Abstract
Southern rice black-streaked dwarf virus (SRBSDV), a new member of the genus Fijivirus, is a double-stranded RNA virus known to lack poly(A) tails. We now showed that some of SRBSDV mRNAs were indeed polyadenylated at the 3' terminus in plant hosts, and investigated the nature of 3' poly(A) tails. The non-abundant presence of SRBSDV mRNAs bearing polyadenylate tails suggested that these viral RNA were subjected to polyadenylation-stimulated degradation. The discovery of poly(A) tails in different families of viruses implies potentially a wide occurrence of the polyadenylation-assisted RNA degradation in viruses.
1. Introduction
RNA of many eukaryotic viruses, ranging from DNA to RNA viruses, have 3' poly(A) tails [1], which are synthesized not only posttranscriptionally, but also by direct transcription from the poly(U) stretched template strand [2,3,4,5]. Regardless of synthesis mechanism used, the viral poly(A) tails have been considered to play crucial roles in RNA stability and translation, resembling roles of the stable poly(A) tails in eukaryotic mRNA [6,7]. Until recently, the function of poly(A) tails in destabilizing the viral RNA was revealed. The viral mRNA containing poly(A) or poly(A)-rich tails were detected in HeLa cells infected with Vaccinia virus (a double-stranded [ds] DNA virus) [8]. Furthermore, the polyadenylate tails were also found in Tobacco mosaic virus (TMV), Cucumber mosaic virus (CMV), Odontoglossum ring-spot virus (ORSV), Cucumber green mottle mosaic virus (CGMMV), Tobacco rattle virus (TRV), Turnip crinkle virus (TCV) and Tobacco necrosis virus (TNV) [9], seven positive-strand RNA viruses known to lack poly(A) tails and terminate 3'-termini with tRNA-like structure (TLS) or non-TLS heteropolymeric sequence [6]. The presence of poly(A) tails suggests that these viral RNAs are subjected to poly(A)-stimulated degradation. In this paper, the poly(A) and poly(A)-rich tails were first reported at the 3'-termini of the mRNAs of a dsRNA virus, Southern rice black-streaked dwarf virus (SRBSDV), generally recognized to lack poly(A) tails.
SRBSDV has been proposed as a new member in the genus Fijivirus of the family Reoviridae [10], which causes a serious rice disease in South China and Vietnam in recent years [11,12]. SRBSDV is most closely related to but distinct from Rice black-streaked dwarf virus (RBSDV), which is also a member of the Fijivirus genus [10,13]. SRBSDV genome contains 10 segments, named as S1-S10 in the descending order of molecular weight. Comparison of 10 genomic segments of SRBSDV with their counterparts in RBSDV suggests that SRBSDV encodes 13 open reading frames (ORFs) and possesses 6 putative structural proteins (P1, P2, P3, P4, P8, and P10) and 7 putative nonstructural proteins (P5-1, P5-2, P6, P7-1, P7-2, P9-1 and P9-2) [13]. At present, the functions of partial genes have been studied. The P6, encoded by S6, has been identified as an RNA silencing suppressor [14]. P7-1 induces the formation of tubules as vehicles for rapid spread of virions through basal lamina from midgut epithelium in its vector, the white-backed planthopper [15]. P9-1 is essential for viroplasm formation and viral replication in non-host insect cells and vector insects [16]. However, no reports are available to date to assign functions to the proteins encoded by other ORFs. The putative function of these proteins can only be postulated based on their RBSDV homologs. P1, P2, P3 and P4 are putative RNA-dependent RNA polymerase (RdRp), core protein, capping enzyme and outer-shell B-spike protein, respectively [13,17]. P8 and P10 are putative core and major outer capsid proteins, respectively [13,18]. SRBSDV mRNAs were considered to lack of poly(A) tails at the 3'-ends. However, in previous experiments, all 13 ORFs of the 10 RNA segments could be amplified via RT-PCR using oligo(dT)18 to prime cDNA synthesis as templates [19], suggesting that each SRBSDV mRNA might bear a potential poly(A) tail at the 3' terminus. In this paper, we confirmed that some of SRBSDV mRNAs were indeed polyadenylated at the 3' terminus in plant hosts.
2. Materials and Methods
2.1 Virus and RNA Extraction
SRBSDV isolate used in the experiment was obtained from rice and maize plants showing typical dwarf symptoms with white waxy galls in 2014 in 8 counties of 4 provinces in China, including Yunnan, Guizhou, Hunan, and Jiangxi provinces. Total RNA from infected rice and maize leaf and stem tissue were extracted following the standard protocol of TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The isolate was identified as SRBSDV excluding RBSDV by reverse transcription RT-PCR using specific primers for distinguishing the two viruses [20].
2.2 Rapid Amplification of cDNA End (RACE) PCR
To confirm characterization of the polyadenylate tails associated with viral mRNAs, the 3' Rapid Amplification of cDNA End (RACE) PCR was performed using BD SMART™ RACE cDNA Amplification Kit (TaKaRa, Dalian, Liaoning, China). In this case, reverse transcription reactions were performed using total RNA (respectively from infected rice and maize) as templates and adapter-oligo(dT) primer (P1) (Table 1) to prime first cDNA strand synthesis. 10 specific upstream primers and 10 nested primers respectively corresponding to SRBSDV each mRNA were designed according to China isolate HuNyy sequence information (GenBank No. JQ034348-JQ034357) (Table 1). Each of upstream primers was paired with adapter primer P2 (as downstream primer) for the 1st PCR amplification using PrimeSTAR HS DNA polymerase (TaKaRa) and cDNA as template. The PCR products from the 1st PCR reaction were subjected to a subsequent the 2nd PCR run with nested primers and adapter primer P3 (Figure 1A). The amplified products were analyzed by 1.5% agarose gel electrophoresis, and the resulting bands, in agreement with the predicted sizes, were individually cloned into pGEM-T Easy vector (Promega, Madison, USA) and subjected to sequence analysis. Approximately 5–10 clones from each isolate were randomly selected and sequenced.

Table 1.
PCR primers used in the experiment.
| Primer | Sequence (5'→3') | Target | Reference GenBank No. |
|---|---|---|---|
| S1-F | TCAGTGCTCAAGGCTCACAAGATTGAAG | S1-mRNA | JQ034348 |
| S1-nested-F | ATTCATGAACTTAATGGGCGCAGAGTG | ||
| S2-F | CGGCACATCTTCACCCGCAGACTTC | S2-mRNA | JQ034349 |
| S2-nested-F | CTGATGAATTGCTCGACCGTTACATTAG | ||
| S3-F | GATGGGATTAGCGAAATTGCATTTGGAG | S3-mRNA | JQ034350 |
| S3-nested-F | TGCATGGACATTCATTTTCAGATCAAG | ||
| S4-F | TAGATTTTGTTATTCCCGGTGTTCGAGAAG | S4-mRNA | JQ034351 |
| S4-nested-F | AGTGCGGATGTGGCTGCAGATAAATTC | ||
| S5-F | TGTGATCAGTGCCATGTCCACTAGCATC | S5-mRNA | JQ034352 |
| S5-nested-F | AATCATCCCTGTGCGCTTCGACTTAG | ||
| S6-F | CGATACTCTGATGAAACAGGCGAAGCTC | S6-mRNA | JQ034353 |
| S6-nested-F | TGAGAACCAATGGAGCGCGTATGGA | ||
| S7-F | ACTACTTCAGCTGAAGATGTCGACGCAC | S7-mRNA | JQ034354 |
| S7-nested-F | TTGGCAAGCGATGGAAAGAAGATGG | ||
| S8-F | CGTATTGGACGATGAGCGCAACTTTG | S8-mRNA | JQ034355 |
| S8-nested-F | TGAATTAGCGTTCGTACCTCATTCGCTG | ||
| S9-F | TTGGACTTGGCTAACTACGTTCGACAAC | S9-mRNA | JQ034356 |
| S9-nested-F | GGAATTGGATGATCGAGTTGAAAAATTGG | ||
| S10-F | CTCCCTGCATCGATTACATCAAACTTGG | S10-mRNA | JQ034357 |
| S10-nested-F | GCCAACAATTTATTGAAGGCGGATCG | ||
| S10-NVP | TTCCATCTCTATCATTCAGTCAAG | S10-mRNA | |
| Adapter-oligo(dT) (P1) | GCTGTCAACGATACGCTACGTAACGGCATGACAGTG(T)18VN | Poly(A) tails | |
| Adapter primer P2 | GCTGTCAACGATACGCTACGTAACG | Adapter | |
| Adapter primer P3 | CGCTACGTAACGGCATGACAGTG | Adapter |
3. Results and Discussion
After 3' RACE, the 3'-termini sequences of viral mRNAs were obtained, and the results indicated that SRBSDV mRNAs indeed possessed ploy(A) or poly(A)-rich tails in plant hosts. Taking S10-mRNA as an example to analyze the nature of poly(A) and poly(A)-rich tails, a total of 42 polyadenylated viral mRNA molecules were cloned from rice and maize plants. In addition to 10 mRNAs bearing poly(A) tails exclusively comprised of adenosines, a large number of mRNAs possessed poly(A)-rich tails (Figure 1B). Notably, the heterogeneity of these poly(A)-rich tails was confined to their 5' ends, and they all terminated in homogenous adenosines (17–23 nt) (Figure 1B), which was possibly due to the 3' bias of oligo(dT)-dependent reverse transcription. Most poly(A)-rich tails were not at the downstream of S10-mRNA entire 3' untranslated region (UTR), and replaced partial 3' UTR sequences. For example, the tail of isolate LX-1 replaced 3' UTR sequence of S10-mRNA from the nucleotide 1753 (Figure 1B). In some poly(A)-rich tails (isolate JH-1, LX-1, PT-1, PT-5, YJ-1 and YJ-4), there were more non-viral nucleotides (35–208 nt) preceded polyadenylates, which was considered to originate from host plants. In order to further certify the presence of poly(A) tails and exclude non-specificity of reverse transcription reaction, these non-viral nucleotides was used to design downstream primers (e.g., S10-NVP) to perform PCR with upstream primer from S10 (Figure 1A), and the result of amplification was positive (data no shown), indicating sufficiently the existence of mRNA bearing ployadenylate tails. Moreover, poly(A) or poly(A)-rich tails were also discovered at the 3'-ends of viral S1-S9 mRNAs (Figure 2). All amplified products based on 3' RACE were weak (data no shown), implying that a small fraction of SRBSDV mRNAs was polyadenylated.
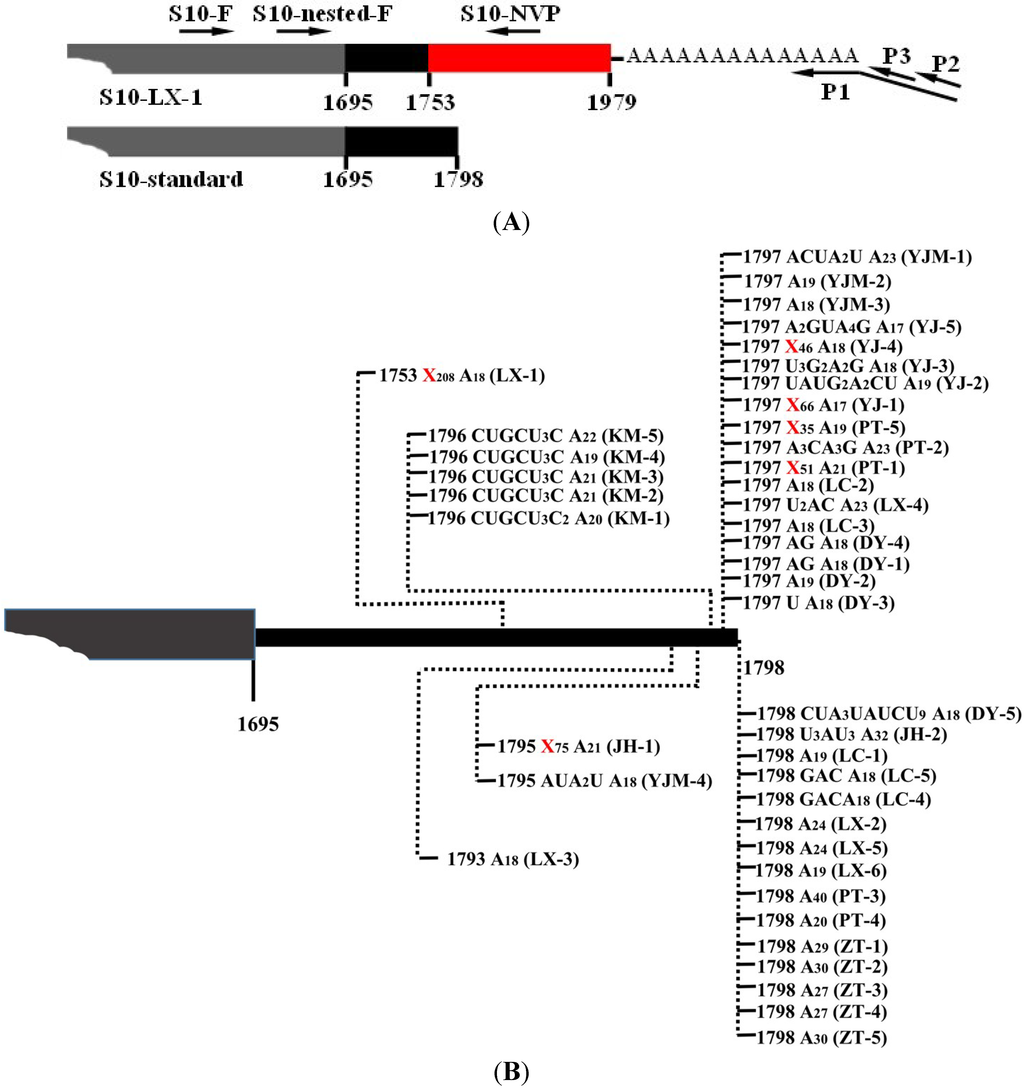
Figure 1.
The 3' Rapid Amplification of cDNA End (RACE) detection of the polyadenylated southern rice black-streaked dwarf virus (SRBSDV) S10-mRNA. (A) Schematic diagram of the primers in S10-mRNA. The primers are displayed as arrowheads (Table 1), and the gray box, black box and red box indicate respectively partial ORF, 3' UTR and non-viral nucleotides in S10-mRNA. (B) Nature of 3' polyadenylate tails associated with S10-mRNA in plant. The poly(A) and poly(A)-rich tails of S10-mRNA are schematically presented, and vertical dashed lines with numbers indicate the exact positions of polyadenylate tails. Nucleotide compositions of the tails are shown, and long non-viral nucleotides are shown with Xn. Isolate names in parentheses, DY: Duyun, PT: Pingtang, Guizhou province; JH: Jianghua, Hunan province; KM: Kunming, LC: Longchuan, YJ (YJM): Yingjiang, ZT: Zhaotong, Yunnan province; LX: Luxi, Jiangxi province. The number after abbreviation is the numbering of isolate clones. YJM isolates are from maize, and others are from rice.
Figure 1.
The 3' Rapid Amplification of cDNA End (RACE) detection of the polyadenylated southern rice black-streaked dwarf virus (SRBSDV) S10-mRNA. (A) Schematic diagram of the primers in S10-mRNA. The primers are displayed as arrowheads (Table 1), and the gray box, black box and red box indicate respectively partial ORF, 3' UTR and non-viral nucleotides in S10-mRNA. (B) Nature of 3' polyadenylate tails associated with S10-mRNA in plant. The poly(A) and poly(A)-rich tails of S10-mRNA are schematically presented, and vertical dashed lines with numbers indicate the exact positions of polyadenylate tails. Nucleotide compositions of the tails are shown, and long non-viral nucleotides are shown with Xn. Isolate names in parentheses, DY: Duyun, PT: Pingtang, Guizhou province; JH: Jianghua, Hunan province; KM: Kunming, LC: Longchuan, YJ (YJM): Yingjiang, ZT: Zhaotong, Yunnan province; LX: Luxi, Jiangxi province. The number after abbreviation is the numbering of isolate clones. YJM isolates are from maize, and others are from rice.
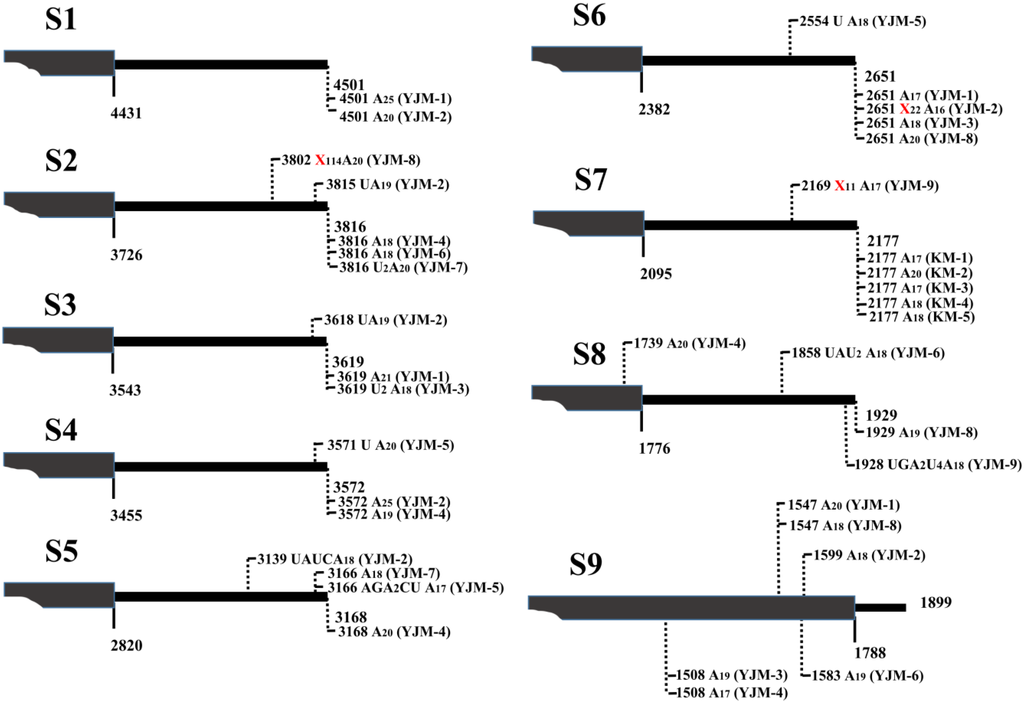
Figure 2.
Nature of 3' polyadenylate tails associated with SRBSDV S1-S9 mRNAs in plant. The poly(A) and poly(A)-rich tails of S1-S9 mRNAs are schematically presented respectively, and vertical dashed lines with numbers indicate the exact positions of polyadenylate tails. Nucleotide compositions of the tails are shown, and long non-viral nucleotides are shown with Xn. Isolate names in parentheses, KM: Kunming (from rice), YJM: Yingjiang (from maize), Yunnan province. The number after abbreviation is the numbering of isolate clones.
Figure 2.
Nature of 3' polyadenylate tails associated with SRBSDV S1-S9 mRNAs in plant. The poly(A) and poly(A)-rich tails of S1-S9 mRNAs are schematically presented respectively, and vertical dashed lines with numbers indicate the exact positions of polyadenylate tails. Nucleotide compositions of the tails are shown, and long non-viral nucleotides are shown with Xn. Isolate names in parentheses, KM: Kunming (from rice), YJM: Yingjiang (from maize), Yunnan province. The number after abbreviation is the numbering of isolate clones.
To our knowledge, dsRNA viruses are lack of poly(A) tails at the 3'-ends of the genome segments and their mRNAs. Interestingly, in this paper, we demonstrated that some viral mRNA molecules were polyadenylated at their 3'-terminus in plant cells infected with SRBSDV (a dsRNA virus). Besides their crucial roles for mRNA stability and translation efficiency, the polyadenylate tails were recently described as involved in viral RNA degradation [8]. The Poly(A)-stimulated RNA degradation occurs throughout the prokaryotic and eukaryotic cells [21,22,23,24,25,26]. Generally, the degradation process comprises three sequential steps: endonucleolytic cleavage, addition of polyadenylate tails to the cleavage products, and exonucleolytic degradation [21,26,27]. The transient poly(A) or poly(A)-rich stretches can act as landing sites to recruit 3'-5' exoribonucleases for further degradation [21,22,26,27], which might be one of ancestral roles of polyadenylation. This evolutionarily conserved mechanism has been confirmed to play critical roles in rapidly removing redundant RNAs in cells, thereby maintaining the stability of gene expression [26,28,29].
In this study, the non-abundant presence of SRBSDV mRNAs bearing polyadenylate tails was considered to represent degradation intermediates of an RNA decay pathway, rather than to convey protection to mRNAs. Recently, a dsDNA virus, Vaccinia virus, was linked with the conserved RNA degradation mechanism, and non-abundant, fragmented viral mRNAs bearing poly(A) or poly(A)-rich tails were detected in human cells infected with this virus [8]. Such polyadenylation-stimulated RNA degradation was also found in seven positive-strand RNA viruses from distinct virus families and genera known to lack poly(A) tails [9]. The discovery of poly(A) tails in three different types of viruses (positive-strand RNA virus, dsDNA and dsRNA virus) implies potentially a wide occurrence of the polyadenylation-assisted RNA degradation in viruses, which might represent a yet-unknown interaction between virus and host.
Acknowledgments
This research was supported by the National Natural Science Foundation of China Project (grant no. 31360528), the Science and Technology Project of Guizhou Province (QKH Major Project, grant no. [2012]6012), the Scientific Research Foundation of Guizhou University (RJH Project, grant no. 2013-28), the Brainstorm Project on Social Development by the Department of Science and Technology of Guizhou Province (grant no. SY[2010]3004), and the Jiangsu Agricultural Scientific Self-innovation Fund (grant no. CX[14]5019).
Author Contributions
P.H., S.L. and M.H. designed the research. P.H., M.H. and Z.J. performed experiments. S.L. and P.H. wrote and finalized the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: SanDiego, CA, USA, 2012. [Google Scholar]
- Sanfaçon, H.; Brodmann, P.; Hohn, T. A dissection of the cauliflower mosaic virus polyadenylation signal. Genes Dev. 1991, 5, 141–149. [Google Scholar] [CrossRef]
- Weichs an der Glon, C.; Ashe, M.; Eggermont, J.; Proudfoot, N.J. Tat-dependent occlusion of the HIVpoly(A) site. EMBO J. 1993, 12, 2119–2128. [Google Scholar]
- Poon, L.L.; Pritlove, D.C.; Fodor, E.; Brownlee, G.G. Direct evidence that the poly(A) tail of influenza A virus mRNA is synthesized by reiterative copying of a U track in the virion RNA template. J. Virol. 1999, 73, 3473–3476. [Google Scholar] [PubMed]
- Steil, B.P.; Kempf, B.J.; Barton, D.J. Poly(A) at the 3' end of positive-strand RNA and VPg-linked poly(U) at the 5' end of negative-strand RNA are reciprocal templates during replication of poliovirus RNA. J. Virol. 2010, 84, 2843–2858. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.W. Funcations of the 3'-untranslated regions of positive strand RNA viral genomes. Annu. Rev. Phytopathol. 1999, 37, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.N.; Fearns, R. How RNA viruses maintain their genome integrity. J. Gen. Virol. 2010, 91, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Slomovic, S.; Fremder, E.; Staals, R.H.; Pruijn, G.J.; Schuster, G. Addition of poly(A) and poly(A)-rich tails during RNA degradation in the cytoplasm of human cells. Proc. Natl. Acad. Sci. USA 2010, 107, 7407–7412. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Zhang, Y.Q.; Zhang, C.; Pei, X.W.; Wang, Z.X.; Jia, S.R. Presence of poly(A) and poly(A)-rich tails in a positive-strand RNA virus known to lack 3' poly(A) tails. Virology 2014, 454–455, 1–10. [Google Scholar]
- Zhou, G.H.; Wen, J.J.; Cai, D.J.; Li, P.; Xu, D.L.; Zhang, S.G. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chin. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Li, S.; Gao, R.Z.; Sun, F.; Liu, W.C.; Zhou, G.H.; Wu, J.X.; Zhou, X.P.; Zhou, Y.J. Distribution and genetic diversity of Southern rice black-streaked dwarf virus in China. Virol. J. 2013, 10, 307. [Google Scholar]
- Hoang, A.T.; Zhang, H.M.; Yang, J.; Chen, J.P.; Hébrard, E.; Zhou, G.H.; Vinh, V.N.; Cheng, J.A. Identification, characterization, and distribution of Southern rice black-streaked dwarf virus in Vietnam. Plant Dis. 2011, 95, 1063–1069. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Zhou, G.H.; Zhang, H.M.; Chen, J.P.; Adams, M.J. The complete genome sequence of two isolates of Southern rice black-streaked dwarf virus, a new member of the genus Fijivirus. J. Phytopathol. 2010, 158, 733–737. [Google Scholar] [CrossRef]
- Lu, Y.H.; Zhang, J.F.; Xiong, R.Y.; Xu, Q.F.; Zhou, Y.J. Identification of an RNA silencing suppressor encoded by Southern rice black-streaked dwarf virus S6. Sci. Agri. Sin. 2011, 44, 2909–2917. [Google Scholar]
- Jia, D.S.; Mao, Q.Z.; Chen, H.Y.; Wang, A.M.; Liu, Y.Y.; Wang, H.T.; Xie, L.H.; Wei, T.Y. Virus-induced tubule: A vehicle for rapid spread of virions through basal lamina from midgut epithelium in the insect vector. J. Virol. 2014, 88, 10488–10500. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.S.; Chen, H.Y.; Zheng, A.L.; Chen, Q.; Liu, Q.F.; Xie, L.H.; Wu, Z.J.; Wei, T.Y. Development of an insect vector cell culture and RNA interference system to investigate the functional role of fijivirus replication protein. J. Virol. 2012, 86, 5800–5807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Chen, J.P.; Adams, M.J. Molecular characterisation of segments 1 to 6 of Rice black-streaked dwarf virus from China provides the complete genome. Arch. Virol. 2001, 146, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Isogai, M.; Uyeda, I.; Lee, B.C. Detection and assignment of proteins encoded by Rice black streaked dwarf fijivirus S7, S8, S9 and S10. J. Gen. Virol. 1998, 79, 1487–1494. [Google Scholar] [PubMed]
- He, P.; Liu, J.J.; He, M.; Wang, Z.C.; Chen, Z.; Guo, R.; Correll, J.C.; Yang, S.; Song, B.A. Quantitative detection of relative expression levels of the whole genome of Southern rice black-streaked dwarf virus and its replication in different hosts. Virol. J. 2013, 10, 136. [Google Scholar] [CrossRef]
- Ji, Y.H.; Gao, R.Z.; Zhang, Y.; Cheng, Z.B.; Zhou, T.; Fan, Y.J.; Zhou, Y.J. A simplified method for quick detection of Rice black-streaked dwarf virus and Southern rice black-streaked dwarf virus. Chin. J. Rice Sci. 2011, 25, 91–94. [Google Scholar]
- Slomovic, S.; Portnoy, V.; Liveanu, V.; Schuster, G. RNA polyadenylation in prokaryotes and organelles; different tails tell different tales. Crit. Rev. Plant Sci. 2006, 25, 65–77. [Google Scholar] [CrossRef]
- Regnier, P.; Hajnsdorf, E. Poly(A)-assisted RNA decay and modulators of RNA stability. Prog. Mol. Biol. Transl. Sci. 2009, 85, 137–185. [Google Scholar] [PubMed]
- Kuai, L.; Das, B.; Sherman, F. A nuclear degradation pathway controls the abundance of normal mRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2005, 102, 13962–13967. [Google Scholar] [CrossRef]
- Wyers, F.; Rougemaille, M.; Badis, G.; Rousselle, J.C.; Dufour, M.E.; Boulay, J.; Régnault, B.; Devaux, F.; Namane, A.; Séraphin, B.; et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 2005, 121, 725–737. [Google Scholar]
- Nakamura, R.; Takeuchi, R.; Takata, K.; Shimanouchi, K.; Abe, Y.; Kanai, Y.; Ruike, T.; Ihara, A.; Sakaguchi, K. TRF4 is involved in polyadenylation of snRNAs in Drosophila melanogaster. Mol. Cell Biol. 2008, 28, 6620–6631. [Google Scholar] [CrossRef]
- Lange, H.; Sement, F.M.; Canaday, J.; Gagliardi, D. Polyadenylation-assisted RNA degradation processes in plants. Trends Plant Sci. 2009, 14, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Slomovic, S.; Portnoy, V.; Yehudai-Resheff, S.; Bronshtein, E.; Schuster, G. Polynucleotide phosphorylase and the archaeal exosome as poly(A)-polymerases. Biochim. Biophys. Acta 2008, 1779, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Houseley, J.; Tollervey, D. The many pathways of RNA degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Gagliardi, D. The exosome and 3'-5' RNA degradation in plants. Adv. Exp. Med. Biol. 2011, 702, 50–62. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
