Review of Intraocular Pharmacokinetics of Anti-Infectives Commonly Used in the Treatment of Infectious Endophthalmitis
Abstract
:1. Introduction: The Infectious Endophthalmitis and the Need for Anti-Infective Treatment
2. Possible Routes of Administration of Anti-Infective Agents in Infectious Endophthalmitis and Intravitreals Currently Available
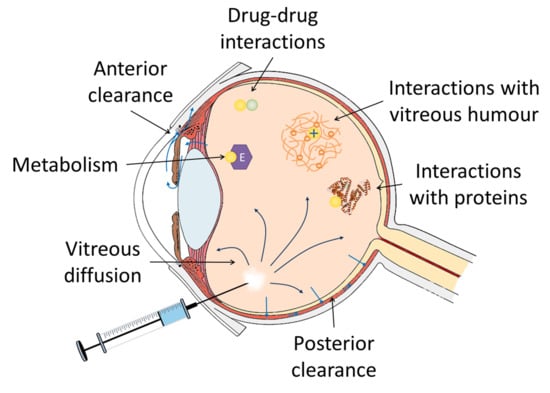
3. Factors Involved in the Intravitreal Pharmacokinetics of Anti-Infectives
3.1. Distribution of Anti-Infectives in the Vitreous
3.1.1. Influence of the Molecular Weight and the Charge of Anti-Infectives
3.1.2. Influence of Vitreous Convection on the Distribution of Anti-Infectives
3.1.3. Physiological and Pathological Conditions of the Vitreous
Influence of Patient Age and Vitreous Composition
Interaction of Drugs with the Components of the Vitreous Humour
Proteins
Vitreous Humour
Consequences of the Vitrectomy
3.1.4. Drug-Drug Interactions in the Vitreous Humour
3.2. Elimination of Anti-Infectives from the Vitreous Humour
3.2.1. Drug Metabolism in the Vitreous Humour
3.2.2. Vitreous Clearance of Drugs
Transporters in the Blood Retinal Barrier (BRB)
Influence on the Clearance of the Status of Ocular Inflammation
3.3. Repetition Rate of Intravitreal Injections
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Vaziri, K.; Schwartz, S.G.; Kishor, K.; Flynn, H.W. Endophthalmitis: State of the art. Clin. Ophthalmol. 2015, 9, 95–108. [Google Scholar] [PubMed]
- Barry, P.; Cordovés, L.; Gardner, S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery: Data, Dilemmas and Conclusions; The European Society for Cataract and Refractive Surgeons: Dublin, Ireland, 2013. [Google Scholar]
- López-Cabezas, C.; Muner, D.S.; Massa, M.R.; Mensa Pueyo, J.M. Antibiotics in endophthalmitis: Microbiological and pharmacokinetic considerations. Curr. Clin. Pharmacol. 2010, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Radhika, M.; Mithal, K.; Bawdekar, A.; Dave, V.; Jindal, A.; Relhan, N.; Albini, T.; Pathengay, A.; Flynn, H.W. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J. Ophthalmic Inflamm. Infect. 2014, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Barcia, M.G. Formulación Magistral en Oftalmología. In Aspectos Prácticos de la Farmacotécnia en un Servicio de Farmacia; Master Line Prodigio: Galpagar, Spain, 2011; ISBN 978-84-938177-3-2. [Google Scholar]
- Fernández-Ferreiro, A.; Barcia, M.G. Patología ocular. In Guía Rápida de Farmacia Hospitalaria; EDIMSA: Madrid, Spain, 2014; pp. 569–589. ISBN 978-84-7714-401-4. [Google Scholar]
- García-Sáenz, M.C.; Arias-Puente, A.; Fresnadillo-Martinez, M.J.; Carrasco-Font, C. Human aqueous humor levels of oral ciprofloxacin, levofloxacin, and moxifloxacin. J. Cataract Refract. Surg. 2001, 27, 1969–1974. [Google Scholar] [CrossRef]
- Fiscella, R.G.; Nguyen, T.K.; Cwik, M.J.; Phillpotts, B.A.; Friedlander, S.M.; Alter, D.C.; Shapiro, M.J.; Blair, N.P.; Gieser, J.P. Aqueous and vitreous penetration of levofloxacin after oral administration. Ophthalmology 1999, 106, 2286–2290. [Google Scholar] [CrossRef]
- Hariprasad, S.M.; Shah, G.K.; Mieler, W.F.; Feiner, L.; Blinder, K.J.; Holekamp, N.M.; Gao, H.; Prince, R.A. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Arch. Ophthalmol. 2006, 124, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Kampougeris, G.; Antoniadou, A.; Kavouklis, E.; Chryssouli, Z.; Giamarellou, H. Penetration of moxifloxacin into the human aqueous humour after oral administration. Br. J. Ophthalmol. 2005, 89, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Comer, G.M.; Peloquin, C.; Wheeler, J. Human vitreous distribution of linezolid after a single oral dose. Retina 2005, 25, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Fiscella, R.G.; Lai, W.W.; Buerk, B.; Khan, M.; Rodvold, K.A.; Pulido, J.S.; Labib, S.; Shapiro, M.J.; Blair, N.P. Aqueous and vitreous penetration of linezolid (Zyvox) after oral administration. Ophthalmology 2004, 111, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Atienza, R.; Sarasa, M.; Soy, D.; Adán, A.; Mensa, J. Pharmacokinetics of linezolid in human non-inflamed vitreous after systemic administration. J. Antimicrob. Chemother. 2009, 63, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 1995, 113, 1479–1496. [Google Scholar]
- Meredith, T.A. Endophthalmitis. In Intraocular Drug Delivery; Taylor & Francis Group: New York, NY, USA, 2006; pp. 349–356. ISBN 978-1-4200-1650-5. [Google Scholar]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Moorfields Eye Hospital (NHS). Ophthalmic Formulary; Moorfields Eye Hospital (NHS): London, UK, 2017. [Google Scholar]
- McElhiney, L.F. Compounding Guide for Ophthalmic Preparations, 1st ed.; American Pharmacists Association: Washington, DC, USA, 2013; ISBN 978-1-58212-177-2. [Google Scholar]
- Herreros, J.M.A. Preparación de Medicamentos y Formulación Magistral Para Oftalmología; Ediciones Díaz de Santos: Madrid, España, 2003; ISBN 978-84-7978-571-0. [Google Scholar]
- Trissel, L.A. A Trissel’s Stability of Compounded Formulations; Amer Pharmaceutical Assn: Washington, DC, USA, 2012; ISBN 978-1-58212-167-3. [Google Scholar]
- Pérez-Santonja, J.J.; Hervás-Hernandis, J.M. Queratitis Infecciosas: Fundamentos, Técnicas Diagnósticas y Tratamiento; Ergon: Townsville, Australia, 2006; ISBN 978-84-8473-447-5. [Google Scholar]
- Garg, A.; Sheppard, J.D.; Donnenfeld, E.D.; Friedlaender, M.H. Clinical Applications of Antibiotics and Anti-Inflammatory Drugs in Ophthalmology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; ISBN 978-0-7817-9123-6. [Google Scholar]
- The PubChem Project. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 14 June 2017).
- Chemicalize. Available online: https://chemicalize.com/#/ (accessed on 11 September 2017).
- Peyman, G.A. Antibiotic administration in the treatment of bacterial endophthalmitis. II. Intravitreal injections. Surv. Ophthalmol. 1977, 21, 339–346. [Google Scholar]
- Baum, J.; Peyman, G.A.; Barza, M. Intravitreal administration of antibiotic in the treatment of bacterial endophthalmitis. III. Consensus. Surv. Ophthalmol. 1982, 26, 204–206. [Google Scholar] [CrossRef]
- Thomas, B.J.; Mehta, N.; Yonekawa, Y.; Sridhar, J.; Kuriyan, A.E.; Relhan, N.; Liang, M.C.; Woodward, M.A.; Witkin, A.J.; Shah, C.; et al. Pars plana vitrectomy for late vitreoretinal sequelae of infectious endophthalmitis. Retina 2017, 37, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Diakonis, V.F.; Tsourdou, A.; Tzatzarakis, M.N.; Tsika, C.; Charisis, S.; Naoumidi, I.; Plainis, S.; Tsilimbaris, M.K. Evaluation of vitreous clearance and potential retinal toxicity of intravitreal lornoxicam (xefo). J. Ocul. Pharmacol. Ther. 2013, 29, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Meredith, T.A. Intravitreal Antimicrobials. In Intraocular Drug Delivery; Taylor & Francis Group: New York, NY, USA, 2006; pp. 85–95. ISBN 978-1-4200-1650-5. [Google Scholar]
- Meredith, T.A. Antimicrobial pharmacokinetics in endophthalmitis treatment: Studies of ceftazidime. Trans. Am. Ophthalmol. Soc. 1993, 91, 653–699. [Google Scholar] [PubMed]
- Wilson, C.G.; Tan, L.E.; Mains, J. Principles of Retinal Drug Delivery from Within the Vitreous. In Drug Product Development for the Back of the Eye; Springer Science & Business Media: Berlin, Germany, 2011; pp. 125–158. ISBN 978-1-4419-9920-7. [Google Scholar]
- Friedrich, S.; Saville, B.; Cheng, Y.L. Drug distribution in the vitreous humor of the human eye: The effects of aphakia and changes in retinal permeability and vitreous diffusivity. J. Ocul. Pharmacol. Ther. 1997, 13, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.-Y.; Nance, E.A.; Yang, J.-C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J. Control. Release 2013, 167, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M. Mishima Ocular Pharmacokinetics. In Pharmacology of the Eye; Springer Science & Business Media: Berlin, Germany, 2012; pp. 19–116. ISBN 978-3-642-69222-2. [Google Scholar]
- Krishnamoorthy, M.K.; Park, J.; Augsburger, J.J.; Banerjee, R.K. Effect of retinal permeability, diffusivity, and aqueous humor hydrodynamics on pharmacokinetics of drugs in the eye. J. Ocul. Pharmacol. Ther. 2008, 24, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bungay, P.M.; Lutz, R.J.; Augsburger, J.J.; Millard, R.W.; Sinha Roy, A.; Banerjee, R.K. Evaluation of coupled convective-diffusive transport of drugs administered by intravitreal injection and controlled release implant. J. Control. Release 2005, 105, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Stay, M.S.; Xu, J.; Randolph, T.W.; Barocas, V.H. Computer simulation of convective and diffusive transport of controlled-release drugs in the vitreous humor. Pharm. Res. 2003, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chirila, T.V.; Hong, Y. Chapter C2 The Vitreous Humor. 2016, 125–134. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Angi, M.; Kalirai, H.; Coupland, S.E.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic Analyses of the Vitreous Humour. Mediat. Inflamm. 2012, 2012, e148039. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.R.; Goel, R.; Subbannayya, Y.; Jacob, H.K.; Murthy, P.R.; Manda, S.S.; Patil, A.H.; Sharma, R.; Sahasrabuddhe, N.A.; Parashar, A.; et al. Proteomic analysis of human vitreous humor. Clin. Proteom. 2014, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Schauersberger, J.; Jager, W. In-vitro Investigation of the Protein Binding of Different Antibiotics in the Human Vitreous. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1853–1853. [Google Scholar]
- Petternel, V.; Krepler, K.; Schauersberger, J.; Wedrich, A. Fosfomycin in human vitreous: -In-vitro investigation of the protein binding of fosfomycin in human vitreous –Fosfomycin levels in the vitreous cavity after intravenous administration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4930–4930. [Google Scholar]
- Loukovaara, S.; Nurkkala, H.; Tamene, F.; Gucciardo, E.; Liu, X.; Repo, P.; Lehti, K.; Varjosalo, M. Quantitative Proteomics Analysis of Vitreous Humor from Diabetic Retinopathy Patients. J. Proteome Res. 2015, 14, 5131–5143. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ferreiro, A.; Luaces-Rodríguez, A.; Aguiar, P.; Pardo-Montero, J.; González-Barcia, M.; García-Varela, L.; Herranz, M.; Silva-Rodríguez, J.; Gil-Martínez, M.; Bermúdez, M.A.; et al. Preclinical PET Study of Intravitreal Injections. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2843–2851. [Google Scholar]
- Mandell, B.A.; Meredith, T.A.; Aguilar, E.; el-Massry, A.; Sawant, A.; Gardner, S. Effects of inflammation and surgery on amikacin levels in the vitreous cavity. Am. J. Ophthalmol. 1993, 115, 770–774. [Google Scholar] [CrossRef]
- Barza, M.; McCue, M. Pharmacokinetics of aztreonam in rabbit eyes. Antimicrob. Agents Chemother. 1983, 24, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Barza, M.; Kane, A.; Baum, J. The effects of infection and probenecid on the transport of carbenicillin from the rabbit vitreous humor. Investig. Ophthalmol. Vis. Sci. 1982, 22, 720–726. [Google Scholar]
- Barza, M.; Kane, A.; Baum, J. Pharmacokinetics of intravitreal carbenicillin, cefazolin, and gentamicin in rhesus monkeys. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1602–1606. [Google Scholar]
- Ficker, L.; Meredith, T.A.; Gardner, S.; Wilson, L.A. Cefazolin levels after intravitreal injection. Effects of inflammation and surgery. Investig. Ophthalmol. Vis. Sci. 1990, 31, 502–505. [Google Scholar]
- Barza, M.; Lynch, E.; Baum, J.L. Pharmacokinetics of newer cephalosporins after subconjunctival and intravitreal injection in rabbits. Arch. Ophthalmol. 1993, 111, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, A.; Meredith, T.A.; Kincaid, M.; Dick, J.; Aguilar, E.; Ritchie, D.J.; Reichley, R.M. Intraocular injection of ceftazidime. Effects of inflammation and surgery. Retina 1995, 15, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Waga, J.; Nilsson-Ehle, I.; Ljungberg, B.; Skarin, A.; Ståhle, L.; Ehinger, B. Microdialysis for pharmacokinetic studies of ceftazidime in rabbit vitreous. J. Ocul. Pharmacol. Ther. 1999, 15, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rootman, D.S.; Savage, P.; Hasany, S.M.; Chisholm, L.; Basu, P.K. Toxicity and pharmacokinetics of intravitreally injected ciprofloxacin in rabbit eyes. Can. J. Ophthalmol. 1992, 27, 277–282. [Google Scholar] [PubMed]
- Pearson, P.A.; Hainsworth, D.P.; Ashton, P. Clearance and distribution of ciprofloxacin after intravitreal injection. Retina 1993, 13, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, F.; Kortunay, S.; Kurt, E.; Ilker, S.S.; Inan, U.U.; Basci, N.E.; Bozkurt, A.; Kayaalp, O. Effects of trauma and infection on ciprofloxacin levels in the vitreous cavity. Retina 1999, 19, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Peyman, G.A.; Liang, C.; Hegazy, H.; Molinari, L.C.; Chen, J.; Brun, S.; Tarcha, P.J. Ocular toxicity of intravitreal clarithromycin. Retina 1999, 19, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Fiscella, R.; Peyman, G.A.; Fishman, P.H. Duration of therapeutic levels of intravitreally injected liposome-encapsulated clindamycin in the rabbit. Can. J. Ophthalmol. 1987, 22, 307–309. [Google Scholar] [PubMed]
- Ozcimen, M.; Sakarya, Y.; Ozcimen, S.; Sakarya, R.; Goktas, S.; Iyisoy, S.; Alpfidan, I.; Erdogan, E. Clearance of intravitreal daptomycin in uveitis-induced rabbit model. Curr. Eye Res. 2015, 40, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Lefèvre, S.; Acar, N.; Bourcier, T.; Marcellin, L.; Prévost, G.; Subilia, A.; Gaucher, D.; Jehl, F. Efficacy of intravitreal administrations of linezolid in an experimental model of S. aureus-related endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4832–4841. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.N.; He, F.; Wensel, T.G.; Mieler, W.F.; Benz, M.S.; Holz, E.R. Clearance of intravitreal moxifloxacin. Investig. Ophthalmol. Vis. Sci. 2006, 47, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, F.; Kortunay, S.; Kurt, E.; Ubeyt Inan, U.; Sami Ilker, S.; Basci, N.E.; Bozkurt, A.; Oguz Kayaalp, S. Ofloxacin levels after intravitreal injection. Effects of trauma and inflammation. Ophthalmic Res. 1999, 31, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, H.E.; Meredith, T.A.; el-Massry, A.; Shaarawy, A.; Kincaid, M.; Dick, J.; Ritchie, D.J.; Reichley, R.M.; Neisman, M.K. Vancomycin levels after intravitreal injection. Effects of inflammation and surgery. Retina 1995, 15, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.M.; López, M.I.; Pastor, J.C.; Nozal, M.J. Pharmacokinetics of intravitreal vancomycin in normal and infected rabbit eyes. J. Ocul. Pharmacol. Ther. 1998, 14, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Vallar, R.V.; Hong, C.H.; von Gunten, S.; Ruoff, K.; D’Amico, D.J. Intravitreal dexamethasone effect on intravitreal vancomycin elimination in endophthalmitis. Arch. Ophthalmol. 1999, 117, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.M.; Lopez, M.I.; Pastor, J.C. Pharmacokinetics of 0.5 mg of a single and a multiple dose of intravitreal vancomycin in infected rabbit eyes. J. Ocul. Pharmacol. Ther. 2000, 16, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Doft, B.H.; Weiskopf, J.; Nilsson-Ehle, I.; Wingard, L.B. Amphotericin clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology 1985, 92, 1601–1605. [Google Scholar] [CrossRef]
- Wingard, L.B.; Zuravleff, J.J.; Doft, B.H.; Berk, L.; Rinkoff, J. Intraocular distribution of intravitreally administered amphotericin B in normal and vitrectomized eyes. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2184–2189. [Google Scholar]
- Shen, Y.-C.; Liang, C.-Y.; Wang, C.-Y.; Lin, K.-H.; Hsu, M.-Y.; Yuen, H.-L.; Wei, L.-C. Pharmacokinetics and safety of intravitreal caspofungin. Antimicrob. Agents Chemother. 2014, 58, 7234–7239. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Velpandian, T.; Dhingra, N.; Jaiswal, J. Intravitreal pharmacokinetics of plain and liposome-entrapped fluconazole in rabbit eyes. J. Ocul. Pharmacol. Ther. 2000, 16, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Wang, M.-Y.; Wang, C.-Y.; Tsai, T.-C.; Tsai, H.-Y.; Lee, Y.-F.; Wei, L.-C. Clearance of intravitreal voriconazole. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.; Gini, G. Ten years after... are findings of the Endophthalmitis Vitrectomy Study still relevant today? Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Aras, C.; Ozdamar, A.; Karacorlu, M.; Ozkan, S. Silicone oil in the surgical treatment of endophthalmitis associated with retinal detachment. Int. Ophthalmol. 2001, 24, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Peyman, G.A.; Vastine, D.W.; Raichand, M. Experimental Aspects and Their Clinical Application. Ophthalmology 1978, 85, 374–385. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Lim, J.I. Aminoglycoside toxicity in the treatment of endophthalmitis. The Aminoglycoside Toxicity Study Group. Arch. Ophthalmol. 1994, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, H.M.; Kivilcim, M.; Peyman, G.A.; Unal, M.H.; Liang, C.; Molinari, L.C.; Kazi, A.A. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir in a silicone oil-filled eye. Retina 1999, 19, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Wakely, L.; Sheard, R. Recent Advances in Endophthalmitis Management; Royal College of Ophthalmologists: London, UK, 2014. [Google Scholar]
- Fiscella, R.G. Physical incompatibility of vancomycin and ceftazidime for intra-vitreal injection. Arch. Ophthalmol. 1993, 11, 730. [Google Scholar] [CrossRef]
- Lifshitz, T.; Lapid-Gortzak, R.; Finkelman, Y.; Klemperer, I. Vancomycin and ceftazidime incompatibility upon intravitreal injection. Br. J. Ophthalmol. 2000, 84, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Hui, M.; Kwok, A.K.H.; Pang, C.P.; Cheung, S.W.; Chan, R.C.Y.; Lam, D.S.C.; Cheng, A.F.B. An in vitro study on the compatibility and precipitation of a combination of ciprofloxacin and vancomycin in human vitreous. Br. J. Ophthalmol. 2004, 88, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ashton, P. Retinal Drug Delivery. In Intraocular Drug Delivery; Taylor & Francis Group: New York, NY, USA, 2006; pp. 1–25. ISBN 978-1-4200-1650-5. [Google Scholar]
- Dias, C.S.; Anand, B.S.; Mitra, A.K. Effect of mono- and di-acylation on the ocular disposition of ganciclovir: Physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J. Pharm. Sci. 2002, 91, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Role of metabolism in ocular drug delivery. Curr. Drug Metab. 2004, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xiang, C.D.; Gale, D.; Carreiro, S.; Wu, E.Y.; Zhang, E.Y. Drug Transporter and Cytochrome P450 mRNA Expression in Human Ocular Barriers: Implications for Ocular Drug Disposition. Drug Metab. Dispos. 2008, 36, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004, 78, 715–721. [Google Scholar] [CrossRef]
- Mannermaa, E.; Vellonen, K.-S.; Urtti, A. Drug transport in corneal epithelium and blood-retina barrier: Emerging role of transporters in ocular pharmacokinetics. Adv. Drug Deliv. Rev. 2006, 58, 1136–1163. [Google Scholar] [CrossRef] [PubMed]
- Vellonen, K.-S.; Hellinen, L.; Mannermaa, E.; Ruponen, M.; Urtti, A.; Kidron, H. Expression, activity and pharmacokinetic impact of ocular transporters. Adv. Drug Deliv. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, H.; Zang, X.; Chen, M.; Jiang, H.; Han, S.; Wu, X. Expression of efflux transporters in human ocular tissues. Drug Metab. Dispos. 2013, 41, 1934–1948. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.G.; Mangini, N.J. P-glycoprotein expression in human retinal pigment epithelium. Mol. Vis. 2002, 8, 422–430. [Google Scholar] [PubMed]
- Chapy, H.; Saubaméa, B.; Tournier, N.; Bourasset, F.; Behar-Cohen, F.; Declèves, X.; Scherrmann, J.-M.; Cisternino, S. Blood-brain and retinal barriers show dissimilar ABC transporter impacts and concealed effect of P-glycoprotein on a novel verapamil influx carrier. Br. J. Pharmacol. 2016, 173, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Setoguchi, C.; Kawazu, K.; Hosoya, K. Impact of P-glycoprotein on blood-retinal barrier permeability: Comparison of blood-aqueous humor and blood-brain barrier using mdr1a knockout rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4650–4658. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Karch, R.; Tournier, N.; Cisternino, S.; Wadsak, W.; Hacker, M.; Marhofer, P.; Zeitlinger, M.; Langer, O. Assessment of P-Glycoprotein Transport Activity at the Human Blood-Retina Barrier with (R)-11C-Verapamil PET. J. Nucl. Med. 2017, 58, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Thamlikitkul, V.; Dubrovskaya, Y.; Manchandani, P.; Ngamprasertchai, T.; Boonyasiri, A.; Babic, J.T.; Tam, V.H. Dosing and Pharmacokinetics of Polymyxin B in Patients with Renal Insufficiency. Antimicrob. Agents Chemother. 2017, 61, e01337-16. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, J.M.; Owusu Obeng, A.; Klein, T.E.; Altman, R.B. PharmGKB summary: Voriconazole pathway, pharmacokinetics. Pharmacogenet. Genom. 2017, 27, 201–209. [Google Scholar] [CrossRef] [PubMed]

| MW | Net Charge | |
|---|---|---|
| INTRAVITREAL ANTIBIOTICS | ||
| Amikacin 0.4 mg/0.1 mL o 0.1 mg/0.1 mL | 585.608 g/mol | +3.80 |
| Ampicillin 5 mg/0.1 mL | 349.405 g/mol | −0.60 |
| Aztreonam 0.1 mg/0.1 mL | 435.426 g/mol | −2.00 |
| Cefazolin 2.25 mg/0.1 mL or 2.5 mg/0.1 mL | 454.498 g/mol | −1.00 |
| Cefotaxime 0.4 mg/0.1 mL | 455.460 g/mol | −1.00 |
| Ceftazidime 2 mg/0.1 mL | 546.573 g/mol | −1.00 |
| Ceftriaxone 2 mg/0.1 mL | 554.571 g/mol | −2.00 |
| Ciprofloxacin 0.1 mg/0.1 mL | 331.347 g/mol | −0.02 |
| Clindamycin 0.5 mg/0.1 mL and 1 mg/0.1 mL | 424.981 g/mol | +0.59 |
| Gentamicin 200 μg/0.1 mL | 477.603 g/mol | +4.52 |
| Levofloxacin 0.625 mg/0.1 mL | 361.373 g/mol | −0.92 |
| Lincomycin 1 mg/0.1mL | 406.538 g/mol | +0.79 |
| Moxifloxacin 160 μg /0.1 mL | 401.438 g/mol | +0.01 |
| Penicillin G 300 units/0.1 mL | 334.390 g/mol | −1.00 |
| Piperacillin/Tazobactam 1.5 mg/0.1 mL | 517.557 g/mol/300.289 g/mol | −1.00 |
| Tobramycin 100 μg /0.1 mL or 200 μg /0.1 mL or 300 μg /0.1 mL or 400 μg /0.1 mL | 467.520 g/mol | +4.42 |
| Vancomycin 1 mg/0.1 mL or 2 mg/0.1 mL | 1449.265 g/mol | +0.89 |
| INTRAVITREAL ANTIFUNGALS | ||
| Colloidal Amphotericin B 5 μg/0.1 mL | 924.091 g/mol | −0.02 |
| Voriconazole 0.05 mg/0.1 mL | 349.317 g/mol | −0.00 |
| INTRAVITREAL ANTIVIRALS | ||
| Ganciclovir 20 mg/mL | 255.234 g/mol | −0.00 |
| Acyclovir 80 μg/0.1 mL or 200 μg/0.1 mL | 225.208 g/mol | −0.00 |
| Foscarnet 1220 μg/0.1 mL | 126.004 g/mol | −2.06 |
| Cidovofir 0.2 mg/mL and 8.1 mg/mL | 279.189 g/mol | −1.38 |
| Anti-Infective | Study Model | Delivered Dose in 0.1 mL | Elimination Route | t1/2 Normal Condition | t1/2 Inflammation | t1/2 Aphakia | t1/2 Aphakia + Vitrectomy | Ref. |
|---|---|---|---|---|---|---|---|---|
| ANTIBIOTICS | ||||||||
| Amikacin | Rabbit | 400 µg/0.1 mL | Anterior | 25.5 h | 15.5 h | 14.3 h | 7.9 h | [46] |
| Aztreonam | Rabbit | 100 µg | Posterior | 7.5 h | [47] | |||
| Carbenicillin | Rabbit | 1000 µg | Posterior | 5 h | 6 h | [48] | ||
| Rhesus monkey | 1000 µg | Posterior | 10 h | [49] | ||||
| Cefazolin | Rhesus monkey | 1000 µg | Posterior | 7 h | [49] | |||
| Rabbit | 2250 µg | Posterior | 6.5 h | 10.4 h | 8.3 h | 6.0 h | [50] | |
| Cefepime | Rabbit | 1000 µg | Anterior | 14.3 h | 15.1 h | [51] | ||
| Ceftazidime | Rabbit | 1000 µg | Anterior | 20 h | 21.5 h | [51] | ||
| Rabbit | 2250 µg | Both | 13.8 h | 10.1 h | 11.8 h | 4.7 h | [52] | |
| Rabbit | 1000 µg | 8.1 h | 11.7 h | [53] | ||||
| Ceftriaxone | Rabbit | 1000 µg | Both | 9.1 h | 13.1 h | [51] | ||
| Ciprofloxacin | Rabbit | 250 µg | 4.5 h | [54] | ||||
| Rabbit | 100 µg | Posterior | 2.2 h | 1 h | [55] | |||
| Rabbit | 200 µg | 6.02 h | 15.06 h | [56] | ||||
| Clarithromycin | Rabbit | 1000 µg | 2 h | [57] | ||||
| Clindamycin | Rabbit | 800 µg | 3 h | [58] | ||||
| Daptomycin | Rabbit | 200 µg * | Both | 25.67 h | 34.6 h | [59] | ||
| Gentamicin | Rhesus monkey | 100 µg | Anterior | 33 h | [49] | |||
| Linezolid | Rabbit | 1, 10, 30 mg | 2 h | [60] | ||||
| Moxifloxacin | Rabbit | 200 µg | Posterior | 1.72 h | [61] | |||
| Ofloxacin | Rabbit | 200 µg | 5.65 h | 9.72 h | [62] | |||
| Vancomycin | Rabbit | 1000 µg | 25.1 h | 8.9 h | 9.0 h | [63] | ||
| Rabbit | 1000 µg | Both | 62.34 h | 14.53 h | [64] | |||
| Rabbit | 1000 µg | 56 h | 48 h | [65] | ||||
| Rabbit | 500 µg | 12.3 h | [66] | |||||
| ANTIFUNGALS | ||||||||
| Amphotericin B | Rabbit | 10 µg | Posterior | 9.1 days | 8.6 days | 4.7 days | 1.4 days | [67] |
| Rabbit | 9.1–13.4 µg | 6.9–15.1 days | 1.8 days | [68] | ||||
| Caspofungin | Rabbit | 50 µg | Posterior | 6.28 h | [69] | |||
| Fluconazole | Rabbit | 100 µg | Posterior | 23 min 3.18 h | [70] | |||
| Voriconazole | Rabbit | 35 µg | Posterior | 2.5 h | [71] | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luaces-Rodríguez, A.; González-Barcia, M.; Blanco-Teijeiro, M.J.; Gil-Martínez, M.; Gonzalez, F.; Gómez-Ulla, F.; Lamas, M.-J.; Otero-Espinar, F.-J.; Fernández-Ferreiro, A. Review of Intraocular Pharmacokinetics of Anti-Infectives Commonly Used in the Treatment of Infectious Endophthalmitis. Pharmaceutics 2018, 10, 66. https://doi.org/10.3390/pharmaceutics10020066
Luaces-Rodríguez A, González-Barcia M, Blanco-Teijeiro MJ, Gil-Martínez M, Gonzalez F, Gómez-Ulla F, Lamas M-J, Otero-Espinar F-J, Fernández-Ferreiro A. Review of Intraocular Pharmacokinetics of Anti-Infectives Commonly Used in the Treatment of Infectious Endophthalmitis. Pharmaceutics. 2018; 10(2):66. https://doi.org/10.3390/pharmaceutics10020066
Chicago/Turabian StyleLuaces-Rodríguez, Andrea, Miguel González-Barcia, María José Blanco-Teijeiro, María Gil-Martínez, Francisco Gonzalez, Francisco Gómez-Ulla, María-Jesús Lamas, Francisco-Javier Otero-Espinar, and Anxo Fernández-Ferreiro. 2018. "Review of Intraocular Pharmacokinetics of Anti-Infectives Commonly Used in the Treatment of Infectious Endophthalmitis" Pharmaceutics 10, no. 2: 66. https://doi.org/10.3390/pharmaceutics10020066
APA StyleLuaces-Rodríguez, A., González-Barcia, M., Blanco-Teijeiro, M. J., Gil-Martínez, M., Gonzalez, F., Gómez-Ulla, F., Lamas, M.-J., Otero-Espinar, F.-J., & Fernández-Ferreiro, A. (2018). Review of Intraocular Pharmacokinetics of Anti-Infectives Commonly Used in the Treatment of Infectious Endophthalmitis. Pharmaceutics, 10(2), 66. https://doi.org/10.3390/pharmaceutics10020066