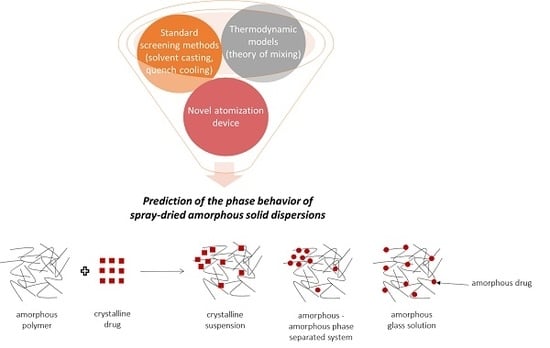
Prediction of Phase Behavior of Spray-Dried Amorphous Solid Dispersions: Assessment of Thermodynamic Models, Standard Screening Methods and a Novel Atomization Screening Device with Regard to Prediction Accuracy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Model Drugs
2.1.2. Polymers
2.2. Methods
2.2.1. Theoretical Models Based on the Thermodynamics of Mixing
2.2.2. Manufacturing/Preparation Methods
2.2.3. Analytical or Characterization Methods
3. Results and Discussion
3.1. Theoretical Models Based on the Thermodynamics of Mixing
3.2. Comparison of Theoretical Models with Experimental Screening Methods with Regards to SDASD Development
3.2.1. Evaluation of Drug–Polymer Miscibility
3.2.2. Evaluation of Glass Solution Thermal Properties
3.2.3. Evaluation of Polymer Ranking
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.L.N.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Marsac, P.J.; Shamblin, S.L.; Taylor, L.S. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm. Res. 2006, 23, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Nies, E.; Van den Mooter, G. Relating hydrogen-bonding interactions with the phase behavior of naproxen/pvp k 25 solid dispersions: Evaluation of solution-cast and quench-cooled films. Mol. Pharm. 2012, 9, 3301–3317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Inbar, P.; Chokshi, H.P.; Malick, A.W.; Choi, D.S. Prediction of the thermal phase diagram of amorphous solid dispersions by flory-huggins theory. J. Pharm. Sci. 2011, 100, 3196–3207. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Van Humbeeck, J.; Van den Mooter, G. Theoretical and experimental investigation on the solid solubility and miscibility of naproxen in poly(vinylpyrrolidone). Mol. Pharm. 2010, 7, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.J.; Williams, A.C.; Timmins, P.; York, P. Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci. 1999, 88, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Thakral, N.K. Prediction of drug-polymer miscibility through the use of solubility parameter based flory-huggins interaction parameter and the experimental validation: Peg as model polymer. J. Pharm. Sci. 2013, 102, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.; Tian, Y.; Potter, C.; Jones, D.S.; Andrews, G.P. Probing the effects of experimental conditions on the character of drug-polymer phase diagrams constructed using flory-huggins theory. Pharm. Res. 2015, 32, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Booth, J.; Meehan, E.; Jones, D.S.; Li, S.; Andrews, G.P. Construction of drug-polymer thermodynamic phase diagrams using flory-huggins interaction theory: Identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol. Pharm. 2013, 10, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Pajula, K.; Lehto, V.P.; Ketolainen, J.; Korhonen, O. Computational approach for fast screening of small molecular candidates to inhibit crystallization in amorphous drugs. Mol. Pharm. 2012, 9, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Degenhardt, M.; Sadowski, G. Influence of low-molecular-weight excipients on the phase behavior of pvpva64 amorphous solid dispersions. Pharm. Res. 2018, 35, 25. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.; Santos, J.L.; Pinto, J.F.; Temtem, M. Screening methodologies for the development of spray-dried amorphous solid dispersions. Pharm. Res. 2015, 32, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.; Matthee, K.; Knop, K.; Kleinebudde, P. Evaluation of predictive models for stable solid solution formation. J. Pharm. Sci. 2011, 100, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huang, J.; Hussain, M.A. Drug-polymer solubility and miscibility: Stability consideration and practical challenges in amorphous solid dispersion development. J. Pharm. Sci. 2010, 99, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.C.; Ran, Y.; Chou, K.J.; Cui, Y.; Sambrone, A.; Chan, C.; Hart, R. Evaluation of drug load and polymer by using a 96-well plate vacuum dry system for amorphous solid dispersion drug delivery. AAPS PharmSciTech 2012, 13, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.G.; Pollock-Dove, C.; Dong, L.C.; Li, S. Advanced screening assays to rapidly identify solubility-enhancing formulations: High-throughput, miniaturization and automation. Adv. Drug Deliv. Rev. 2008, 60, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, N.; Janas, C.; Siam, M.; Lauer, M.E.; Jacob, L.; Scheubel, E.; Page, S. Miniaturized screening of polymers for amorphous drug stabilization (spads): Rapid assessment of solid dispersion systems. Eur. J. Pharm. Biopharm. 2013, 84, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, M.; Roine, J.; Murtomaa, M.; Mohan Sankaran, R.; Santos, H.A.; Salonen, J. Solid state transformations in consequence of electrospraying—A novel polymorphic form of piroxicam. Eur. J. Pharm. Biopharm. 2015, 89, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wulsten, E.; Kiekens, F.; van Dycke, F.; Voorspoels, J.; Lee, G. Levitated single-droplet drying: Case study with itraconazole dried in binary organic solvent mixtures. Int. J. Pharm. 2009, 378, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; De Zeure, A.; Paudel, A.; Van Humbeeck, J.; Rombaut, P.; Van den Mooter, G. Influence of preparation methods on solid state supersaturation of amorphous solid dispersions: A case study with itraconazole and eudragit e100. Pharm. Res. 2010, 27, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients, 5th ed.; Pharmaceutical Press: London, UK, 2006; ISBN 978-1582120584. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953; ISBN 978-0801401343. [Google Scholar]
- Rubinstein, M.; Colby, R.H. Polymer Physics, 1st ed.; Oxford University Press: New York, NY, USA, 2003; ISBN 978-0198520597. [Google Scholar]
- Barton, A.F.M. Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991; ISBN 978-0849301766. [Google Scholar]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Theoretical and experimental investigation of drug-polymer interaction and miscibility and its impact on drug supersaturation in aqueous medium. Eur. J. Pharm. Biopharm. 2016, 107, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Van Krevelen, D. Properties of Polymers: Their Estimation and Correlation with Chemical Structure; Elsevier: Amsterdam, The Netherlands, 1976; ISBN 978-0-444-82874. [Google Scholar]
- Huang, Y.; Dai, W.G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharmacol. Sin. 2014, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Just, S.; Sievert, F.; Thommes, M.; Breitkreutz, J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. Eur. J. Pharm. Biopharm. 2013, 85, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Higashi, K.; Yamamoto, K.; Moribe, K. The effect of hpmcas functional groups on drug crystallization from the supersaturated state and dissolution improvement. Int. J. Pharm. 2014, 464, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, E.; Panayiotou, C. A new expanded solubility parameter approach. Int. J. Pharm. 2012, 426, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Six, K.; Murphy, J.; Weuts, I.; Craig, D.Q.M.; Verreck, G.; Peeters, J.; Brewster, M.; Van den Mooter, G. Identification of phase separation in solid dispersions of itraconazole and eudragit® e100 using microthermal analysis. Pharm. Res. 2003, 20, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Taylor, L.S. An ab initio polymer selection methodology to prevent crystallization in amorphous solid dispersions by application of crystal engineering principles. CrystEngComm 2011, 13, 61–71. [Google Scholar] [CrossRef]
- Worku, Z.A.; Aarts, J.; Singh, A.; Van den Mooter, G. Drug-polymer miscibility across a spray dryer: A case study of naproxen and miconazole solid dispersions. Mol. Pharm. 2014, 11, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. 2016, 374, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D. Predicting solubility/miscibility in amorphous dispersions: It is time to move beyond regular solution theories. J. Pharm. Sci. 2018, 107, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Bartels, M.; Degenhardt, M.; Sadowski, G. Physical stability of api/polymer-blend amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2017, 124, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Yang, M.; van den Berg, F.; Pajander, J.; Rades, T.; Rantanen, J. Influence of solvent evaporation rate and formulation factors on solid dispersion physical stability. Eur. J. Pharm. Sci. 2011, 44, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Engers, D.; Teng, J.; Jimenez-Novoa, J.; Gent, P.; Hossack, S.; Campbell, C.; Thomson, J.; Ivanisevic, I.; Templeton, A.; Byrn, S.; et al. A solid-state approach to enable early development compounds: Selection and animal bioavailability studies of an itraconazole amorphous solid dispersion. J. Pharm. Sci. 2010, 99, 3901–3922. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Loyson, Y.; Van den Mooter, G. An investigation into the effect of spray drying temperature and atomizing conditions on miscibility, physical stability, and performance of naproxen–PVP K 25 solid dispersions. J. Pharm. Sci. 2013, 102, 1249–1267. [Google Scholar] [CrossRef] [PubMed]














| Drug | Abbreviation | Mw (g/mol) | logP | H-Bond Donor Sites | H-Bond Acceptor Sites | Tg (°C) | Tm (°C) | GFA/GS |
|---|---|---|---|---|---|---|---|---|
| Ibuprofen | IBU | 206 | 3.97 | 1 | 2 | −44 | 76 | III |
| Naproxen | NAP | 230 | 3.18 | 1 | 3 | 6 | 158 | I |
| Carbamazepine | CAR | 236 | 2.45 | 1 | 1 | 50 | 177 (III)–193 (I) | I |
| Itraconazole | ITR | 705 | 5.66 | 0 | 9 | 59 | 168 | III |
| Polymer | Abbreviation | Mw (g/mol) | True Density (g/cm3) | Dissolution pH | Tg (°C) | T Degradation (°C) |
|---|---|---|---|---|---|---|
| HPMCP-HP50 | HP50 | 78,000 | 1.82 | >5.0 | 140 | 160 |
| HPMCAS-LF | HAS | 18,167 | 1.29 | >5.5 | 122 | 170 |
| PVPVA | PVA | 57,500 | 1.27 | - | 112 | 215 |
| PVPK30 | PK30 | 50,000 | 1.18 | - | 162 | >300 |
| Soluplus | SOL | 115,000 | 1.03 | - | 80 | 190 |
| Eudragit L100 | EUD | 125,000 | 1.28 | >6.0 | 192 | 165 |
| Eudragit L100-55 | EUD55 | 320,000 | 1.25 | >5.5 | 122 | 165 |
| MPa½ | cm3 mol−1 | ||||||
|---|---|---|---|---|---|---|---|
| δ(F) | δ(VK) | δd(VK) | δp(VK) | δh(VK) | v(F) | v(VK) | |
| IBU | 20.9 | 19.4 | 17.9 | 2.2 | 7.2 | 195.5 | 195.5 |
| NAP | 23.4 | 21.9 | 20.1 | 3.0 | 8.0 | 178.3 | 203.1 |
| CAR | 28.1 | 24.9 | 22.0 | 6.6 | 9.6 | 154.1 | 168.8 |
| ITR | 26.6 | 26.0 | 22.8 | 6.0 | 10.9 | 403.6 | 434.5 |
| HP50 a | 26.8 | 26.7 | 20.8 | 3.8 | 16.4 | 384.5 | 384.5 |
| HAS a | 25.9 | 26.3 | 19.4 | 4.3 | 17.2 | 344.5 | 344.5 |
| PK30 | 27.4 | 26.3 | 20.4 | 13.7 | 9.3 | 71.7 | 81.2 |
| PVA b | 25.1 | 24.4 | 19.2 | 11.2 | 9.7 | 69.7 | 75.4 |
| SOL c | 23.1 | 22.6 | 18.6 | 9.2 | 8.7 | 83.9 | 89.3 |
| EUD d | 23.0 | 22.6 | 18.5 | 6.6 | 11.1 | 70.4 | 70.4 |
| EUD55 d | 23.3 | 22.5 | 18.4 | 6.6 | 11.1 | 70.8 | 70.8 |
| Drug | Thermodynamic Model | HP50 | HAS | PV30 | PVA | SOL | EUD | EUD55 |
|---|---|---|---|---|---|---|---|---|
| IBU | Δδ(F) | 5.9 | 5.0 | 6.5 | 4.2 | 2.2 | 2.1 | 2.4 |
| Δδ(VK) | 7.4 | 6.9 | 6.9 | 5.0 | 3.2 | 3.2 | 3.1 | |
| χ(F) | 3.1 | 2.3 | 3.6 | 1.7 | 0.7 | 0.7 | 0.8 | |
| χ(VK) | 4.6 | 4.1 | 4.1 | 2.3 | 1.2 | 1.2 | 1.1 | |
| EUC-d | 9.8 | 10.2 | 6.9 | 4.9 | 3.2 | 4.3 | 4.3 | |
| NAP | Δδ(F) | 3.4 | 2.5 | 4.0 | 1.7 | 0.3 | 0.4 | 0.1 |
| Δδ(VK) | 4.9 | 4.4 | 4.4 | 2.5 | 0.7 | 0.7 | 0.6 | |
| χ(F) | 1.2 | 0.8 | 1.5 | 0.6 | 0.4 | 0.4 | 0.3 | |
| χ(VK) | 2.3 | 1.9 | 1.9 | 0.9 | 0.4 | 0.4 | 0.4 | |
| EUC-d | 8.4 | 9.2 | 4.4 | 2.5 | 0.8 | 3.2 | 3.2 | |
| CAR | Δδ(F) | 1.3 | 2.2 | 0.7 | 2.9 | 5.0 | 5.1 | 4.8 |
| Δδ(VK) | 1.8 | 1.3 | 1.4 | 0.5 | 2.3 | 2.3 | 2.4 | |
| χ(F) | 0.4 | 0.6 | 0.4 | 0.9 | 1.9 | 1.9 | 1.8 | |
| χ(VK) | 0.6 | 0.5 | 0.5 | 0.4 | 0.7 | 0.7 | 0.7 | |
| EUC-d | 7.1 | 8.3 | 1.6 | 0.8 | 2.4 | 3.8 | 3.8 | |
| ITR | Δδ(F) | 0.3 | 0.6 | 0.9 | 1.4 | 3.4 | 3.5 | 3.2 |
| Δδ(VK) | 0.7 | 0.3 | 0.3 | 1.6 | 3.4 | 3.4 | 3.5 | |
| χ(F) | 0.4 | 0.4 | 0.5 | 0.7 | 2.3 | 2.4 | 2.1 | |
| χ(VK) | 0.4 | 0.4 | 0.4 | 0.8 | 2.4 | 2.4 | 2.5 | |
| EUC-d | 6.0 | 7.3 | 1.9 | 1.8 | 3.6 | 4.0 | 4.1 |
| Predictive and Manufacturing Methods | IBU | NAP | CAR | ITR | |
|---|---|---|---|---|---|
| Δδ(F)/χ(F) | Polymer ranking | EUD > SOL > EUD55 | EUD55 > SOL > EUD | PK30 > HP50 > HAS | HP50 > HAS > K30 |
| f1/f2 | 0/0.33 | 0.33/0.33 | 0/0.33 | 0/0.33 | |
| Δδ(VK)/χ(VK) | Polymer ranking | EUD55 > EUD > SOL | EUD55 > EUD > SOL | PVA > HAS > PK30 | HAS > K30 > HP |
| f1/f2 | 0.33/0.33 | 0/0.33 | 0/0.33 | 0.33/0.33 | |
| EUC-d | Polymer ranking | SOL > EUD55 > EUD | SOL > PVA > EUD | PVA > PK30 > SOL | PVA > PK30 > SOL |
| f1/f2 | 0/0.33 | 0.66/0.66 | 0.33/0.33 | 0/0 | |
| Film casting-RT | Polymer ranking | PK30 > PVA > SOL | SOL > PK30 > HAS | EUD > PK30 > HAS | EUD > EUD55 > HP50 |
| f1/f2 | 1/1 | 0/0.33 | 0.66/0.66 | 1/1 | |
| Film casting-reduced pressure | Polymer ranking | PVA > PK30 > SOL | SOL > PVA > PK30 | PK30 > EUD > HAS | HAS > HP50 > EUD55 |
| f1/f2 | 0.33/1 | 0.33/0.66 | 0/0.66 | 0/0.66 | |
| Quench cooling | Polymer ranking | PK30 > PVA > HAS | PK30 > PVA > SOL | PK30 > PVA > SOL | EUD > EUD55 > HAS |
| f1/f2 | 0.66/0.66 | 0.66/0.66 | 0/0.33 | 0.66/0.66 | |
| Atomization device | Polymer ranking | PK30 > PVA > SOL | PK30 > PVA > SOL | EUD > PK30 > EU55 | EUD > EUD55 > HP50 |
| f1/f2 | 1/1 | 0.66/0.66 | 1/1 | 1/1 | |
| Spray dryer | Polymer ranking | PK30 > PVA > SOL | PK30 > PVA > EUD | EUD > PK30 > EUD55 | EUD > EUD55 > HP50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ousset, A.; Chavez, P.-F.; Meeus, J.; Robin, F.; Schubert, M.A.; Somville, P.; Dodou, K. Prediction of Phase Behavior of Spray-Dried Amorphous Solid Dispersions: Assessment of Thermodynamic Models, Standard Screening Methods and a Novel Atomization Screening Device with Regard to Prediction Accuracy. Pharmaceutics 2018, 10, 29. https://doi.org/10.3390/pharmaceutics10010029
Ousset A, Chavez P-F, Meeus J, Robin F, Schubert MA, Somville P, Dodou K. Prediction of Phase Behavior of Spray-Dried Amorphous Solid Dispersions: Assessment of Thermodynamic Models, Standard Screening Methods and a Novel Atomization Screening Device with Regard to Prediction Accuracy. Pharmaceutics. 2018; 10(1):29. https://doi.org/10.3390/pharmaceutics10010029
Chicago/Turabian StyleOusset, Aymeric, Pierre-François Chavez, Joke Meeus, Florent Robin, Martin Alexander Schubert, Pascal Somville, and Kalliopi Dodou. 2018. "Prediction of Phase Behavior of Spray-Dried Amorphous Solid Dispersions: Assessment of Thermodynamic Models, Standard Screening Methods and a Novel Atomization Screening Device with Regard to Prediction Accuracy" Pharmaceutics 10, no. 1: 29. https://doi.org/10.3390/pharmaceutics10010029
APA StyleOusset, A., Chavez, P.-F., Meeus, J., Robin, F., Schubert, M. A., Somville, P., & Dodou, K. (2018). Prediction of Phase Behavior of Spray-Dried Amorphous Solid Dispersions: Assessment of Thermodynamic Models, Standard Screening Methods and a Novel Atomization Screening Device with Regard to Prediction Accuracy. Pharmaceutics, 10(1), 29. https://doi.org/10.3390/pharmaceutics10010029