z.; Kassenov, M.; Zakarya, K.; Sultankulova, K. Complete Genome Sequence of the Highly Pathogenic Strain A/Domestic Goose/Pavlodar/1/05 (H5N1) of the Avian In-fluenza Virus, Isolated in Kazakhstan in 2005. Microbiol. Resour. Announc. 2020, 9, e00109–e00120. [Google Scholar] [CrossRef]
- The World Organization for Animal Health (WOAH). Available online: https://rr-europe.woah.org/app/uploads/2021/05/8_kazakhstan_ai-nd_ru.pdf (accessed on 27 December 2024). (In Russian).
- Baikara, B.; Seidallina, A.; Baimakhanova, B.; Kasymbekov, Y.; Sabyrzhan, T.; Daulbaeva, K.; Nuralibekov, S.; Khan, Y.; Karamendin, K.; Sultanov, A.; et al. Genome Sequence of Highly Pathogenic Avian Influenza Virus A/Chicken/North Kazakhstan/184/2020 (H5N8). Microbiol. Resour. Announc. 2023, 12, e0115122. [Google Scholar] [CrossRef]
- Bopi, A.K.; Omarova, Z.D.; Rystayeva, R.; Tulendibaev, A.B.; Argimbayeva, T.U.; Alibekova, D.A.; Aubakir, N.A.; Ermekbay, T.T.; Ermekbay, A.A.; Orynbayev, M.B.; et al. Monitoring of highly pathogenic avian influenza in Kazakhstan. Biosaf. Biotechnol. 2022, 10, 24–30. (In Russian) [Google Scholar] [CrossRef]
- EBird. Available online: https://ebird.org/home (accessed on 20 March 2025).
- Potential Risk of Highly Pathogenic Avian Influenza (HPAI) Spreading Through Wild Water Bird Migration. Available online: https://openknowledge.fao.org/items/d6185856-dae8-4e8a-a345-1eb34e591a36 (accessed on 25 January 2025).
- Anonymous. Kazakhstan’s Wetlands are of International Importance. Available online: https://online.zakon.kz/Document/?doc_id=30024524&pos=4;-135#pos=4;-135/ (accessed on 30 December 2024).
- Sultankulova, K.T.; Argimbayeva, T.U.; Aubakir, N.A.; Bopi, A.; Omarova, Z.D.; Melisbek, A.M.; Karamendin, K.; Kydyrmanov, A.; Chervyakova, O.V.; Kerimbayev, A.A.; et al. Reassortants of the Highly Pathogenic Influenza Virus A/H5N1 Causing Mass Swan Mortality in Kazakhstan from 2023 to 2024. Animals 2024, 14, 3211. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.P.; An, Q.; Gao, X.; Wang, H. Bin Risk Factors Contributing to Highly Pathogenic Avian Influenza H5N6 in China, 2014–2021: Based on a MaxEnt Model. Transbound. Emerg. Dis. 2023, 2023, 6449392. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Alkhamis, M.; Hijmans, R.J.; Al-Enezi, A.; Martínez-López, B.; Peres, A.M. The Use of Spatial and Spatiotemporal Modeling for Surveillance of H5N1 Highly Pathogenic Avian Influenza in Poultry in the Middle East. Avian Dis. 2016, 60, 146–155. [Google Scholar] [CrossRef]
- Belkhiria, J.; Hijmans, R.J.; Boyce, W.; Crossley, B.M.; Martínez-López, B. Identification of High Risk Areas for Avian Influenza Outbreaks in California Using Disease Distribution Models. PLoS ONE 2018, 13, e0190824. [Google Scholar] [CrossRef] [PubMed]
- Kyuyoung, L.; Yu, D.; Martínez-López, B.; Belkhiria, J.; Kang, S.; Yoon, H.; Hong, S.-K.; LEE, I.; Son, H.-M.; Lee, K. Identification of Highly Pathogenic Avian Influenza Suitable Areas for Wild Birds Using Species Distribution Models in South Korea. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Huang, L.; Tiwari, R.C.; Zou, Z.; Kulldorff, M.; Feuer, E.J. Weighted Normal Spatial Scan Statistic for Heterogeneous Population Data. J. Am. Stat. Assoc. 2009, 104, 886–898. [Google Scholar] [CrossRef]
- Steven, J.; Phillips; Dudík, M.; Robert, E. Schapire. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 15 January 2025).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- Kulldorff, M. SaTScan™ v10.0: Software for the Spatial and Space-Time Scan Statistics [www.satscan.org]; Information Management Services, Inc.: Boston, MA, USA, 2021. [Google Scholar]
- Kydyrmanov, A.; Sayatov, M.; Karamendin, K.; Zhumatov, K.; Asanova, S.; Daulbayeva, K.; Starick, E.; Fereidouni, S. Monitoring of Influenza A Viruses in Wild Bird Populations in Kazakhstan in 2002–2009. Arch. Virol. 2017, 162, 147–155. [Google Scholar] [CrossRef]
- Li, X.H.; Tian, H.D.; Heiner, M.; Li, D.M. Global Occurrence and Spread of Highly Pathogenic Avian Influenza Virus of the Subtype H5N1. Avian Dis. Dig. 2011, 6, e9–e12. [Google Scholar] [CrossRef]
- Karamendin, K.; Kydyrmanov, A.; Kasymbekov, Y.; Daulbayeva, K.; Khan, E.; Seidalina, A.; Sayatov, M. A Highly Pathogenic H5N1 Influenza A Virus Isolated from a Flamingo on the Caspian Sea Shore. Microbiol. Resour. Announc. 2020, 9, e00508–e00520. [Google Scholar] [CrossRef]
- Paul, M.; Wongnarkpet, S.; Gasqui, P.; Poolkhet, C.; Thongratsakul, S.; Ducrot, C.; Roger, F. Risk Factors for Highly Pathogenic Avian Influenza (HPAI) H5N1 Infection in Backyard Chicken Farms, Thailand. Acta Trop. 2011, 118, 209–216. [Google Scholar] [CrossRef]
- Velkers, F.C.; Manders, T.T.M.; Vernooij, J.C.M.; Stahl, J.; Slaterus, R.; Stegeman, J.A. Association of Wild Bird Densities around Poultry Farms with the Risk of Highly Pathogenic Avian Influenza Virus Subtype H5N8 Outbreaks in the Netherlands, 2016. Transbound. Emerg. Dis. 2021, 68, 76–87. [Google Scholar] [CrossRef]
- Jung Kjær, L.; Ward, M.P.; Boklund, A.E.; Larsen, L.E.; Hjulsager, C.K.; Kirkeby, C.T. Using Surveillance Data for Early Warning Modelling of Highly Pathogenic Avian Influenza in Europe Reveals a Seasonal Shift in Transmission, 2016–2022. Sci. Rep. 2023, 13, 15396. [Google Scholar] [CrossRef]
- Yoo, D.-S.; Lee, K.-N.; Chun, B.-C.; Lee, H.-S.; Park, H.; Kim, J.-K. Preventive Effect of On-Farm Biosecurity Practices against Highly Pathogenic Avian Influenza (HPAI) H5N6 Infection on Commercial Layer Farms in the Republic of Korea during the 2016-17 Epidemic: A Case-Control Study. Prev. Vet. Med. 2022, 199, 105556. [Google Scholar] [CrossRef]
- Ibáñez-Porras, P.; de la Torre, A.; Gómez-Pérez, J.I.; Tomás-Tenllado, C.; García, E.; Cáceres, G.; Pérez, A.; Iglesias, I. DiFLUsion: A Novel Space-Time Alert System for HPAI. Ger. J. Vet. Res. 2024, 4, 31–42. [Google Scholar] [CrossRef]
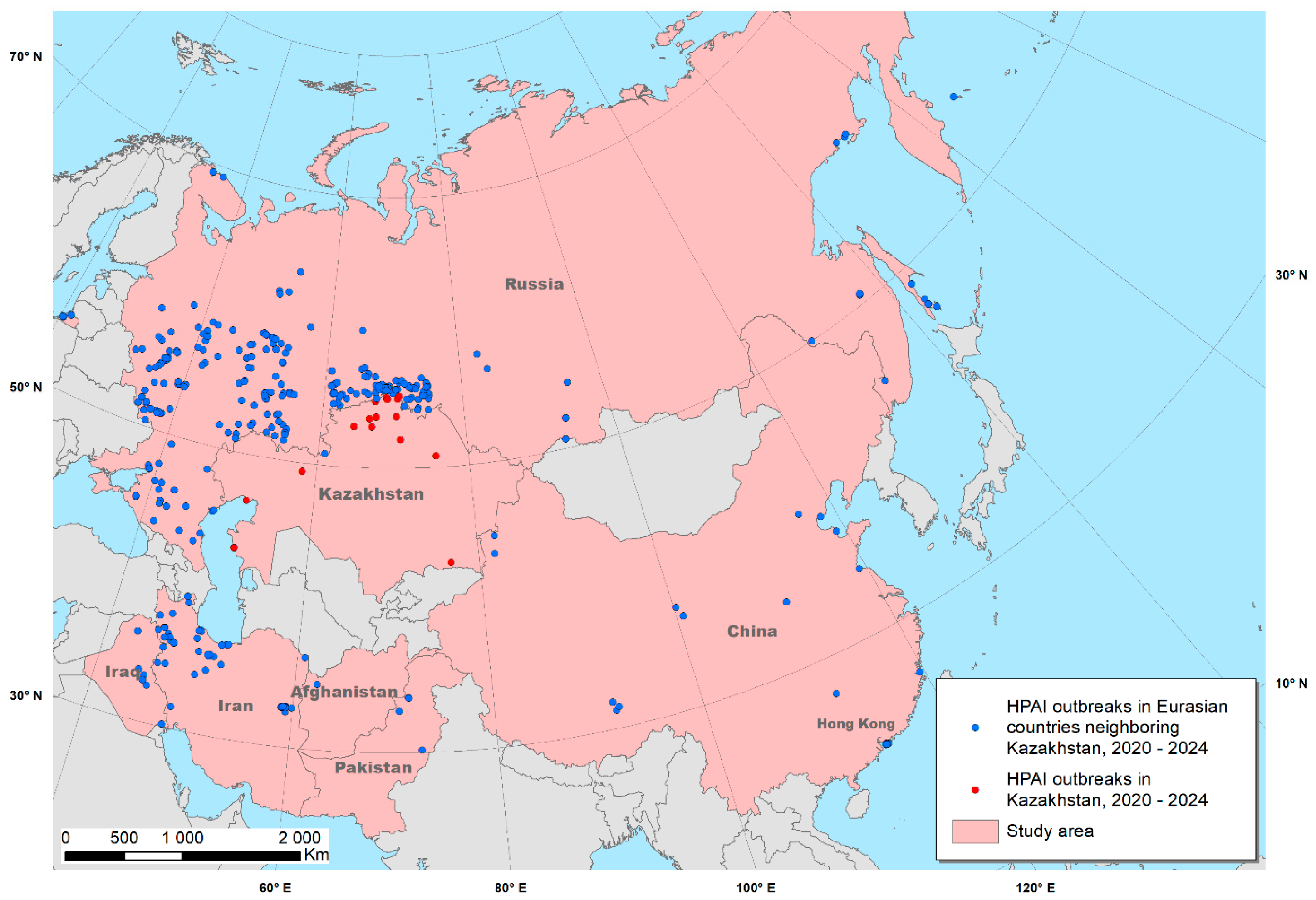

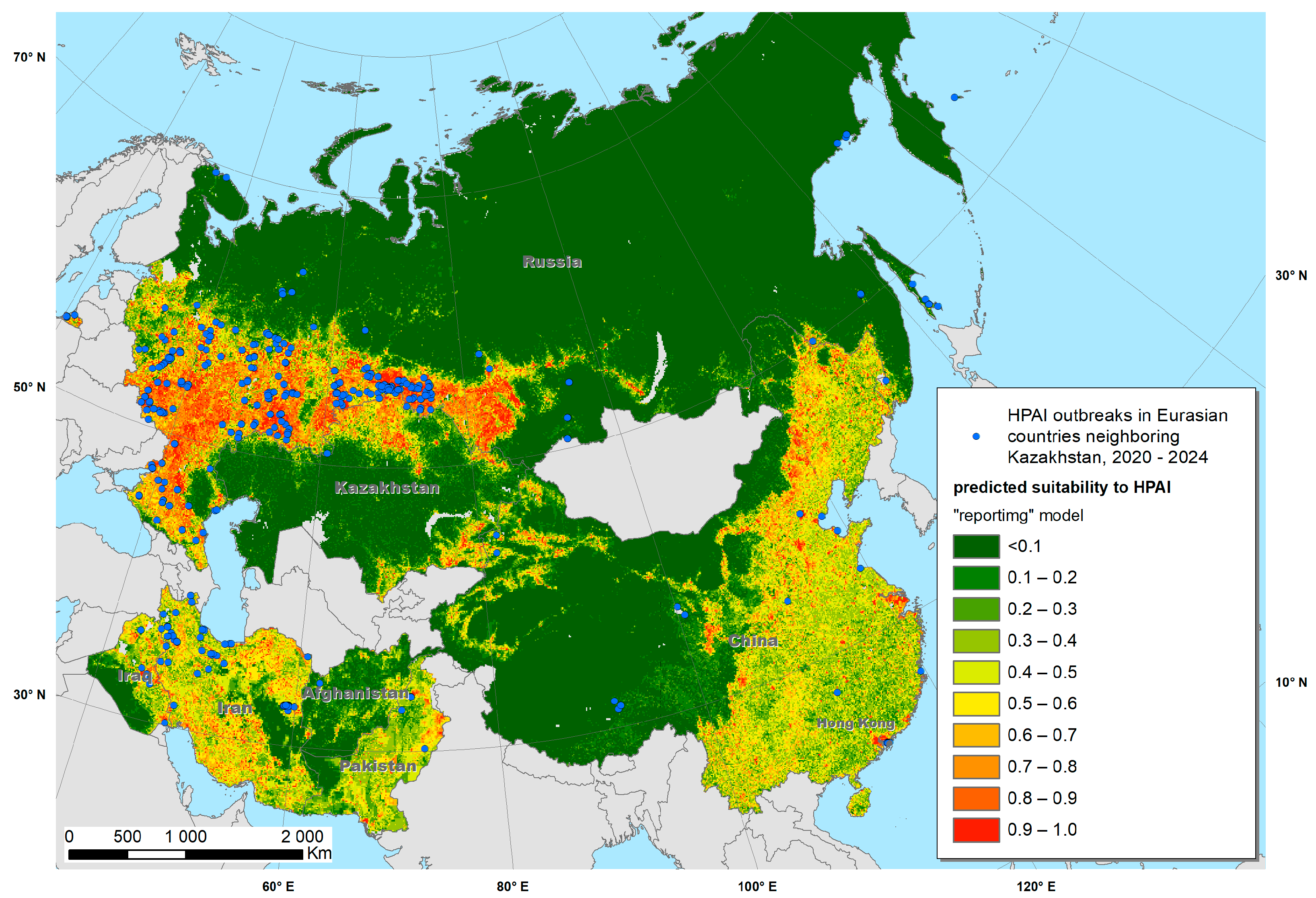
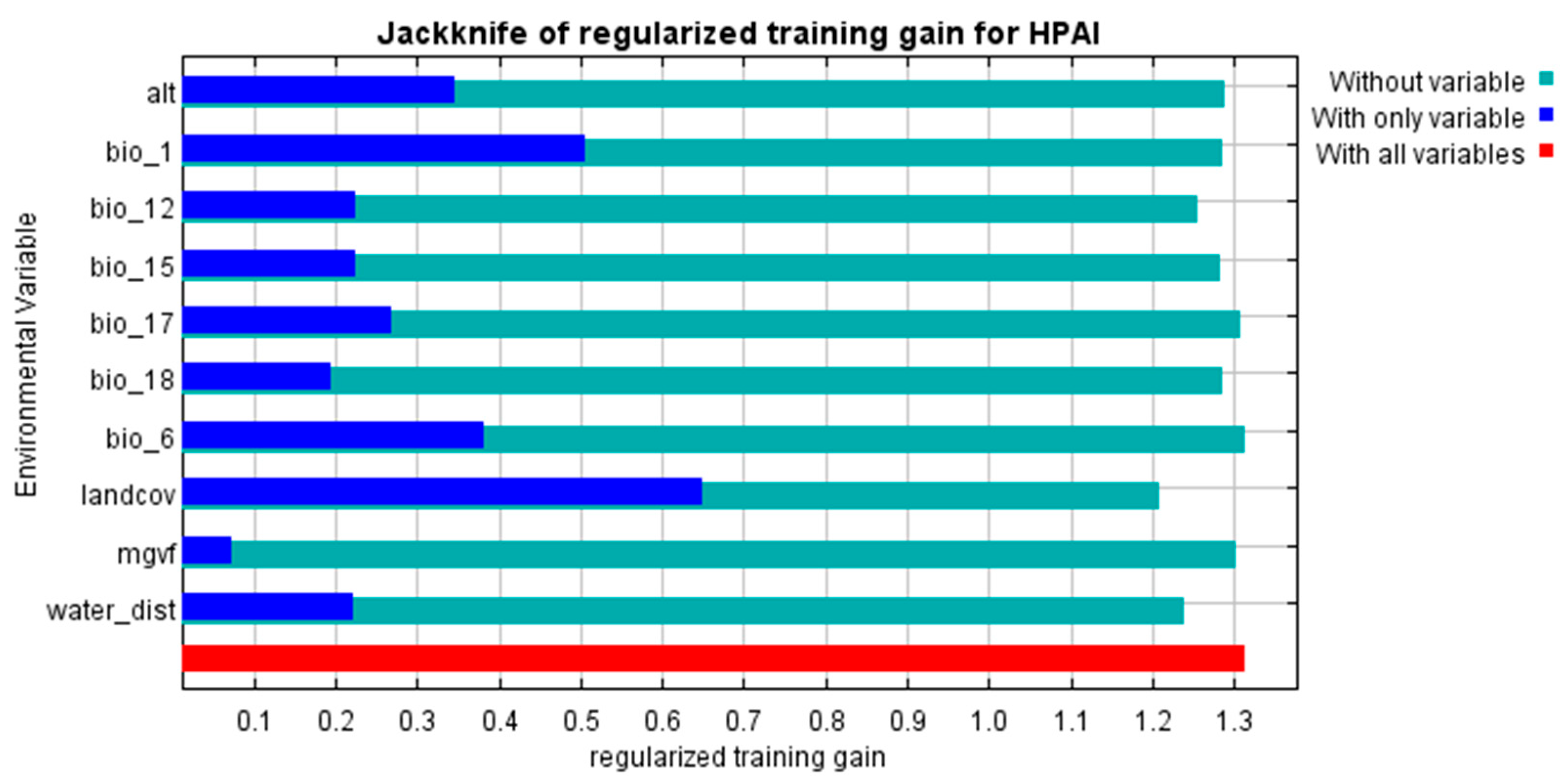
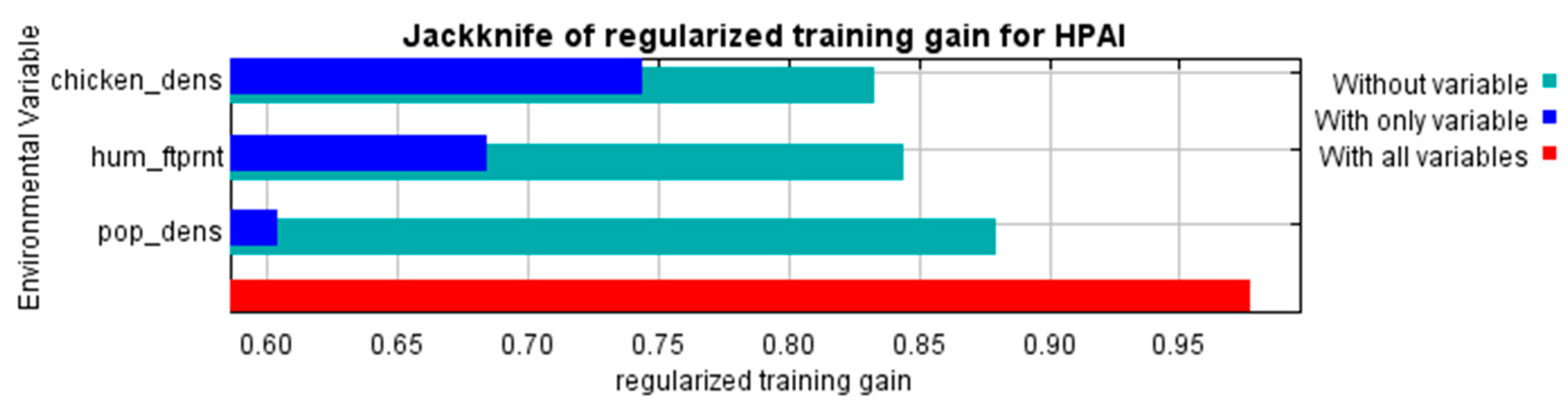

| Number of HPAI Outbreaks | In Domestic Birds | In Wild Birds |
|---|---|---|
| Afghanistan | 1 | 0 |
| China | 1 | 13 |
| Hong Kong | 0 | 12 |
| Iran | 57 | 5 |
| Iraq | 8 | 0 |
| Kazakhstan | 14 | 3 |
| Pakistan | 5 | 0 |
| Russia | 241 | 101 |
| Variable Name | Variable Meaning | Factor Type | Measurement Unit |
|---|---|---|---|
| Alt | Altitude above sea level | environmental | meters |
| landcov | Type of land cover | environmental | categories |
| Mgvf | Maximum green vegetation fraction | environmental | % |
| pop_dens | Population density | population | persons/km2 |
| water_dist | Distance to water bodies | environmental | kilometers |
| hum_ftprnt | Human footprint index (a measure of human influence on the terrestrial systems of the Earth) | population | index |
| chicken_dens | Chicken density | population | head/km2 |
| bio_1 | Annual mean air temperature | climatic | °C × 10 |
| bio_6 | Minimum air temperature of the coldest month | climatic | °C × 10 |
| bio_12 | Annual precipitation | climatic | millimeters |
| bio_15 | Precipitation seasonality | climatic | % |
| bio_17 | Precipitation of the driest quarter | climatic | millimeters |
| bio_18 | Precipitation of the warmest quarter | climatic | Millimeters |
| Country | Summary “Occurrence” Suitability | Summary “Reporting” Suitability | Number of HPAI Outbreaks Reported |
|---|---|---|---|
| Afghanistan | 180 | 267 | 1 |
| China | 671 | 17,840 | 14 |
| Hong Kong | 1 | 2 | 12 |
| Iran | 2024 | 6891 | 62 |
| Iraq | 718 | 1054 | 8 |
| Kazakhstan | 2069 | 2970 | 17 |
| Pakistan | 108 | 1745 | 5 |
| Russia | 23,653 | 22,174 | 342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abenova, A.Z.; Mukhanbetkaliyev, Y.Y.; Kadyrov, A.S.; Sytnik, I.I.; Shevtsov, A.B.; Korennoy, F.I.; Martin, I.I.; Perez, A.M.; Abdrakhmanov, S.K. Environmental Suitability of Kazakhstan to Highly Pathogenic Avian Influenza Using Data on Eurasian Outbreaks, 2020–2024. Viruses 2025, 17, 574. https://doi.org/10.3390/v17040574
Abenova AZ, Mukhanbetkaliyev YY, Kadyrov AS, Sytnik II, Shevtsov AB, Korennoy FI, Martin II, Perez AM, Abdrakhmanov SK. Environmental Suitability of Kazakhstan to Highly Pathogenic Avian Influenza Using Data on Eurasian Outbreaks, 2020–2024. Viruses. 2025; 17(4):574. https://doi.org/10.3390/v17040574
Chicago/Turabian StyleAbenova, Asem Zh., Yersyn Y. Mukhanbetkaliyev, Ablaikhan S. Kadyrov, Igor I. Sytnik, Alexander B. Shevtsov, Fedor I. Korennoy, Irene Iglesias Martin, Andres M. Perez, and Sarsenbay K. Abdrakhmanov. 2025. "Environmental Suitability of Kazakhstan to Highly Pathogenic Avian Influenza Using Data on Eurasian Outbreaks, 2020–2024" Viruses 17, no. 4: 574. https://doi.org/10.3390/v17040574
APA StyleAbenova, A. Z., Mukhanbetkaliyev, Y. Y., Kadyrov, A. S., Sytnik, I. I., Shevtsov, A. B., Korennoy, F. I., Martin, I. I., Perez, A. M., & Abdrakhmanov, S. K. (2025). Environmental Suitability of Kazakhstan to Highly Pathogenic Avian Influenza Using Data on Eurasian Outbreaks, 2020–2024. Viruses, 17(4), 574. https://doi.org/10.3390/v17040574






