Flow Virometry in Wastewater Monitoring: Comparison of Virus-like Particles to Coliphage, Pepper Mild Mottle Virus, CrAssphage, and Tomato Brown Rugose Fruit Virus
Abstract
1. Introduction
2. Materials and Methods
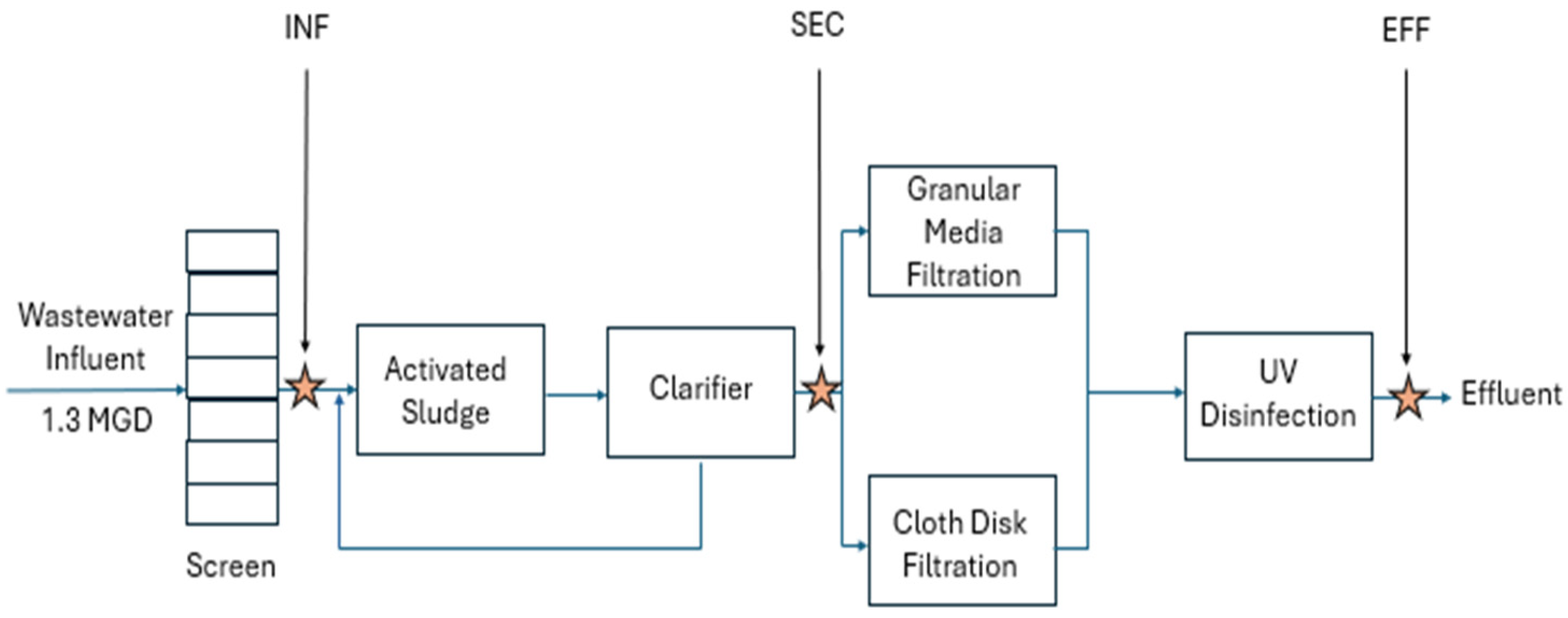
2.1. Sample Collection and Processing
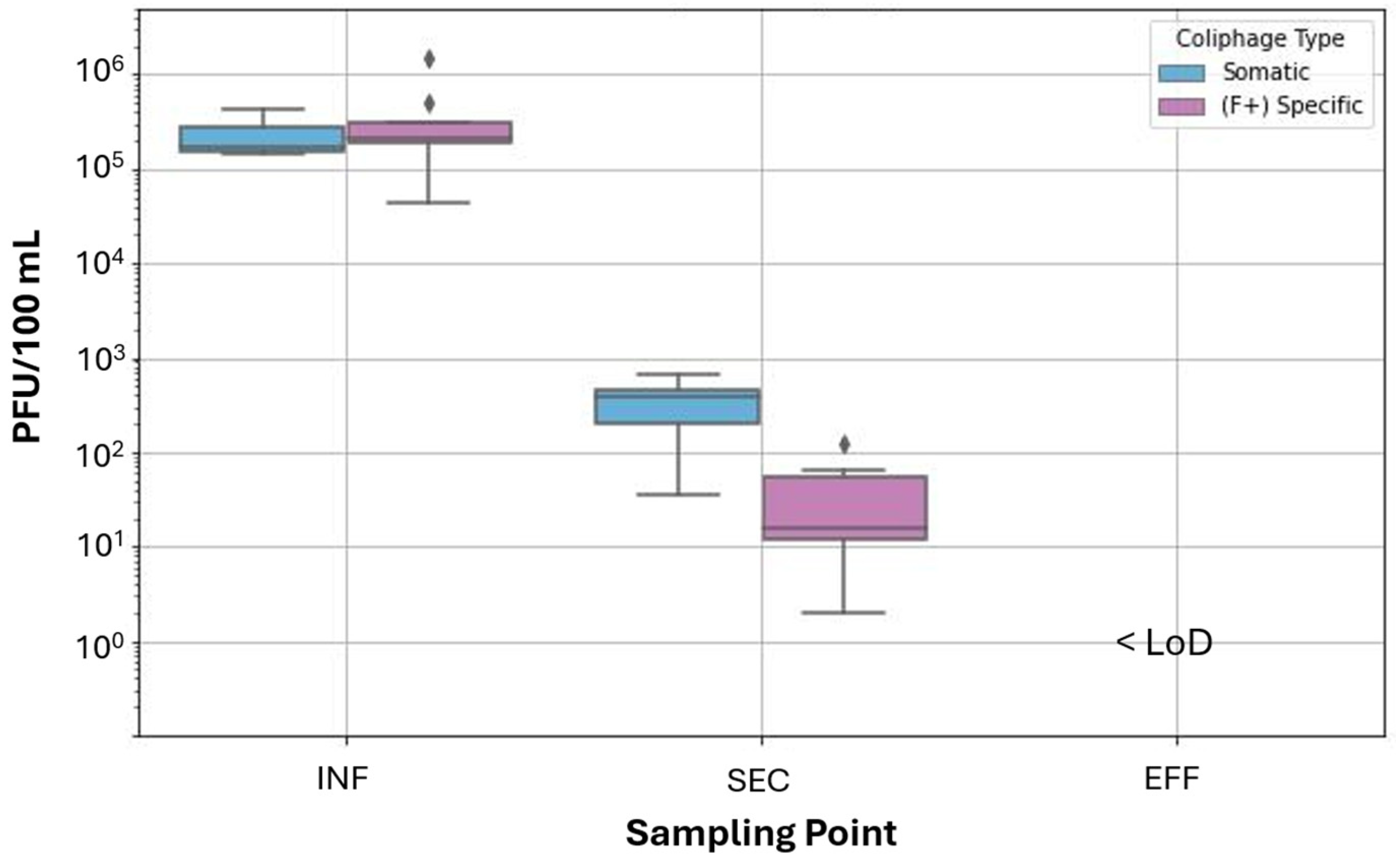
2.2. (F+) Specific and Somatic Coliphage Plaque Assays
2.3. FCM Sample Preparation and Enumeration
2.4. FVM Method Validation and Quality Control
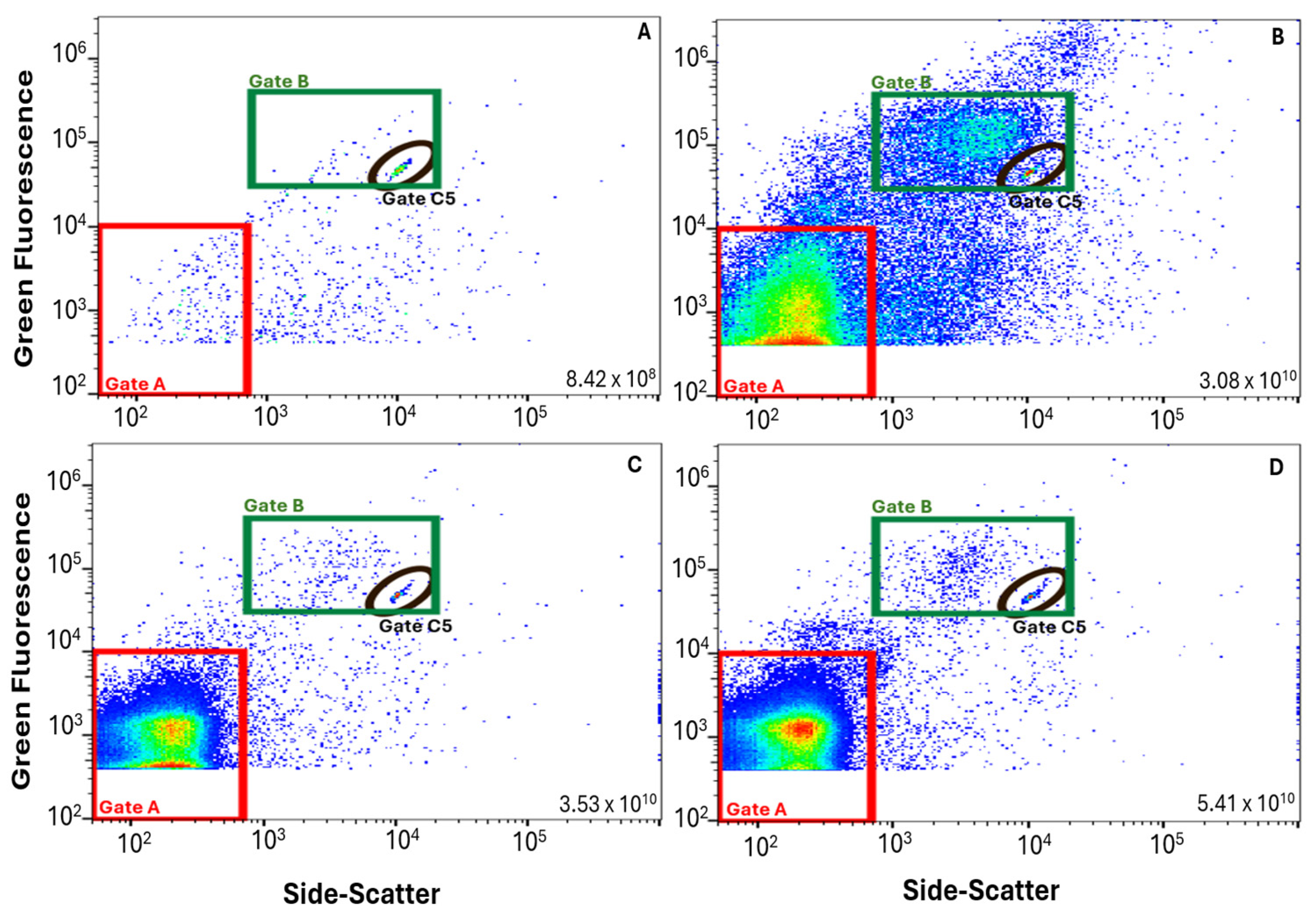
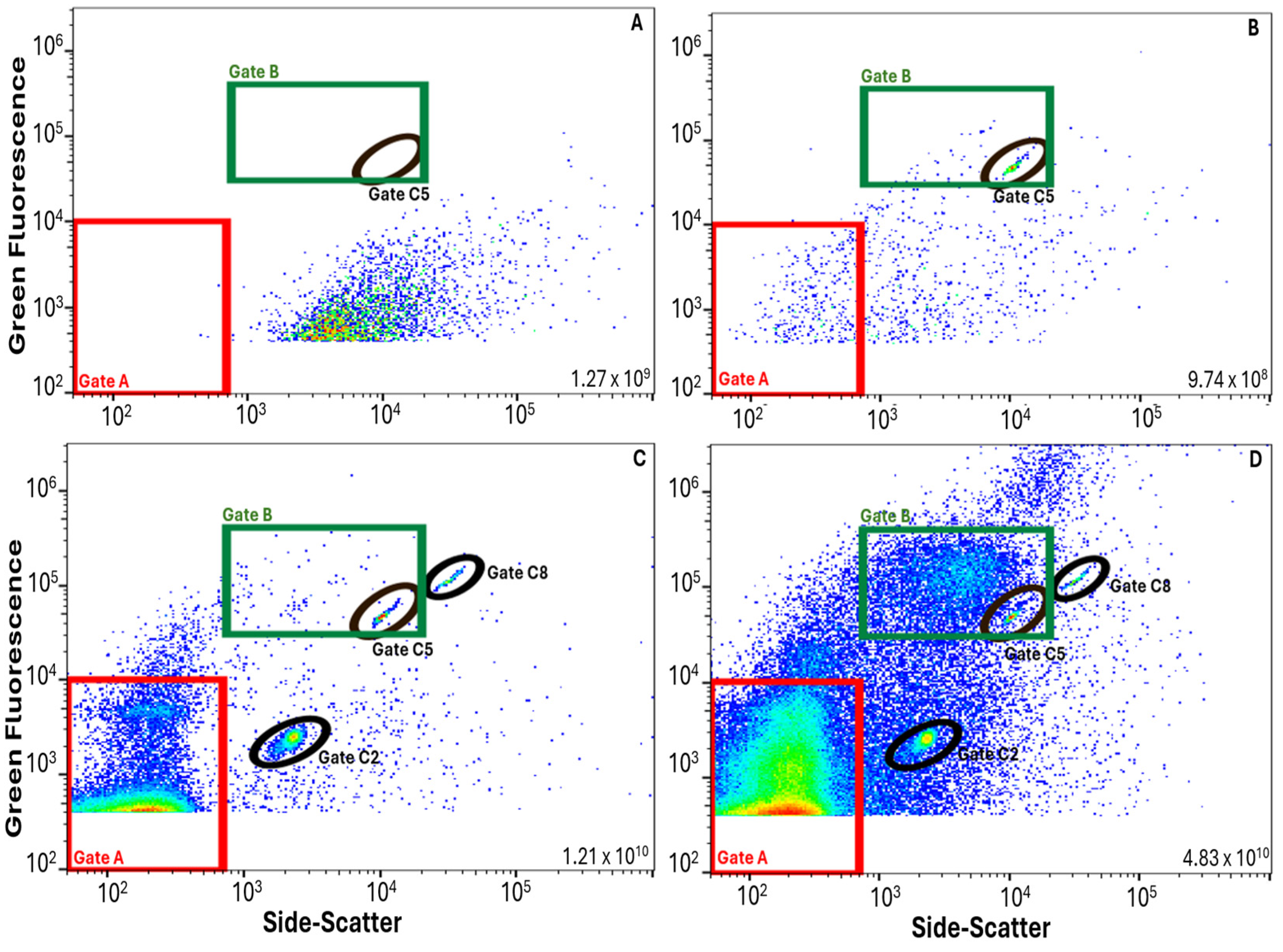
2.5. FCM Data Management and VLP Gate
2.6. Tertiary Treatment Exploration with UV-Influent Samples
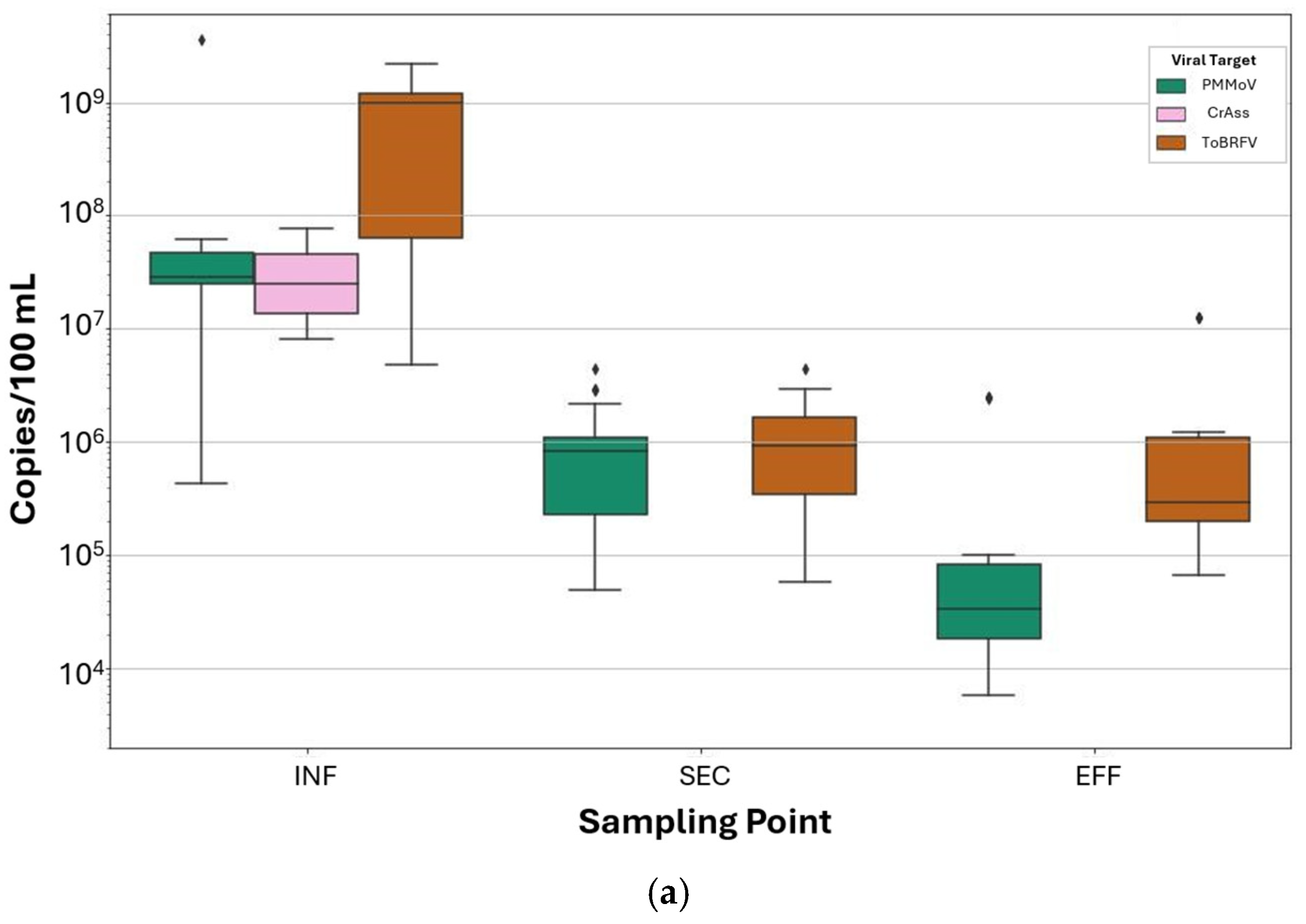
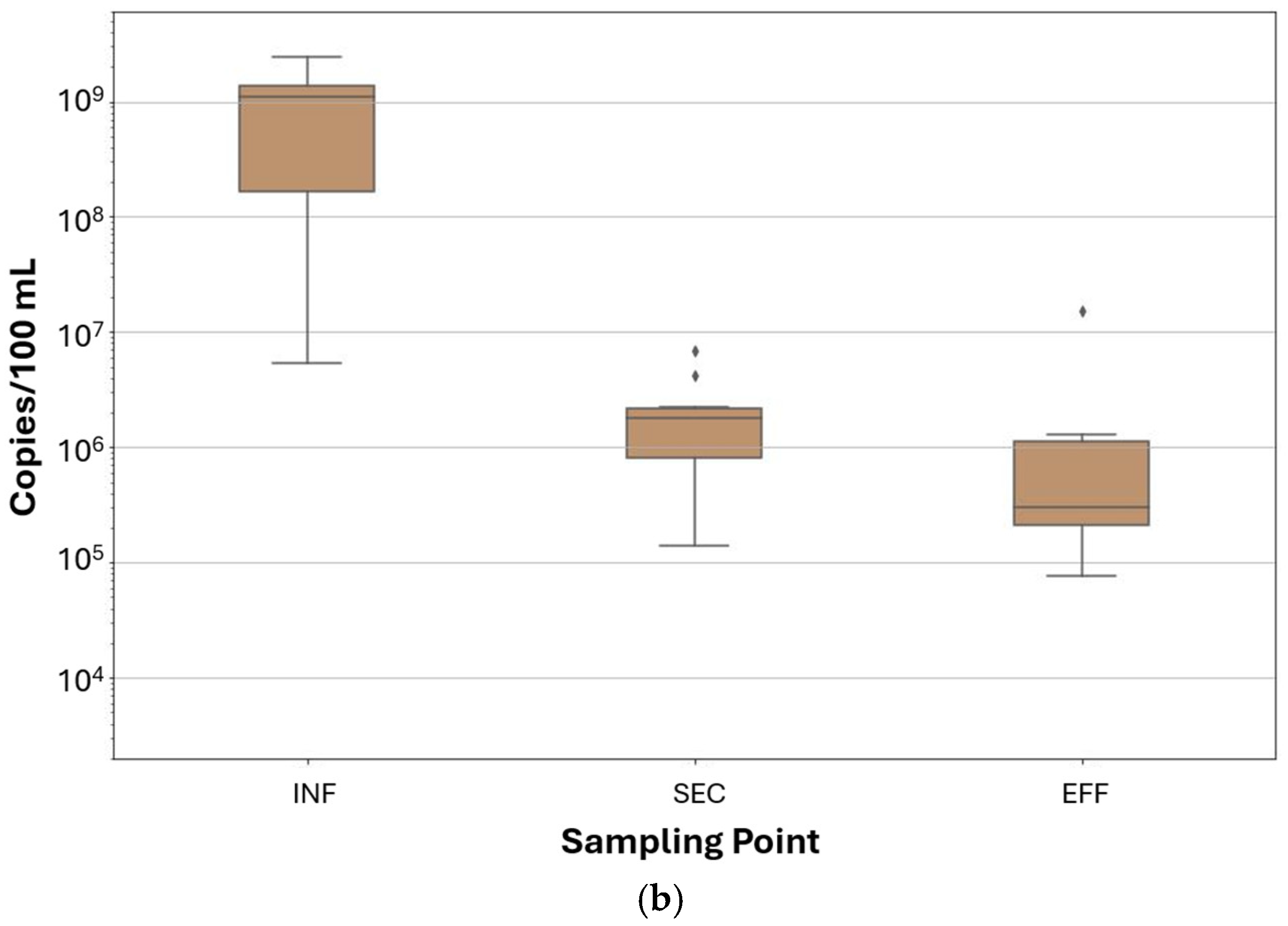
2.7. Molecular Analysis by ddPCR
2.8. Determination of Limits of Detection (LoD)
3. Results and Discussion
3.1. FVM Method Validation
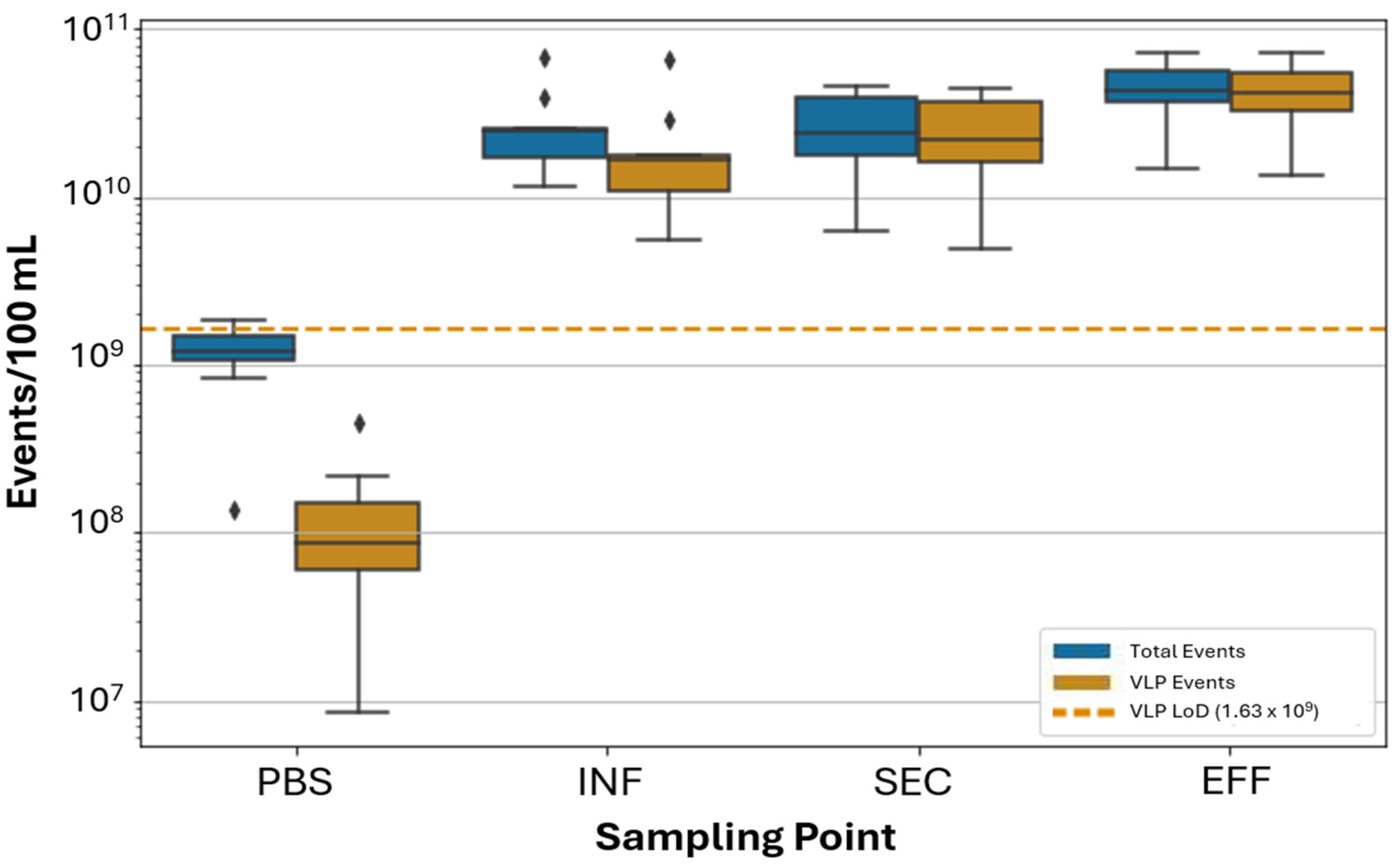
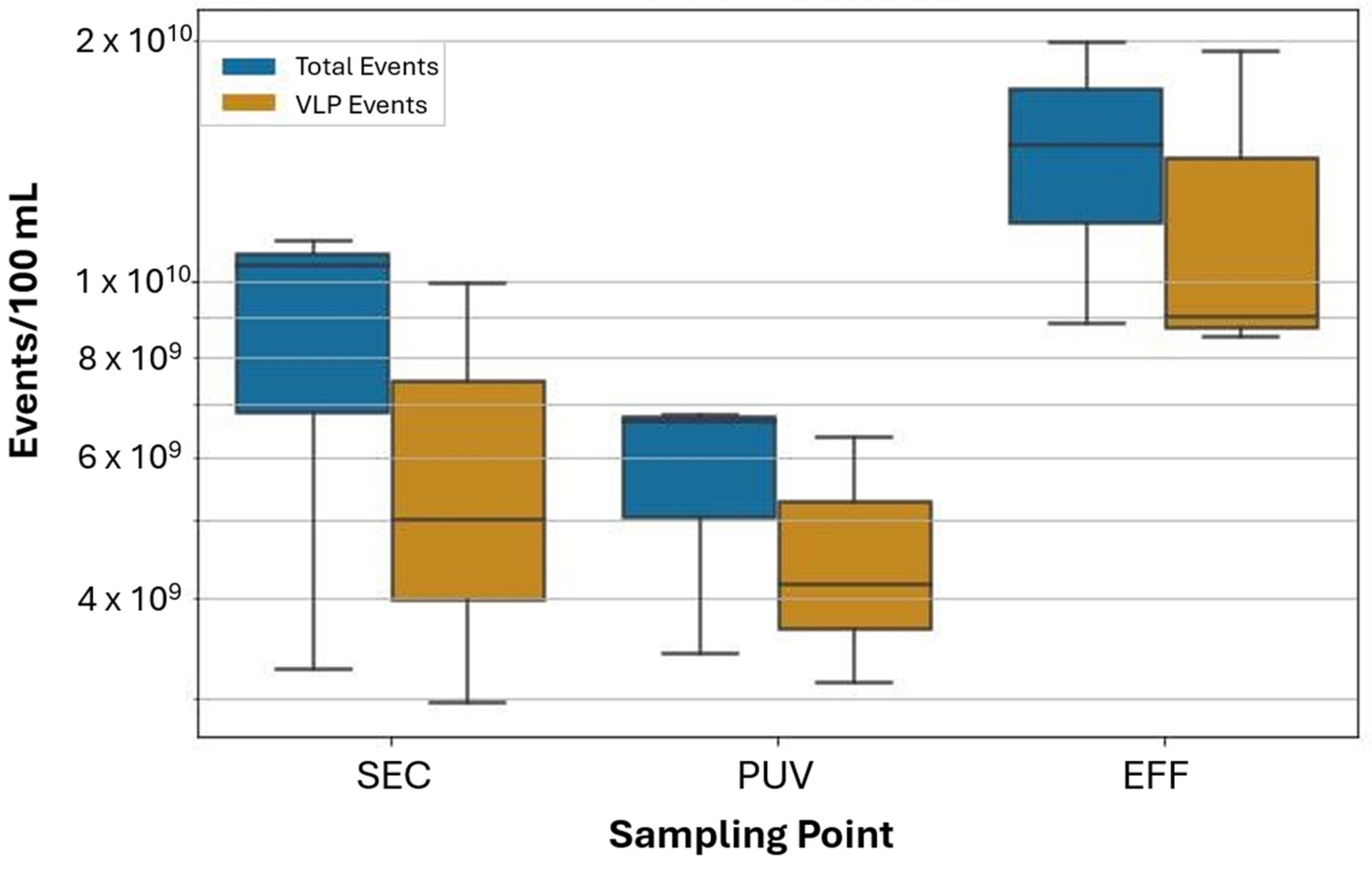
3.2. Application of FVM Protocol for Wastewater Monitoring
3.3. Comparison of VLPs by FVM with Somatic and F+ Specific Coliphage
3.4. Comparison Between FVM and ddPCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picot, J.; Guerin, C.L.; Le Van Kim, C.; Boulanger, C.M. Flow cytometry: Retrospective, fundamentals and recent instrumentation. Cytotechnology 2012, 64, 109–130. [Google Scholar] [CrossRef]
- Zamora, J.L.R.; Aguilar, H.C. Flow virometry as a tool to study viruses. Methods 2018, 134–135, 87–97. [Google Scholar] [CrossRef]
- Robinson, J.P. Flow cytometry: Past and future. Biotechniques 2022, 72, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Safford, H.R.; Bischel, H.N. Flow cytometry applications in water treatment, distribution, and reuse: A review. Water Res. 2019, 151, 110–133. [Google Scholar] [CrossRef]
- Hercher, M.; Mueller, W.; Shapiro, H.M. Detection and discrimination of individual viruses by flow cytometry. J. Histochem. Cytochem. 1979, 27, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.E.; Corzo, K.A.; Drissi, C.L.; Buckingham, J.M.; Thompson, C.P.; Helton, R.R. Estimates of viral abundance in soils are strongly influenced by extraction and enumeration methods. Biol. Fertil. Soils 2013, 49, 857–869. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, X.; Te, S.H.; Goh, S.G.; Mani, K.; He, Y.; Gin, K.Y.-H. Occurrence and distribution of viruses and picoplankton in tropical freshwater bodies determined by flow cytometry. Water Res. 2019, 149, 342–350. [Google Scholar] [CrossRef]
- Duhamel, S.; Jacquet, S. Flow cytometric analysis of bacteria- and virus-like particles in lake sediments. J. Microbiol. Methods 2006, 64, 316–332. [Google Scholar] [CrossRef]
- Heinrichs, M.E.; De Corte, D.; Engelen, B.; Pan, D. An advanced protocol for the quantification of marine sediment viruses via flow cytometry. Viruses 2021, 13, 102. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Hernandez, D.; Jiang, S.C. Near real-time flow cytometry monitoring of bacterial and viral removal efficiencies during water reclamation processes. Water 2016, 8, 464. [Google Scholar] [CrossRef]
- Safford, H.R.; Johnson, M.M.; Bischel, H.N. Flow virometry for water-quality assessment: Protocol optimization for a model virus and automation of data analysis. NPJ Clean Water 2023, 6, 28. [Google Scholar] [CrossRef]
- Dowdell, K.S.; Olsen, K.; Paz, E.F.M.; Sun, A.; Keown, J.; Lahr, R.; Steglitz, B.; Busch, A.; LiPuma, J.J.; Olson, T.; et al. Investigating the suitability of online flow cytometry for monitoring full-scale drinking water ozone system disinfection effectiveness. Water Res. 2024, 257, 121702. [Google Scholar] [CrossRef] [PubMed]
- Sano, D.; Amarasiri, M.; Hata, A.; Watanabe, T.; Katayama, H. Risk management of viral infectious diseases in wastewater reclamation and reuse: Review. Environ. Int. 2016, 91, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Corpuz, M.V.A.; Buonerba, A.; Vigliotta, G.; Zarra, T.; Ballesteros, F., Jr.; Campiglia, P.; Belgiorno, V.; Korshin, G.; Naddeo, V. Viruses in wastewater: Occurrence, abundance and detection methods. Sci. Total Environ. 2020, 6, 100238. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef]
- Ma, L.; Mao, G.; Liu, J.; Yu, H.; Gao, G.; Wang, Y. Rapid quantification of bacteria and viruses in influent, settled water, activated sludge and effluent from a wastewater treatment plant using flow cytometry. Water Sci. Technol. 2013, 68, 1763–1769. [Google Scholar] [CrossRef]
- Waturangi, D.E. Enumeration of Bacteriophages by Plaque Assay. In Methods in Molecular Biology; Springer: New York, NY, USA, 2024; pp. 147–153. [Google Scholar]
- Lipps, W.C.; Baxter, T.E.; Braun-Howland, E.B. Standard Methods for the Examination of Water and Wastewater, 24th ed.; American Public Health Association, American Water Works Association, Water Environment Federation; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Petterson, S.; Grøndahl-Rosado, R.; Nilsen, V.; Myrmel, M.; Robertson, L.J. Variability in the recovery of a virus concentration procedure in water: Implications for QMRA. Water Res. 2015, 87, 79–86. [Google Scholar] [CrossRef]
- Polanco, J.; Safarik, J.; Plumlee, M.H. Demonstrating Virus Log Removal Credit for Wastewater Treatment and Reverse Osmosis for Potable Reuse at OCWD; City of Fullerton Metropolitan Water District of Southern California Orange County Water District; The Water Research Foundation: Alexandria, VA, USA, 2022. [Google Scholar]
- Djoulissa, L.-J.; Tandukar, S.; Schmitz, B.W.; Innes, G.K.; Gerba, C.P.; Pepper, I.L.; Sherchan, S.P. Abundance and possibilities of crAssphage and PMMoV as a viral indicator in raw sewage in wastewater treatment plants. Sci. Total Environ. 2025, 963, 611–622. [Google Scholar] [CrossRef]
- Freen-Van Heeren, J.J. Flow-fish as a tool for studying bacteria, fungi and viruses. BioTech 2021, 10, 21. [Google Scholar] [CrossRef]
- Tabler, C.O.; Tilton, J.C. Analysis of Individual Viral Particles by Flow Virometry. Viruses 2024, 16, 802. [Google Scholar] [CrossRef]
- Bhat, T.; Cao, A.; Yin, J. Virus-like Particles: Measures and Biological Functions. Viruses 2022, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Dlusskaya, E.; Dey, R.; Pollard, P.C.; Ashbolt, N.J. Outer Limits of Flow Cytometry to Quantify Viruses in Water. ACS ES T Water 2021, 1, 1127–1135. [Google Scholar] [CrossRef]
- Strange, J.E.S.; Leekitcharoenphon, P.; Møller, F.D.; Aarestrup, F.M. Metagenomics analysis of bacteriophages and antimicrobial resistance from global urban sewage. Sci. Rep. 2021, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.D.; Dong, X.; Liu, Z.; Yang, Y.; Lin, J.G.; Li, M.; Gu, J.D.; Zhu, L.Z.; Zhao, H.P. A mixed blessing of viruses in wastewater treatment plants. Water Res. 2022, 215, 118237. [Google Scholar] [CrossRef]
- McCall, C.; Wu, H.; Miyani, B.; Xagoraraki, I. Identification of multiple potential viral diseases in a large urban center using wastewater surveillance. Water Res. 2020, 184, 116160. [Google Scholar] [CrossRef]
- Jiang, S.C.; Bischel, H.N.; Goel, R.; Rosso, D.; Sherchan, S.P.; Whiteson, K.L.; Yan, T.; Solo-Gabriele, H.M. Integrating Virus Monitoring Strategies for Safe Non-Potable Water Reuse. Water 2022, 14, 1187. [Google Scholar] [CrossRef]
- Regents of the University of California. National Pollutant Discharge Elimination System (NPDES); CA0077895; Regents of the University of California: Oakland, CA, USA, 2021. [Google Scholar]
- United States Environmental Protection Agency. Method 1602: Male-Specific (F+) and Somatic Coliphage in Water by Single Agar Layer (SAL) Procedure; EPA Document 821-R-01-029; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Wandro, S.; Ghatbale, P.; Attai, H.; Hendrickson, C.; Samillano, C.; Suh, J.; Dunham, S.J.; Pride, D.T.; Whiteson, K. Phage Cocktails Constrain the Growth of Enterococcus. Msystems 2022, 7, e00019-22. [Google Scholar] [CrossRef]
- Monsibais, A.N.; Tea, O.; Ghatbale, P.; Dunham, S.J.; Zünd, M.; Phan, J.; Lam, K.; Paulson, M.; Tran, N.; Suder, D.S.; et al. Enhanced Suppression of Stenotrophomonas maltophilia by a Three-Phage Cocktail: Genomic Insights and Kinetic Profiling. Antimicrob. Agents Chemother. 2024, e01162-24. [Google Scholar] [CrossRef]
- Santos, M.; Oliveira, H.; Pereira, J.L.; Pereira, M.J.; Gonçalves, F.J.; Vidal, T. Flow cytometry analysis of low/high DNA content (LNA/HNA) bacteria as bioindicator of water quality evaluation. Ecol. Indic. 2019, 103, 774–781. [Google Scholar] [CrossRef]
- Muralidharan, A.; Olson, R.; Bess, C.W.; Bischel, H.N. Equity-centered adaptive sampling in sub-sewershed wastewater surveillance using census data. Environ. Sci. 2024, 11, 136–151. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Kishida, N.; Konno, Y.; Katayama, H.; Asami, M.; Akiba, M. Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl. Environ. Microbiol. 2013, 79, 7413–7418. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Payyappat, S.; Cassidy, M.; Besley, C. A duplex PCR assay for the simultaneous quantification of Bacteroides HF183 and crAssphage CPQ_056 marker genes in untreated sewage and stormwater. Environ. Int. 2019, 126, 252–259. [Google Scholar] [CrossRef]
- Caruso, A.G.; Bertacca, S.; Parrella, G.; Rizzo, R.; Davino, S.; Panno, S. Tomato brown rugose fruit virus: A pathogen that is changing the tomato production worldwide. Ann. Appl. Biol. 2022, 181, 258–274. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar]
- Qiao, Z.; Ye, Y.; Chang, P.H.; Thirunarayanan, D.; Wigginton, K.R. Nucleic Acid Photolysis by UV254 and the Impact of Virus Encapsidation. Environ. Sci. Technol. 2018, 52, 10408–10415. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.E.; Rodriguez, R.A.; Hawkins, M.A.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Comparison of UV-induced inactivation and RNA damage in MS2 phage across the germicidal UV spectrum. Appl. Environ. Microbiol. 2016, 82, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Ballesté, E.; Blanch, A.R.; Muniesa, M.; García-Aljaro, C.; Rodríguez-Rubio, L.; Martín-Díaz, J.; Pascual-Benito, M.; Jofre, J. Bacteriophages in sewage: Abundance, roles, and applications. FEMS Microbes 2022, 3, xtac009. [Google Scholar] [CrossRef]
- Wang, S.; Tian, R.; Bi, Y.; Meng, F.; Zhang, R.; Wang, C.; Wang, D.; Liu, L.; Zhang, B. A review of distribution and functions of extracellular DNA in the environment and wastewater treatment systems. Chemosphere 2024, 359, 142264. [Google Scholar] [CrossRef]
- Wu, H.; Brighton, K.; Chen, J.; Shuai, D.; Aw, T.G. Quantification of Particle-Associated Viruses in Secondary Treated Wastewater Effluent. Food Environ. Virol. 2025, 17, 19. [Google Scholar] [CrossRef]
- Renner, T.M.; Tang, V.A.; Burger, D.; Langlois, M.-A. Intact Viral Particle Counts Measured by Flow Virometry Provide Insight into the Infectivity and Genome Packaging Efficiency of Moloney Murine Leukemia Virus. J. Virol. 2020, 94, e01600-19. [Google Scholar] [CrossRef]
- Gaudin, R.; Barteneva, N.S. Sorting of small infectious virus particles by flow virometry reveals distinct infectivity profiles. Nat. Commun. 2015, 6, 6022. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J. Extracell. Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef] [PubMed]
- Dlusskaya, E. Fluorescence-Based Methods of Virus Estimation in Wastewater: Current Challenges and Future Perspectives; University of Alberta: Edmonton, Alberta, 2021. [Google Scholar]
| Assay (Target) | Sampling Point | Equivalent Wastewater Volume | # Replicates | LOD |
|---|---|---|---|---|
| Somatic Coliphage (PFU) | INF | 5 μL | 4 | 2 × 104 PFU/100 mL |
| SEC | 10 mL | 1 | 1 × 101 PFU/100 mL | |
| EFF | 100 mL | 1 | 1 × 100 PFU/100 mL | |
| (F+) Specific Coliphage (PFU) | INF | 5 μL | 4 | 2 × 104 PFU/100 mL |
| SEC | 100 mL | 1 | 1 × 100 PFU/100 mL | |
| EFF | 100 mL | 1 | 1 × 100 PFU/100 mL | |
| Flow Virometry (VLPs) | INF | 0.22 µL | 4 | 1.63 × 109 VLP/100 mL |
| SEC | 0.22 µL | 4 | 1.63 × 109 VLP/100 mL | |
| EFF | 0.22 µL | 4 | 1.63 × 109 VLP/100 mL | |
| ddPCR (PMMoV) | INF | 2.78 µL | 3 | 5.54 × 105 gc/100 mL |
| SEC | 27.8 µL | 3 | 5.54 × 104 gc/100 mL | |
| EFF | 278 µL | 3 | 5.54 × 103 gc/100 mL | |
| ddPCR (CrAss) | INF | 2.78 μL | 3 | 6.81 × 105 gc/100 mL |
| SEC | 27.8 μL | 3 | 6.81 × 104 gc/100 mL | |
| EFF | 278 μL | 3 | 6.81 × 103 gc/100 mL | |
| ddPCR (ToBRFV) | INF | 2.78 μL | 3 | 4.68 × 105 gc/100 mL |
| SEC | 27.8 μL | 3 | 4.68 × 104 gc/100 mL | |
| EFF | 278 μL | 3 | 4.68 × 103 gc/100 mL |
| VLP Events/100 mL (n = 11, ±1 SD) | PFU/100 mL (n = 2) |
|---|---|
| 3.2 × 1010 (±3.5 × 1010) | ~1.3 × 108 |
| 2.8 × 109 (±3.1 × 109) | ~1.3 × 107 |
| 5.0 × 108 (±5.4 × 108) | ~1.3 × 106 |
| Sample | Un-Spiked Mean (±1 SD) | Spiked Mean (±1 SD) | T-Statistic | p-Value |
|---|---|---|---|---|
| PBS (control) | 1.27 × 108 (±1.24 × 108) | 3.20 × 1010 (±3.53 × 1010) | −3.00 | 0.013 |
| INF | 1.94 × 1010 (±1.68 × 1010) | 7.86 × 1010 (±6.95 × 1010) | −3.01 | 0.013 |
| SEC | 2.49 × 1010 (±1.41 × 1010) | 7.64 × 1010 (±8.13 × 1010) | −2.13 | 0.059 |
| EFF | 4.3 × 1010 (±1.74 × 1010) | 9.60 × 1010 (±7.39 × 1010) | −2.56 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, M.M.; Bess, C.W.; Olson, R.; Bischel, H.N. Flow Virometry in Wastewater Monitoring: Comparison of Virus-like Particles to Coliphage, Pepper Mild Mottle Virus, CrAssphage, and Tomato Brown Rugose Fruit Virus. Viruses 2025, 17, 575. https://doi.org/10.3390/v17040575
Johnson MM, Bess CW, Olson R, Bischel HN. Flow Virometry in Wastewater Monitoring: Comparison of Virus-like Particles to Coliphage, Pepper Mild Mottle Virus, CrAssphage, and Tomato Brown Rugose Fruit Virus. Viruses. 2025; 17(4):575. https://doi.org/10.3390/v17040575
Chicago/Turabian StyleJohnson, Melis M., C. Winston Bess, Rachel Olson, and Heather N. Bischel. 2025. "Flow Virometry in Wastewater Monitoring: Comparison of Virus-like Particles to Coliphage, Pepper Mild Mottle Virus, CrAssphage, and Tomato Brown Rugose Fruit Virus" Viruses 17, no. 4: 575. https://doi.org/10.3390/v17040575
APA StyleJohnson, M. M., Bess, C. W., Olson, R., & Bischel, H. N. (2025). Flow Virometry in Wastewater Monitoring: Comparison of Virus-like Particles to Coliphage, Pepper Mild Mottle Virus, CrAssphage, and Tomato Brown Rugose Fruit Virus. Viruses, 17(4), 575. https://doi.org/10.3390/v17040575






