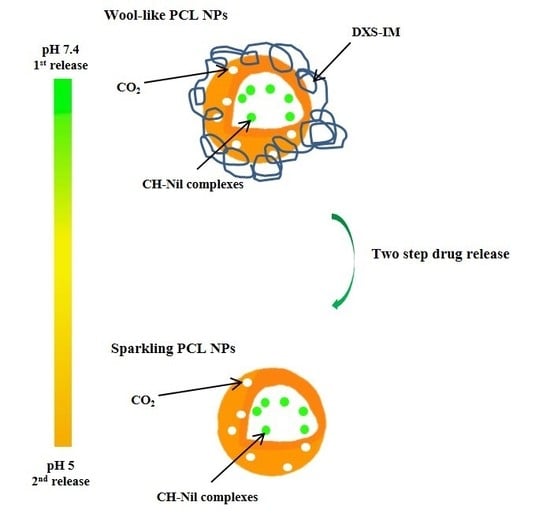
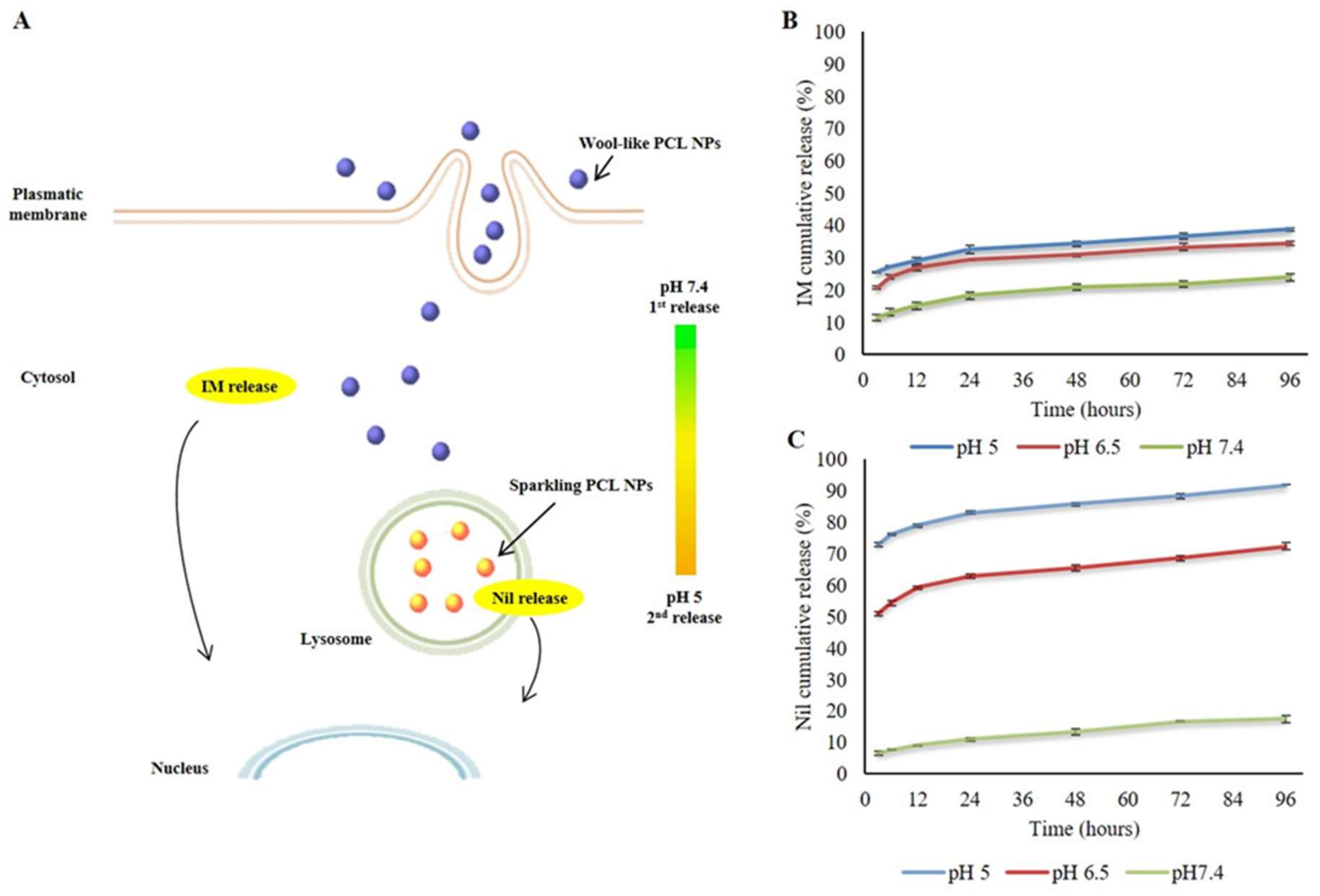
Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Synthesis and Characterization of Sparkling and Wool-Like PCL NPs
2.3. Drugs Entrapment Efficacy and In vitro Release
2.4. Wool-Like PCL NPs Cellular Uptake and Intracellular Localization
2.5. Quantitative Study of Co-Localization
2.6. In Vitro Combinatorial Anti-Leukaemia Efficacy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Physico-Chemical Characterization of Drug Loaded Sparkling and Wool-Like Hollow PCL NPs
3.2. Cellular Uptake and Intracellular Localization of Wool-Like PCL NPs
3.3. Combinatorial Cytotoxicity of Nil and IM in CML Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vardiman, J.W. Chronic myelogenous leukemia, BCR-ABL1+. Am. J. Clin. Pathol. 2009, 132, 250–260. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Zabriskie, M.S.; Eiring, A.M.; Deininger, M.W. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat. Rev. Cancer 2012, 12, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.J.; Melo, J.V. Primitive, quiescent and difficult to kill: The role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle 2006, 5, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Van Etten, R.A. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 2005, 353, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Le Coutre, P.; Tassi, E.; Varella-Garcia, M.; Barni, R.; Mologni, L.; Cabrita, G.; Marchesi, E.; Supino, R.; Gambacorti-Passerini, C. Induction of resistance to the abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood 2000, 95, 1758–1766. [Google Scholar] [PubMed]
- Jørgensen, H.G.; Holyoake, T.L. Characterization of cancer stem cells in chronic myeloid leukaemia. Biochem. Soc. Trans. 2007, 35, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Gorre, M.E.; Mohammed, M.; Ellwood, K.; Hsu, N.; Paquette, R.; Rao, P.N.; Sawyers, C.L. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001, 293, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Nicoll, J.M.; Nagar, B.; Gorre, M.E.; Paquette, R.L.; Kuriyan, J.; Sawyers, C.L. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002, 2, 117–125. [Google Scholar] [CrossRef]
- Soverini, S.; Colarossi, S.; Gnani, A.; Rosti, G.; Castagnetti, F.; Poerio, A.; Iacobucci, I.; Amabile, M.; Abruzzese, E.; Orlandi, E.; et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of philadelphia-positive patients: By the GIMEMA working party on chronic myeloid leukemia. Clin. Cancer Res. 2006, 12, 7374–7379. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Corbin, A.S.; Druker, B.J. Targeted CML therapy: Controlling drug resistance, seeking cure. Curr. Opin. Genet. Dev. 2006, 16, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.; Manley, P.W.; Breitenstein, W.; Brüggen, J.; Cowan-Jacob, S.W.; Ray, A.; Huntly, B.; Fabbro, D.; Fendrich, G.; Hall-Meyers, E.; et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005, 7, 129–141. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Walters, D.K.; Stoffregen, E.P.; Jia, T.; Manley, P.W.; Mestan, J.; Cowan-Jacob, S.W.; Lee, F.Y.; Heinrich, M.C.; Deininger, M.W.N.; et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005, 65, 4500–4505. [Google Scholar] [CrossRef] [PubMed]
- Pricl, S.; Fermeglia, M.; Ferrone, M.; Tamborini, E. T315i-mutated BCR-ABL in chronic myeloid leukemia and imatinib: Insights from a computational study. Mol. Cancer Ther. 2005, 4, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Almaguer, D.; Tarín-Arzaga, L.; Cantú-Rodríguez, O.; Ceballos-López, A. More about imatinib and nilotinib combination therapy in chronic myeloid leukemia. Acta Haematol. 2013, 129, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Almaguer, D.; Saldaña-Vázquez, R.; Tarín-Arzaga, L.; Herrera-Rojas, M.A.; Vázquez-Mellado, L.A.; Cantú-Rodríguez, O.G.; Gutiérrez-Aguirre, C.H.; Jaime-Pérez, J.C. Combination of low-dose imatinib plus nilotinib for the treatment of chronic-phase chronic myeloid leukaemia after imatinib failure. Hematology 2016, 21, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pan, L.Q.; Hong, M.; Liu, W.X.; Qiao, C.; Li, J.Y.; Qian, S.X. The combination therapy of imatinib and dasatinib achieves long-term molecular response in two imatinib-resistant and dasatinibintolerant patients with advanced chronic myeloid leukemia. J. Biomed. Res. 2016, 30, 525–528. [Google Scholar] [PubMed]
- Zhang, L.; Radovic-Moreno, A.F.; Alexis, F.; Gu, F.X.; Basto, P.A.; Bagalkot, V.; Jon, S.; Langer, R.S.; Farokhzad, O.C. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle–aptamer bioconjugates. ChemMedChem 2007, 2, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cai, C.; Lin, J.; Chen, T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Hu, C.-M.J.; Zhang, L. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol. Pharm. 2011, 8, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, L.; Gu, W.; Xi, Y.; Lin, L.; Li, Y. Targeted nanoassembly loaded with docetaxel improves intracellular drug delivery and efficacy in murine breast cancer model. Mol. Pharm. 2008, 5, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Santander-Ortega, M.J.; Csaba, N.; González, L.; Bastos-González, D.; Ortega-Vinuesa, J.L.; Alonso, M.J. Protein-loaded plga–peo blend nanoparticles: Encapsulation, release and degradation characteristics. Colloid Polym. Sci. 2010, 288, 141–150. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B.; Wang, Y.; Lou, D. Dual drug release from core–shell nanoparticles with distinct release profiles. J. Pharm. Sci. 2014, 103, 3205–3216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D.; et al. Co-delivery of doxorubicin and curcumin by ph-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef] [PubMed]
- Naderinezhad, S.; Amoabediny, G.; Haghiralsadat, F. Co-delivery of hydrophilic and hydrophobic anticancer drugs using biocompatible ph-sensitive lipid-based nano-carriers for multidrug-resistant cancers. RSC Adv. 2017, 7, 30008–30019. [Google Scholar] [CrossRef]
- Song, X.R.; Cai, Z.; Zheng, Y.; He, G.; Cui, F.Y.; Gong, D.Q.; Hou, S.X.; Xiong, S.J.; Lei, X.J.; Wei, Y.Q. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in plga nanoparticles. Eur. J. Pharm. Sci. 2009, 37, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Pakunlu, R.I.; Brannan, A.; Bates, F.; Minko, T.; Discher, D.E. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J. Control. Release 2006, 116, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Subr, V.; Ulbrich, K.; Peschke, P.; Huber, P.E.; Hennink, W.E.; Storm, G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials 2009, 30, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.-T.; Benoît, J.-P.; Venier-Julienne, M.-C. Why and how to prepare biodegradable, monodispersed, polymeric microparticles in the field of pharmacy? Int. J. Pharm. 2011, 407, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Chen, F.-M.; Zhao, Y.-M.; Wu, H.; Deng, Z.-H.; Wang, Q.-T.; Zhou, W.; Liu, Q.; Dong, G.-Y.; Li, K.; Wu, Z.-F.; et al. Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-i from dextran–co-gelatin microspheres. J. Control. Release 2006, 114, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-J.; Yang, H.-H.; Chen, C.-H.; Lin, W.-W.; Chen, S.-C.; Lai, P.-H.; Chang, Y.; Sung, H.-W. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J. Control. Release 2007, 120, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Yashchenok, A.; Videnova, K.; Kreft, O.; Möhwald, H.; Skirtach, A.G. Multicompartmental micro- and nanocapsules: Hierarchy and applications in biosciences. Macromol. Biosci. 2010, 10, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, H.; Georgieva, R. Coupled enzyme reactions in multicompartment microparticles. Biomacromolecules 2010, 11, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Palama, I.E.; Leporatti, S.; de Luca, E.; Di Renzo, N.; Maffia, M.; Gambacorti-Passerini, C.; Rinaldi, R.; Gigli, G.; Cingolani, R.; Coluccia, A.M.L. Imatinib-loaded polyelectrolyte microcapsules for sustained targeting of BCR-ABL(+) leukemia stem cells. Nanomedicine 2010, 5, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Palama, I.E.; Coluccia, A.M.L.; Gigli, G. Uptake of imatinib-loaded polyelectrolyte complexes by BCR-ABL(+) cells: A long-acting drug-delivery strategy for targeting oncoprotein activity. Nanomedicine 2014, 9, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; D’Amone, S.; Gigli, G.; Palama, I.E. Sustained anti-BCR-ABL activity with ph responsive imatinib mesylate loaded PCL nanoparticles in cml cells. MedChemComm 2015, 6, 212–221. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Kim, S.; Park, K.; Kim, K.; Woo, D.G.; Kwon, I.C.; Chung, H.-M.; Park, K.-H. Heparin/poly(L-lysine) nanoparticle-coated polymeric microspheres for stem-cell therapy. J. Am. Chem. Soc. 2007, 129, 5788–5789. [Google Scholar] [CrossRef] [PubMed]
- Palama, I.E.; Cortese, B.; D’Amone, S.; Arcadio, V.; Gigli, G. Coupled delivery of imatinib mesylate and doxorubicin with nanoscaled polymeric vectors for a sustained downregulation of BCR-ABL in chronic myeloid leukemia. Biomater. Sci. 2015, 3, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Palama, I.E.; Cortese, B.; D’Amone, S.; Gigli, G. mRNA delivery using non-viral PCL nanoparticles. Biomater. Sci. 2015, 3, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Manders, E.M.; Stap, J.; Brakenhoff, G.J.; Van Driel, R.; Aten, J.A. Dynamics of three-dimensional replication patterns during the s-phase, analysed by double labelling of DNA and confocal microscopy. J. Cell Sci. 1992, 103, 857–862. [Google Scholar] [PubMed]
- Li, Q.; Lau, A.; Morris, T.J.; Guo, L.; Fordyce, C.B.; Stanley, E.F. A syntaxin 1, gα(o), and N-type calcium channel complex at a presynaptic nerve terminal: Analysis by quantitative immunocolocalization. J. Neurosci. 2004, 24, 4070–4081. [Google Scholar] [CrossRef] [PubMed]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- Talalay, T.C.C.A.P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. In Cancer Nanotechnology: Methods and protocols; Grobmyer, S.R., Moudgil, B.M., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 25–37. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular PH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.D.; Nori, A.; Tijerina, M.; Kopečková, P.; Kopeček, J. Cytoplasmic delivery and nuclear targeting of synthetic macromolecules. J. Control. Release 2003, 87, 89–105. [Google Scholar] [CrossRef]
- Panyam, J.; Zhou, W.-Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Kogure, K.; Akita, H.; Harashima, H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006, 58, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, M.; Malamas, A.; Shin, T.; Jin, E.; Sun, Y.; Lu, Z.-R. Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic sirna release. Mol. Pharm. 2014, 11, 2734–2744. [Google Scholar] [CrossRef] [PubMed]






| Sample | IC50 IM (nM) | IC50 Nil (nM) |
|---|---|---|
| Free IM | 150 nM | - |
| Free Nil | - | 30 nM |
| Free IM/Nil combination | 130 nM | 28 nM |
| IM released from PCL NPs | 70 nM | - |
| Nil released from PCL NPs | - | 18 nM |
| IM/Nil released from PCL NPs | 50 nM | 15 nM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortese, B.; D’Amone, S.; Palamà, I.E. Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy. Pharmaceutics 2018, 10, 52. https://doi.org/10.3390/pharmaceutics10020052
Cortese B, D’Amone S, Palamà IE. Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy. Pharmaceutics. 2018; 10(2):52. https://doi.org/10.3390/pharmaceutics10020052
Chicago/Turabian StyleCortese, Barbara, Stefania D’Amone, and Ilaria Elena Palamà. 2018. "Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy" Pharmaceutics 10, no. 2: 52. https://doi.org/10.3390/pharmaceutics10020052
APA StyleCortese, B., D’Amone, S., & Palamà, I. E. (2018). Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy. Pharmaceutics, 10(2), 52. https://doi.org/10.3390/pharmaceutics10020052