Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs
Abstract
:1. Introduction
2. History and Definition of Cocrystals
- (1)
- The neutrality of the ingredients,
- (2)
- The solid state of the components in ambient conditions, and
- (3)
- The homogeneity of the crystalline material and the stoichiometry of the components.
3. Intermolecular Interactions in Cocrystals
- (1)
- All good proton donors that are available in the molecule will be used for hydrogen bond formation in the crystalline structure of the compound.
- (2)
- All good acceptors will be used for hydrogen bond formation, when there are available hydrogen bond donors.
- (3)
- Intramolecular hydrogen bonds of six-member rings preferably form bimolecular hydrogen bonds.
- (4)
4. Prediction and Screening of Cocrystal Formation
5. Cocrystals of Direct Pharmaceutical Interest
- Entresto® (sacubitril-valsartan), which was approved by the FDA in 2015 for the treatment of heart failure. It is the first drug in a class combining valsartan (angiotensin receptor) and sacubitril (inhibitor neprilysinis) to reduce cardiovascular mortality [50].
- Lexapro® (escitalopram oxalate), which was approved in 2009 for the treatment of depression and anxiety. It belongs to the selective serotonin reuptake inhibitors [23].
- Depakote® (valproate sodium cocrystal with valproic acid). It is used to treat seizure disorders, manic depression, and to prevent disorders [51].
6. Drug Properties That Can Be Altered by Cocrystallization
- (i)
- Relative Humidity (RH): In solid forms, changes in RH must be considered when developing a cocrystal. Studies on automated humidity sorption/desorption are usually performed to determine the “problematic” conditions and give directions for more detailed studies, if necessary. Moisture uptake can be controlled through the exposure of the cocrystal to a particular RH using an appropriate humidity chamber and then analyzing the sample after reaching equilibrium. A systematic study in which caffeine was cocrystallized with various carboxylic acids, namely oxalic, malonic, maleic and glutaric acid, showed that the cocrystals produced exhibited reduced hygroscopicity compared to the raw API. The samples were placed in four RH conditions and analyzed after 1, 3 and 7 weeks. The caffeine-oxalic acid (2:1) cocrystals (Figure 8) exhibited complete stability to moisture in all RH conditions [54].
- (ii)
- Thermal stress: Stability (physical and chemical) of the solid API under high temperature conditions is always evaluated. A study examining the cocrystal of a monophosphate salt with phosphoric acid at 60 °C showed no detectable degradation or transitions between forms [60].
- (iii)
- Photostability: Carbamazepine undergoes photodegradation, with the mechanism depending on the distances between the rings in the crystal lattice (it requires < 4.1 Å). The carbamazepine-saccharin and carbamazepine-nicotinamide cocrystals have longer ring distances, eliminating the mechanism of photodegradation. Thus, the cocrystal can be protected from unwanted processes, since cocrystallization may affect chemical stability through the rearrangement of the molecules in the crystal lattice [59,61].
- (iv)
- Solution stability: This is defined as the ability of the cocrystal components to remain in the solution and to not readily crystallize. This is an important parameter to evaluate during development, both for solutions and suspensions, as well as for solid dosage forms that will dissolve in the gastrointestinal tract. Since cocrystal dissociation may occur, the stability in solution is a key element in their development. A study on carbamazepine cocrystals with 18 coformers evaluated the formation of carbamazepine hydrate when the cocrystals were slurried in water for 24–48 h. Of the studied cocrystals, seven maintained their crystalline structures, and the rest were converted into carbamazepine hydrate. The aqueous solubility of the coformer appeared to be an important parameter for the formation of the hydrate. It was noted that cocrystals containing coformers with relatively high solubility in water resulted in the hydrated form, while cocrystals with coformers of relatively low solubility remained stable in aqueous media [43].
7. Methods of Cocrystal Preparation
- Thermal methods that require melting need high temperatures, which can affect the integrity of heat-sensitive compounds.
- Mechanical methods, such as grinding, require energy consumption and can produce amorphous materials, limiting their effectiveness if a suitable solvent is not used.
- Methods based on precipitation from solution require continuous and precise control of the supersaturation level of the components’ concentration and necessitate the creation of phase diagrams, while the use of a solvent is not environmentally friendly.
- (i)
- Cocrystallization with supercritical solvent (CSS): The active substance and the coformers are dissolved in the supercritical CO2 (sc-CO2) inside a stainless-steel vessel, and depressurization then leads to the loss of dissolving dominance of sc-CO2, to supersaturation and eventually to the formation of cocrystals. The application of CSS requires sufficient (ideally equal) solubility of the pure components in sc-CO2. Its main disadvantage is its low performance in pure products.
- (ii)
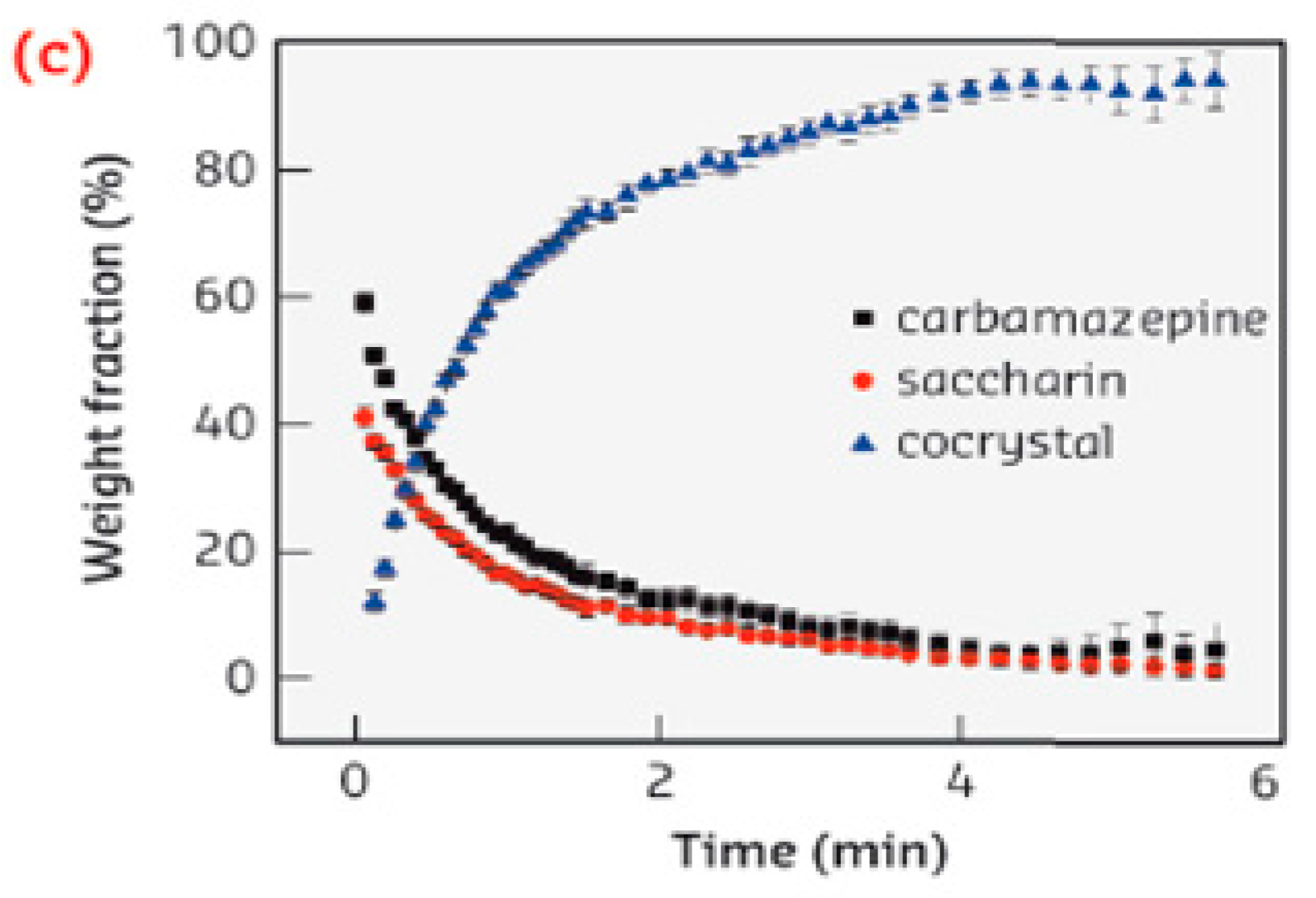
- Supercritical antisolvent (SAS): If a substance is not soluble in sc-CO2, the sc-CO2 can be used as an anti-solvent for a solution of cocrystal components (coformer and API) in an organic solvent. Therefore, the active substance and the coformers dissolve into an organic solvent (primary solvent). This is followed by its dropwise mixing with the sc-CO2 by passing the organic solution through a nozzle. The sc-CO2 dissolves quickly in the droplets of the organic solution, reduces the dissolving power of the solvent and simultaneously extracts it, causing saturation and supersaturation, during which cocrystallization nuclei are formed and the precipitation of cocrystals by the anti-solvent effect of sc-CO2 takes place. This technique requires complete miscibility of the organic solvent with the sc-CO2 and lower solubility for the solute in the mixture. Then, the organic solvent is removed, and a product without solvent is obtained. Itraconazole (antifungal drug of poor bioavailability) and succinic acid cocrystals preparation, with sc-CO2 as an antisolvent, is an example of such [102], while the same method was used for the patent of the carbamazepine-aspirin cocrystal [103].
- (iii)
- Atomized anti-solvent (AAS): In the AAS technique, the sc-CO2 enhances the atomization of the organic solution, producing particles by two different mechanisms: antisolvent crystallization and spray-drying crystallization. The solution containing the API and coformer is pumped through a coaxial nozzle, where it mixes with the sc-CO2 or N2 in the mixing chamber prior to its depressurization into the precipitator vessel (for SAS technique, the precipitator is filled with CO2 at high pressure, whereas in the AAS technique, it is at ambient pressure). Pure indomethacin–saccharin cocrystals have been produced by the AAS technique [100].
8. Characterization of Cocrystals
- (i)
- Differential scanning calorimetry (DSC): For cocrystals, DSC is useful for the construction of binary phase diagrams in the screening of cocrystal formation or the existence of a eutectic mixture or eutectic impurities, which reduce the melting point [119]. It measures the heat of fusion, heat of transition in solid-solid transitions, and heat capacity. It can also be used for the determination of the degree of crystallinity (measurement of enthalpy of fusion of the sample and comparison to the value of the fully crystalline material).
- (ii)
- Hot-stage microscopy (ΗSΜ): HSM is a combination of microscopy and thermal analysis to study the physical characteristics of materials in solid form as a function of temperature and time. When the drug crystals are heated, they undergo changes that can be quickly and easily observed through the microscope. In this way, thermal changes such as melting point, melting range, crystal growth, crystalline transformations, etc. can be visualized. HSM is a simple and relatively inexpensive technique. The systems of HSM allow the controlled heating of the sample, which is placed on a glass slide and viewed under the microscope. Heating is achieved by heat transfer from a metal element, which is heated thermoelectrically. The HSM instrument can be combined with other devices, such as Fourier-transform infrared spectroscope (FTIR), DSC, or a heating-cooling system for regulating the flow of hot or cold air. An important application of thermal microscopy, besides those reported by Stieger et al. [122], is the in situ formation of cocrystals, which is also known as the Kofler contact preparation method (Figure 16) [123].
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Byrn, S.R.; Pfeiffer, R.R.; Stowell, J.G. Solid-State Chemistry of Drugs, 2nd ed.; SSCI, Inc.: West Lafayette, IN, USA, 1999; ISBN 0967067103 9780967067100. [Google Scholar]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Grant, D.J.W. Theory and origin of polymorphism. In Polymorphism in Pharmaceutical Solids, 2nd ed.; Brittain, H.G., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; Volume 95, pp. 1–33. ISBN 9781420073218. [Google Scholar]
- Byrn, S.R.; Zografi, G.; Xiaoming, S.C. Solvates and Hydrates. In Solid-State Properties of Pharmaceutical Materials, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 38–47. [Google Scholar]
- Food and Drug Administration. Regulatory Classification of Pharmaceutical Co-Crystals, Guidance for Industry. April 2013. Available online: https://www.fda.gov/downloads/Drugs/Guidances/UCM281764.pdf (accessed on 2 November 2017).
- Hilfiker, R.; Blatter, F.; von Raumer, M. Relevance of solid-state properties for pharmaceutical products. In Polymorphism in the Pharmaceutical Industry; Hilfiker, R., Ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 2. [Google Scholar]
- Wohler, F. Untersuchungen über das Chinon. Ann. Chem. Pharm. 1844, 51, 145–163. [Google Scholar] [CrossRef]
- Stahly, G.P. A Survey of Cocrystals Reported Prior to 2000. Cryst. Growth Des. 2009, 9, 4212–4229. [Google Scholar] [CrossRef]
- Pfeiffer, P. Organische Molekulverbindungen; Verlag Enke: Stuttgart, Germany, 1922. [Google Scholar]
- Swiss Patent F. Hoffmann-La Roche & Co; Basel (Switzerland). Verfahren zur Darstellung von 2,4-Dioxo-3,3-diäthyl-tetrahydropyridin. CH 187826, 1 March 1937. [Google Scholar]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal and co-crystal. CrystEngComm 2003, 5, 466–467. [Google Scholar] [CrossRef]
- Dunitz, J.D. Crystal and co-crystal: A second opinion. CrystEngComm 2003, 5, 506. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Bond, A.D. What is a co-crystal? CrystEngComm 2007, 9, 833–834. [Google Scholar] [CrossRef]
- Stahl, P.H.; Wermuth, C.G. Monographs on Acids and Bases. In Handbook of Pharmaceutical Salts: Properties, Selection and Use; Stahl, P.H., Wermuth, C.G., Eds.; Verlag Helvetica Chimica Acta: Zurich, Switzerland; VHCA-Wiley-VCH: Weinheim, Germany; New York, NY, USA, 2002; pp. 265–327. ISBN 3906390268. [Google Scholar]
- Jones, W.; Motherwell, W.D.S.; Trask, A.V. Pharmaceutical Cocrystals: An Emerging Approach to Physical Property Enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The salt-cocrystal continuum: The influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Seliger, J.; Zagar, V. Hydrogen bonding and proton transfer in cocrystals of 4,4′-bipyridyl and organic acids studied using nuclear quadrupole resonance. Phys. Chem. Chem. Phys. 2014, 16, 18141–18147. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.T.A.; Yathirajan, H.S.; Bindya, S.; Anilkumar, H.G.; Devaraju, M. Escitalopram oxalate: Co-existence of oxalate dianions and oxalic acid molecules in the same crystal. Acta Crystallogr. C 2007, 63, 129–131. [Google Scholar] [CrossRef]
- European Medicines Agency. Reflection Paper on the Use of Cocrystals and Other Solid State Forms of Active Substances in Medicinal Products. February 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/07/WC500170467.pdf (accessed on 2 November 2017).
- European Medicines Agency. Reflection Paper on the Use of Cocrystals of Active Substances in Medicinal Products. May 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/07/WC500189927.pdf (accessed on 2 November 2017).
- Aakeröy, C.B.; Beatty, A.M.; Helfrich, B.A.; Nieuwenhuyzen, M. Do Polymorphic Compounds Make Good Cocrystallizing Agents? A Structural Case Study That Demonstrates the Importance of Synthon Flexibility. Cryst. Growth Des. 2003, 3, 159–165. [Google Scholar] [CrossRef]
- Corey, E.J. General methods for the construction of complex molecules. Pure Appl. Chem. 1967, 14, 19–38. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Bis, J.A.; Vishweshwar, P.; Weyna, D.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Hydroxyl·Pyridine Hydrogen Bonds in Cocrystals That Contain a Cyano Acceptor. Mol. Pharm. 2007, 4, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.A.; Chisholm, J.A.; Jones, W.; Motherwell, W.D.S. Supramolecular synthon competition in organic sulfonates: A CSD survey. CrystEngComm 2004, 6, 584–588. [Google Scholar] [CrossRef]
- Martínez-Alejo, J.M.; Domínguez-Chávez, J.G.; Rivera-Islas, J.; Herrera-Ruiz, D.; Höpfl, H.; Morales-Rojas, H.; Senosiain, J.P. A twist in cocrystals of salts: Changes in packing and chloride coordination lead to opposite trends in the biopharmaceutical performance of fluoroquinolone hydrochloride cocrystals. Cryst. Growth Des. 2014, 14, 3078–3095. [Google Scholar] [CrossRef]
- Krawczuk, A.; Macchi, P. Charge density analysis for crystal engineering. Chem. Cent. J. 2014, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hathwar, V.R.; Thakur, T.S.; Guru Row, T.N.; Desiraju, G.R. Transferability of multipole charge density parameters for supramolecular synthons: A new tool for quantitative crystal engineering. Cryst. Growth Des. 2011, 11, 616–623. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Gavezzotti, A. Supramolecular Synthons: Validation and Ranking of Intermolecular Interaction Energies. Cryst. Growth Des. 2012, 12, 5873–5877. [Google Scholar] [CrossRef]
- Gavezzotti, A.; Colombo, V.; Presti, L.L. Facts and factors in the formation and stability of binary crystals. Cryst. Growth Des. 2016, 16, 6095–6104. [Google Scholar] [CrossRef]
- Colombo, V.; Pesti, L.L.; Gavezzotti, A. Two–component organic crystals without hydrogen bonding: Structure and intermolecular interaction in bimolecular stacking. CrystEngComm 2017, 19, 2413–2423. [Google Scholar] [CrossRef]
- Malamatari, M.; Ross, S.A.; Douroumis, D.; Velaga, S.P. Experimental cocrystal screening and solution based scale-up cocrystallization methods. Adv. Drug Deliv. Rev. 2017, 117, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Kendrick, J.; Neumann, M.A.; Leusen, F.J. Towards ab initio screening of co-crystal formation through lattice energy calculations and crystal structure prediction of nicotinamide, isonicotinamide, picolinamide and paracetamol multi-component crystals. CrystEngComm 2013, 15, 3799–3807. [Google Scholar] [CrossRef]
- Musumeci, D.; Hunter, A.C.; Prohens, R.; Scuderi, S.; McCabe, F.J. Virtual cocrystal screening. Chem. Sci. 2011, 2, 883–890. [Google Scholar] [CrossRef]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Hirakura, Y.; Yuda, M.; Teramura, T.; Terada, K. Detection of Cocrystal Formation Based on Binary Phase Diagrams Using Thermal Analysis. Pharm. Res. 2013, 30, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Rodriguez-Hornedo, N.; Reddy, L.S.; Jayasankar, A.; Maheshwari, C.; McCausland, L.; Shipplett, R.; Stahly, B.C. Screening Strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. CrystEngComm 2008, 10, 856–864. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Kumar, D.; Chavan, R.B.; Shastri, N.R. Multidrug co-crystals: Towards the development of effective therapeutic hybrids. Drug Discov. Today 2016, 21, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Drug-drug co-crystals. DARU J. Pharm. Sci. 2012, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Cheney, M.L.; Weyna, D.R.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Coformer selection in pharmaceutical cocrystal development: A case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Zhang, G.G.; Flanagan, D.R. Co-crystal intrinsic dissolution behavior using a rotating disk. J. Pharm. Sci. 2011, 100, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.M.; Azim, Y.; Thakur, T.S.; Desiraju, G.R. Co-crystals of the anti-HIV drugs lamivudine and zidovudine. Cryst. Growth Des. 2009, 9, 951–957. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef] [PubMed]
- Petrusevski, G.; Naumov, P.; Jovanovski, G.; Bogoeva-Gaceva, G.; Ng, S.W.; Weng, N. Solid-state forms of sodium valproate, active component of the anticonvulsant drug epilim. ChemMedChem 2008, 3, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.K.; Bak, A. Physicochemical Properties of Pharmaceutical Co-Crystals: A Case Study of Ten AMG 517 Co-Crystals. Cryst. Growth Des. 2008, 8, 3856–3862. [Google Scholar] [CrossRef]
- Stanton, M.K.; Tufekcic, S.; Morgan, C.; Bak, A. Drug Substance and Former Structure Property Relationships in 15 Diverse Pharmaceutical Co-Crystals. Cryst. Growth Des. 2009, 9, 1344–1352. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Pharmaceutical Cocrystallization: Engineering a Remedy for Caffeine Hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical stability enhancement of theophylline via co crystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Basavoju, S.; Bostrom, D.; Velaga, S.P. Indomethacin-saccharin cocrystal: Design, synthesis and preliminary pharmaceutical characterization. Pharm. Res. 2008, 25, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Gore, A.; Yanez, E.; Stanton, M.; Tufekcic, S.; Sysed, R.; Akrami, A.; Rose, M.; Surapaneni, S.; Bostick, T.; et al. The co-crystal approach to improve the exposure of a water-insoluble compound: AMG 517 sorbic acid co-crystal characterization and pharmacokinetics. J. Pharm. Sci. 2008, 97, 3942–3956. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’Donnell, E.; Park, A. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Spong, B. Enhancing the Pharmaceutical Behavior of Carbamazepine through the Formation of Cocrystals. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2005. [Google Scholar]
- Chen, A.M.; Ellison, M.E.; Peresypkin, A.; Wenslow, R.M.; Variankaval, N.; Savarin, C.G.; Natishan, T.K.; Mathre, D.J.; Dormer, P.G.; Euler, D.H.; et al. Development of a pharmaceutical cocrystal of a monophosphate salt with phosphoric acid. Chem. Commun. 2007, 4, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Akazawa, R.; Teraoka, R.; Otsuka, M. Pharmaceutical evaluation of carbamazepine modifications: Comparative study for photostability of carbamazepine polymorphs by using Fourier-transformed reflection-absorption infrared spectroscopy and colorimetric measurement. J. Pharm. Pharmacol. 1994, 46, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Alsenz, J.; Kansy, M. High throughput solubility measurement in drug discovery and development. Adv. Drug Deliv. Rev. 2007, 59, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Zuck, D.A. Investigation of Some Complexes Formed in Solution by Caffeine. II. Benzoic Acid and Benzoate Ion. J. Am. Pharm. Assoc. 1953, 42, 132–138. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase-Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Nehm, S.J.; Rodriguez-Spong, B.; Rodriguez-Hornedo, N. Phase solubility diagrams of cocrystals are explained by solubility product and solution complexation. Cryst. Growth Des. 2006, 6, 592–600. [Google Scholar] [CrossRef]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Oberoi, L.M.; Alexander, K.S.; Riga, A.T. Study of Interaction between Ibuprofen and Nicotinamide Using Differential Scanning Calorimetry, Spectroscopy, and Microscopy and Formulation of a Fast-Acting and Possibly Better Ibuprofen Suspension for Osteoarthritis Patients. J. Pharm. Sci. 2005, 94, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nernst, W. Theorie der Reaktionsgeschwindigkeit in heterogenen systemen. Z. Phys. Chem. 1904, 47, 52–55. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morisette, S.L.; Vetter, A.; Guzman, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Haley, S.; et al. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Variankaval, N.; Wenslow, R.; Murry, J.; Hartman, R.; Helmy, R.; Kwong, E.; Clas, S.; Dalton, C.; Santos, I. Preparation and Solid-State Characterization of Nonstoichiometric Cocrystals of a Phosphodiesterase-IV Inhibitor and l-Tartaric Acid. Cryst. Growth Des. 2006, 6, 690–700. [Google Scholar] [CrossRef]
- Jung, M.S.; Kim, J.S.; Kim, M.S.; Alhalaweh, A.; Cho, W.; Hwang, S.J.; Velaga, S.P. Bioavailability of indomethacin-saccharin cocrystals. J. Pharm. Pharmacol. 2010, 62, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Cheney, M.L.; Shan, N.; Healey, E.R.; Hanna, M.; Wojtas, L.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal Engineering Case Study of Lamotrigine. Cryst. Growth Des. 2009, 10, 394–405. [Google Scholar] [CrossRef]
- Karki, S.; Frisic, T.; Fabian, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving mechanical properties of crystalline solids by cocrystal formation: New compressible forms of paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Widjojokusumo, E.; Soewandhi, S.N.; Wikarsa, S.; Tjandrawinata, R.R. Physicochemical and mechanical properties of paracetamol cocrystal with 5-nitroisophthalic acid. Int. J. Pharm. 2016, 497, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.R.; Shi, L.; Bag, P.P.; Sun, C.C.; Reddy, C.M. Correlation among crystal structure, mechanical behavior, and tabletability in the co-crystals of vanillin isomers. Cryst. Growth Des. 2015, 15, 1827–1832. [Google Scholar] [CrossRef]
- Rahman, Z.; Agarabi, C.; Zidan, A.S.; Khan, S.R.; Khan, M.A. Physico-mechanical and stability evaluation of carbamazepine cocrystal with nicotinamide. AAPS PharmSciTech 2011, 12, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or salt: Does it really matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Chow, P.S.; Tan, R.P.H. Polymorphism in cocrystals: A review and assessment of its significance. CrystEngComm 2014, 16, 3451–3465. [Google Scholar] [CrossRef]
- Prohens, R.; Barbas, R.; Portell, A.; Font-Bardia, M.; Alcobé, X.; Puigjaner, C. Polymorphism of Cocrystals: The Promiscuous Behavior of Agomelatine. Cryst. Growth Des. 2016, 16, 1063–1070. [Google Scholar] [CrossRef]
- Mandala, V.S.; Loewus, S.J.; Mehta, M.A. Monitoring cocrystal formation via in situ solid-state NMR. J. Phys. Chem. Lett. 2014, 5, 3340–3344. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Imai, Y.; Tajima, N. Generation of a co-crystal phase with novel coloristic properties via solid state grinding procedures. Chem. Commun. 2002, 23, 2848–2849. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Bassi, P.S.; Chadha, S.L. Mechanism of the reaction between hydrocarbons and picric acid in the solid state. J. Phys. Chem. 1963, 67, 2569–2573. [Google Scholar] [CrossRef]
- Gagnière, E.; Mangin, D.; Puel, F.; Valour, J.P.; Klein, J.P.; Monnier, O. Cocrystal formation in solution: Inducing phase transition by manipulating the amount of cocrystallizing agent. J. Cryst. Growth. 2011, 316, 118–125. [Google Scholar] [CrossRef]
- Gagnière, E.; Mangin, D.; Veesler, S.; Puel, F. Co-crystallization in solution and scale-up issues. In Pharmaceutical Salts and Co-Crystals, 1st ed.; Wouters, J., Quéré, L., Thurston, D.E., Eds.; RSC Publishing: Cambridge, UK, 2011; Volume 16, pp. 188–211. ISBN 1849731586. [Google Scholar]
- Blagden, N.; Berry, D.J.; Parkin, A.; Javed, H.; Ibrahim, A.; Gavan, P.T.; De Matosa, L.L.; Seatona, C.C. Current directions in co-crystal growth. New J. Chem. 2008, 32, 1659–1672. [Google Scholar] [CrossRef]
- Chiarella, R.A.; Davey, R.J.; Peterson, M.L. Making Co-Crystals-The Utility of Ternary Phase Diagrams. Cryst. Growth Des. 2007, 7, 1223–1226. [Google Scholar] [CrossRef]
- Chadwick, K.; Davey, R.; Cross, W. How does grinding produce co-crystals? Insights from the case of benzophenone and diphenylamine. CrystEngComm 2007, 9, 732–734. [Google Scholar] [CrossRef]
- Shan, N.; Toda, F.; Jones, W. Mechanochemistry and co-crystal formation: Effect of solvent on reaction kinetics. Chem. Commun. 2002, 20, 2372–2373. [Google Scholar] [CrossRef]
- Weyna, D.R.; Shattock, T.; Vishweshwar, P.; Zaworotko, M.J. Synthesis and Structural Characterization of Cocrystals and Pharmaceutical Cocrystals: Mechanochemistry vs. Slow Evaporation from Solution. Cryst. Growth Des. 2009, 9, 1106–1123. [Google Scholar] [CrossRef]
- Childs, S.L. Novel Cocrystallization of Hydrochloric Acid Salt of an Active Agent. Can. Patent CA2514092 A1, 5 August 2004. [Google Scholar]
- Trask, A.V.; van de Streek, J.; Motherwell, W.D.S.; Jones, W. Achieving Polymorphic and Stoichiometric Diversity in Cocrystal Formation: Importance of Solid-State Grinding, Powder X-ray Structure Determination, and Seeding. Cryst. Growth Des. 2005, 5, 2233–2241. [Google Scholar] [CrossRef]
- Schultheiss, N.C.; Bethune, S.J. Pterostilbene Cocrystals. U.S. Patent US20110189275 A1, 4 August 2011. [Google Scholar]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Solvent-drop grinding: Green polymorph control of cocrystallisation. Chem. Commun. 2004, 7, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Bethunea, S.; Hencka, J.O. Nutraceutical cocrystals: Utilizing pterostilbene as a cocrystal former. CrystEngComm 2010, 12, 2436–2442. [Google Scholar] [CrossRef]
- Takata, N.; Shiraki, K.; Takano, R.; Hayashi, Y.; Terada, K. Cocrystal screening of stanolone and mestanolone using slurry crystallization. Cryst. Growth Des. 2008, 8, 3032–3037. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R.; Videla, C.S.; Tesson, N.; Trilla Castano, M. Co-Crystals of Venlafaxine and Celecoxib. Eur. Patent EP2515892 A2, 31 October 2012. [Google Scholar]
- Wang, I.C.; Lee, M.J.; Sim, S.J.; Kim, W.S.; Chun, N.H.; Choi, G.J. Anti-solvent crystallization of carbamazepine and saccharin. Int. J. Pharm. 2013, 450, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Chun, N.H.; Wang, I.C.; Lee, M.J.; Jung, Y.T.; Lee, S.; Kim, W.S.; Choi, G.J. Characteristics of indomethacin-saccharin (IMC-SAC) co-crystals by antisolvent crystallization process. Eur. J. Pharm. Biopharm. 2013, 85, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, A.M.; Velaga, P.S.; Matos, A.H.; de Azevedo, E.G. Formation of indomethacin–saccharin cocrystals using supercritical fluid technology. Eur. J. Pharm. Sci. 2009, 38, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Pando, C.; Cabañas, A.; Cuadra, I.A. Preparation of pharmaceutical co-crystals through sustainable processes using supercritical carbon dioxide: A review. RSC Adv. 2016, 6, 71134–71150. [Google Scholar] [CrossRef]
- Ober, C.A.; Gupta, R.B. Formation of Itraconazole–Succinic Acid Cocrystals by Gas Antisolvent Cocrystallization. AAPS PharmSciTech 2012, 13, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Mazen, H.; Townend, G. Method of Creating Crystalline Substances. U.S. Patent US20080280858 A1, 13 November 2008. [Google Scholar]
- Shekar, H.S.; Rajamma, A.J.; Sateesha, S.B. Application of Ultrasound to Pharmaceutical Industry: An Overview. J. Pharm. Drug Deliv. Res. 2017, 6. [Google Scholar] [CrossRef]
- Childs, S.L.; Mougin, P.; Stahly, B.C. Screening for Solid Forms by Ultrasound Crystallization and Cocrystallization Using Ultrasound. Eur. Patent EP2292585 A1, 9 March 2011. [Google Scholar]
- Jayasankar, A.; Somwangthanaroj, A.; Shao, Z.J.; Rodríguez-Hornedo, N. Cocrystal Formation during Cogrinding and Storage is mediated by Amorphous Phase. Pharm. Res. 2006, 23, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Halasz, I.; Puškaric, A.; Kimber, S.A.J.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Honkimäki, V.; Dinnebier, R.E.; Patel, B.; Jones, W.; et al. Real-Time In Situ Powder X-ray Diffraction Monitoring of Mechanochemical Synthesis of Pharmaceutical Cocrystals. Angew. Chem. Int. Ed. Engl. 2013, 52, 11538–11541. [Google Scholar] [CrossRef] [PubMed]
- Kalofonos, I.; Stahly, G.P.; Martin-Doyle, W.; Kalofonos, D.; Stults, J.S.; Houston, T.L. Novel Choline Cocrystal of Epalrestat. Eur. Patent EP2326632B1, 31 May 2017. [Google Scholar]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Jones, W.; Krebs, A.; Mack, J.; Maini, L.; Orpen, A.G.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Alhalaweh, A.; Velaga, S.P. Formation of cocrystals from stoichiometric solutions of incongruently saturating systems by spray drying. Cryst. Growth Des. 2010, 10, 3302–3305. [Google Scholar] [CrossRef]
- Am Ende, D.J.; Anderson, S.R.; Salan, J.S. Development and Scale-Up of Cocrystals Using Resonant Acoustic Mixing. Org. Process Res. Dev. 2014, 18, 331–341. [Google Scholar] [CrossRef]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, A.; Pagire, S. Effervescent Compositions Containing Co-Crystals of the Acid Part. U.S. Patent US20170128359 A1, 11 May 2017. [Google Scholar]
- Medina, C.; Daurio, D.; Nagapudi, K.; Alvarez-Nunez, F. Manufacture of pharmaceutical co-crystals using twin screw extrusion: A solvent-less and scalable process. J. Pharm. Sci. 2010, 99, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Boksa, K.; Otte, A.; Pinal, R. Matrix-assisted cocrystallization (MAC) simultaneous production and formulation of pharmaceutical cocrystals by hot-melt extrusion. J. Pharm. Sci. 2014, 103, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Pindelska, E.; Sokal, A.; Kolodziejski, W. Pharmaceutical cocrystals, slats and polymorphs: Advanced characterization techniques. Adv. Drug Deliv. Rev. 2017, 1, 111–146. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; de Azevedo, E.G.; Velaga, S.P. Powder X-ray diffraction method for the quantification of cocrystals in the crystallization mixture. Drug Dev. Ind. Pharm. 2012, 38, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Stoler, E.; Warner, J.C. Non-covalent derivatives: Cocrystals and eutectics. Molecules 2015, 20, 14833–14848. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, K.; Miller, J.; Alvarez-Núez, F.; Rodríguez-Hornedo, N. Crystallization pathways and kinetics of carbamazepine-nicotinamide cocrystals from the amorphous state by in situ thermomicroscopy, spectroscopy, and calorimetry studies. J. Pharm. Sci. 2007, 96, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Buanz, A.B.M.; Parkinson, G.N.; Gaisford, S. Characterization of Carbamazepine-Nicatinamide Cocrystal Polymorphs with Rapid Heating DSC and XRPD. Cryst. Growth Des. 2011, 11, 1177–1181. [Google Scholar] [CrossRef]
- Stieger, N.; Aucamp, M.; Zhang, S.-W.; de Villiers, M.M. Hot-stage Optical Microscopy as an Analytical Tool to Understand Solid-state Changes in Pharmaceutical Materials. Am. Pharm. Rev. 2012, 15, 32–36. [Google Scholar]
- Kofler, L.; Kofler, A. Thermal Micromethods for the Study of Organic Compounds and Their Mixtures; Wagner: Innsbrook, Austria, 1952. [Google Scholar]
- Vogt, F.G.; Clawson, J.S.; Strohmeier, M.; Edwards, A.J.; Pham, T.N.; Watson, S.A. Solid-State NMR Analysis of Organic Cocrystals and Complexes. Cryst. Growth Des. 2009, 9, 921–937. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Salmon, D.J.; Smith, M.M.; Desper, J. Cyanophenyloximes: Reliable and versatile tools for hydrogen-bond directed supramolecular synthesis of co-crystals. Cryst. Growth Des. 2006, 6, 1033–1042. [Google Scholar] [CrossRef]
- Saganowska, P.; Wesolowski, M.J. Principal component and cluster analyses as supporting tools for co-crystals detection. Therm. Anal. Calorim. 2017, 130, 45–55. [Google Scholar] [CrossRef]
- Kelly, A.L.; Gough, T.; Dhumal, R.S.; Halsey, S.A.; Paradkar, A. Monitoring ibuprofen-nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int. J. Pharm. 2012, 426, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chun, N.H.; Kim, M.J.; Kim, P.; Song, K.H.; Choi, G.J. In Situ Monitoring of Antisolvent Cocrystallization by Combining Near-Infrared and Raman Spectroscopies. Cryst. Growth Des. 2015, 15, 4385–4393. [Google Scholar] [CrossRef]
- Allesø, M.; Velaga, S.; Alhalaweh, A.; Cornett, C.; Rasmussen, M.A.; van den Berg, F.; de Diego, H.L.; Rantanen, J. Near-infrared spectroscopy for cocrystal screening. A comparative study with Raman spectroscopy. Anal. Chem. 2008, 80, 7755–7764. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Raijada, D.; Rantanen, J. Raman spectroscopy in pharmaceutical product design. Adv. Drug Deliv. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Elbagerma, M.A.; Edwards, H.G.M.; Munshi, T.; Hargreaves, M.D.; Matousek, P.; Scowen, I.J. Characterization of New Cocrystals by Raman Spectroscopy, Powder X-ray Diffraction, Differential Scanning Calorimetry, and Transmission Raman Spectroscopy. Cryst. Growth Des. 2010, 10, 2360–2371. [Google Scholar] [CrossRef]
- Langkilde, F.W.; Sjoblom, J.; Tekenbergs-Hjelte, L.; Mrak, J. Quantitative FT-Raman analysis of two crystal forms of a pharmaceutical compound. J. Pharm. Biomed. Anal. 1997, 15, 687–696. [Google Scholar] [CrossRef]
- Farias, M.; Carneiro, R. Simultaneous Quantification of Three Polymorphic Forms of Carbamazepine in the Presence of Excipients Using Raman Spectroscopy. Molecules 2014, 19, 14128–14138. [Google Scholar] [CrossRef] [PubMed]
- Müllers, K.C.; Paisana, M.; Wahl, M.A. Simultaneous formation and micronization of pharmaceutical cocrystals by Rapid Expansion of Supercritical Solutions (RESS). Pharm. Res. 2015, 32, 702–713. [Google Scholar] [CrossRef] [PubMed]
















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. https://doi.org/10.3390/pharmaceutics10010018
Karagianni A, Malamatari M, Kachrimanis K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics. 2018; 10(1):18. https://doi.org/10.3390/pharmaceutics10010018
Chicago/Turabian StyleKaragianni, Anna, Maria Malamatari, and Kyriakos Kachrimanis. 2018. "Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs" Pharmaceutics 10, no. 1: 18. https://doi.org/10.3390/pharmaceutics10010018
APA StyleKaragianni, A., Malamatari, M., & Kachrimanis, K. (2018). Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics, 10(1), 18. https://doi.org/10.3390/pharmaceutics10010018






