Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process
Abstract
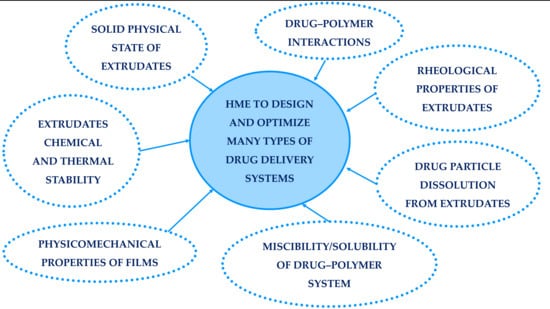
:1. Introduction
- The chemical and thermal stability of extrudates
- The solid physical state of extrudates
- The drug-polymer interaction
- The miscibility/solubility of the drug-polymer systems
- The rheological properties of extrudates
- The physicomechanical properties of films produced by hot melt extrusion
- The drug particle dissolution from extrudates
1.1. Hot Melt Extrusion: General Aspects
1.1.1. Hot Melt Extrusion Technologies
1.1.2. Hot Melt Extrusion Scale-Up
1.2. Pre-Formulation, Formulation and Process, Post-Formulation
1.3. The Chemical and Thermal Stability of Extrudates
1.4. The Solid Physical State of Extrudates
1.5. The Drug-Polymer Interaction
1.6. The Miscibility/Solubility of Drug-Polymer Systems
1.7. The Rheological Properties of Extrudates
1.8. Physicomechanical Properties of Films Produced by Hot Melt Extrusion
1.9. The Drug Particle Dissolution from Extrudates
2. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.; Margetson, D.; Jones, D.; McAllister, S.; Diak, O. A Basic Guide: Hot-Melt Extrusion; UKICRS: Belfast, UK, 2008; Volume 13. [Google Scholar]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Koleng, J.J.; McGinity, J.W. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 2002, 23, 4241–4248. [Google Scholar] [CrossRef]
- Melocchi, A.; Loreti, G.; Del Curto, M.D.; Maroni, A.; Gazzaniga, A.; Zema, L. Evaluation of hot-melt extrusion and injection molding for continuous manufacturing of immediate-release tablets. J. Pharm. Sci. 2015, 104, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Feng, X.; Pimparade, M.; Ye, X.; Kim, D.W.; Martin, S.T.; Repka, M.A. Dual-mechanism gastroretentive drug delivery system loaded with an amorphous solid dispersion prepared by hot-melt extrusion. Eur. J. Pharm. Sci. 2017, 102, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albarahmieh, E.; Qi, S.; Craig, D.Q.M. Hot melt extruded transdermal films based on amorphous solid dispersions in Eudragit RS PO: The inclusion of hydrophilic additives to develop moisture-activated release systems. Int. J. Pharm. 2016, 514, 270–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosse, A.; Konig, C.; Lamprecht, A.; Wagner, K.G. Hot Melt Extrusion for Sustained Protein Release: Matrix Erosion and In Vitro Release of PLGA-Based Implants. AAPS PharmSciTech 2017, 18, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Rothen-Weinhold, A.; Oudry, N.; Schwach-Abdellaoui, K.; Frutiger-Hughes, S.; Hughes, G.J.; Jeannerat, D.; Burger, U.; Besseghir, K.; Gurny, R. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur. J. Pharm. Biopharm. 2000, 49, 253–257. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Sibilia, F.; Henschel, V.G.; Fare, S.; Tanzi, M.C. Structure and properties of polycaprolactone/ibuprofen rods prepared by melt extrusion for implantable drug delivery. Polym. Bull. 2017, 74, 4973–4987. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. A review on the taste masking of bitter APIs: Hot-melt extrusion (HME) evaluation. Drug Dev. Ind. Pharm. 2014, 40, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Pimparade, M.B.; Morott, J.T.; Park, J.B.; Kulkarni, V.I.; Majumdar, S.; Murthy, S.N.; Lian, Z.Y.; Pinto, E.; Bi, V.; Dung, T.; et al. Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro-in vivo evaluations. Int. J. Pharm. 2015, 487, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Javeer, S.D.; Patole, R.; Amin, P. Enhanced solubility and dissolution of simvastatin by HPMC-based solid dispersions prepared by hot melt extrusion and spray-drying method. J. Pharm. Investig. 2013, 43, 471–480. [Google Scholar] [CrossRef]
- Liu, X.; Lu, M.; Guo, Z.F.; Huang, L.; Feng, X.; Wu, C.B. Improving the Chemical Stability of Amorphous Solid Dispersion with Cocrystal Technique by Hot Melt Extrusion. Pharm. Res. 2012, 29, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs - An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Vynckier, A.K.; De Beer, M.; Monteyne, T.; Voorspoels, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Enteric protection of naproxen in a fixed-dose combination product produced by hot-melt co-extrusion. Int. J. Pharm. 2015, 49, 243–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorackova, K.; Dolezel, P.; Maskova, E.; Muselik, J.; Kejdusova, M.; Vetchy, D. The effect of acid pH modifiers on the release characteristics of weakly basic drug from hydrophlilic-lipophilic matrices. AAPS PharmSciTech 2013, 14, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Follonier, N.; Doelker, E.; Cole, E.T. Various Ways of Modulating the Release of Diltiazem Hydrochloride from Hot-Melt Extruded Sustained-Release Pellets Prepared Using Polymeric Materials. J. Control. Release 1995, 36, 243–250. [Google Scholar] [CrossRef]
- Bhagurkar, A.M.; Repka, M.A.; Murthy, S.N. A Novel Approach for the Development of a Nanostructured Lipid Carrier Formulation by Hot-Melt Extrusion Technology. J. Pharm. Sci. 2017, 106, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.; Spahn-Langguth, H.; Vergnault, G.; Grenier, P.; Tubic Grozdanis, M.; Lenhardt, T.; Langguth, P. Pharmacokinetic evaluation of oral fenofibrate nanosuspensions and SLN in comparison to conventional suspensions of micronized drug. Adv. Drug Deliv. Rev. 2007, 59, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Feng, X.; Ye, X.; Majumdar, S.; Repka, M.A. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: A systematic study based on a quality by design approach. AAPS J. 2015, 17, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.C.; Kumar, M.S.; Mathivanan, N.; Rao, M.E. Nanosuspensions as the most promising approach in nanoparticulate drug delivery systems. Pharmazie 2004, 59, 5–9. [Google Scholar] [PubMed]
- Rauwendaal, C. Polymer Extrusion; Hanser Publishers: Munchen, Germany, 1986; pp. 20–25. [Google Scholar]
- McGinity, J.W.; Zhang, F.; Koleng, J.; Repka, M. Hot-melt extrusion as a pharmaceutical process. Am. Pharm. Rev. 2001, 4, 25–37. [Google Scholar]
- Stankovic, M.; Frijlink, H.W.; Hinrichs, W.L.J. Polymeric formulations for drug release prepared by hot melt extrusion: Application and characterization. Drug Discov. Today 2015, 20, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; Krier, F.; Evrard, B. A review of pharmaceutical extrusion: Critical process parameters and scaling-up. Int. J. Pharm. 2015, 479, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Bialleck, S.; Rein, H. Preparation of starch-based pellets by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 79, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.J.; Sandhu, H.K.; Iyer, R.M.; Shah, N.H.; Malick, A.W.; Zia, H. Characterization of physico-mechanical properties of indomethacin and polymers to assess their suitability for hot-melt extrusion processs as a means to manufacture solid dispersion/solution. J. Pharm. Sci. 2005, 94, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Martin, C. Continuous mixing of solid dosage forms via hot-melt extrusion. Pharm. Technol. 2008, 32, 76–86. [Google Scholar]
- Hengsawas Surasarang, S.; Keen, J.M.; Huang, S.; Zhang, F.; McGinity, J.W.; Williams, R.O., III. Hot melt extrusion versus spray drying: Hot melt extrusion degrades albendazole. Drug Dev. Ind. Pharm. 2017, 43, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Ghebremeskel, A.N.; Vemavarapu, C.; Lodaya, M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer-surfactant combinations using solubility parameters and testing the processability. Int. J. Pharm. 2007, 328, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Low, A.Q.J.; Parmentier, J.; Khong, Y.M.; Chai, C.C.E.; Tun, T.Y.; Berania, J.E.; Liu, X.; Gokhale, R.; Chan, S.Y. Effect of type and ratio of solubilising polymer on characteristics of hot-melt extruded orodispersible films. Int. J. Pharm. 2013, 455, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, W.S.; Piao, J.; Yoon, I.S.; Kim, D.D.; Cho, H.J. Soluplus(R)/TPGS-based solid dispersions prepared by hot-melt extrusion equipped with twin-screw systems for enhancing oral bioavailability of valsartan. Drug Des. Dev. Ther. 2015, 9, 2745–2756. [Google Scholar]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T.; Maniruzzaman, M.; Halsey, S.A.; Chowdhry, B.Z.; Douroumis, D. Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach. Drug Deliv. Transl. Res. 2014, 4, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Feng, X.; Morott, J.T.; Pimparade, M.B.; Tiwari, R.V.; Zhang, F.; Repka, M.A. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2016, 98, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jaeghere, W.; De Beer, T.; Van Bocxlaer, J.; Remon, J.P.; Vervaet, C. Hot-melt extrusion of polyvinyl alcohol for oral immediate release applications. Int. J. Pharm. 2015, 492, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, D.Q.M. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm. 2002, 231, 131–144. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Rana, M.M.; Boateng, J.S.; Mitchell, J.C.; Douroumis, D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev. Ind. Pharm. 2013, 39, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process technology to pharmaceutical products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef] [PubMed]
- Hitzer, P.; Bauerle, T.; Drieschner, T.; Ostertag, E.; Paulsen, K.; van Lishaut, H.; Lorenz, G.; Rebner, K. Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions. Anal. Bioanal. Chem. 2017, 409, 4321–4333. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lu, M.; Li, Y.; Pang, H.; Lin, L.; Liu, X.; Wu, C. The utilization of drug-polymer interactions for improving the chemical stability of hot-melt extruded solid dispersions. J. Pharm. Pharmacol. 2014, 66, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; Lebrun, P.; Vinassa, C.; Adam, M.; Netchacovitch, L.; Ziemons, E.; Hubert, P.; Krier, F.; Evrard, B. Continuous production of itraconazole-based solid dispersions by hot melt extrusion: Preformulation, optimization and design space determination. Int. J. Pharm. 2016, 515, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Fule, R.; Meer, T.; Amin, P.; Dhamecha, D.; Ghadlinge, S. Preparation and characterisation of lornoxicam solid dispersion systems using hot melt extrusion technique. J. Pharm. Investig. 2014, 44, 41–59. [Google Scholar] [CrossRef]
- Jaiswar, D.R.; Jha, D.; Amin, P.D. Preparation and characterizations of stable amorphous solid solution of azithromycin by hot melt extrusion. J. Pharm. Investig. 2016, 46, 655–668. [Google Scholar] [CrossRef]
- Griff, A.L. Plastics Extrusion Technology; Reinhold Book Corp.: London, UK, 1968. [Google Scholar]
- Sauceau, M.; Fages, J.; Common, A.; Nikitine, C.; Rodier, E. New challenges in polymer foaming: A review of extrusion processes assisted by supercritical carbon dioxide. Prog. Polym. Sci. 2011, 36, 749–766. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for industry, PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance; Food and Drug Administration: Silver Spring, MD, USA, 2004. Available online: http://www.fda.gov/cder/guidance/published.html (accessed on 10 July 2018).
- World Health Organization. Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products; WHO Technical Report Series; WHO: Geneva, Switzerland, 2009; Volume 953, pp. 87–123. [Google Scholar]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Meena, A.; Parikh, T.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-I: Polyvinylpyrrolidone and related polymers. J. Excip. Food Chem. 2016, 5, 1001. [Google Scholar]
- Martino, P.D.; Magnoni, F.; Peregrina, D.V.; Gigliobianco, M.R.; Censi, R.; Malaj, L. Formation, Physicochemical Characterization, and Thermodynamic Stability of the Amorphous State of Drugs and Excipients. Curr. Pharm. Des. 2016, 22, 4959–4974. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Hempenstall, J.; Tucker, T.; Rades, T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int. J. Pharm. 2001, 226, 147–161. [Google Scholar]
- Wu, Y. The use of liquid chromatography-mass spectrometry for the identification of drug degradation products in pharmaceutical formulations. Biomed. Chromatogr. 2000, 14, 384–396. [Google Scholar] [CrossRef]
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O., III. A New Extrudable Form of Hypromellose: AFFINISOL HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; O’Donnell, K.P.; Delpon de Vaux, S.M.; O’Brien, J.; Stutzman, J.; Williams, R.O., III. Processing thermally labile drugs by hot-melt extrusion: The lesson with gliclazide. Eur. J. Pharm. Biopharm. 2017, 119, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Haser, A.; Huang, S.; Listro, T.; White, D.; Zhang, F. An approach for chemical stability during melt extrusion of a drug substance with a high melting point. Int. J. Pharm. 2017, 524, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Karandikar, H.; Ambardekar, R.; Kelly, A.; Gough, T.; Paradkar, A. Systematic identification of thermal degradation products of HPMCP during hot melt extrusion process. Int. J. Pharm. 2015, 486, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haser, A.; Cao, T.; Lubach, J.; Listro, T.; Acquarulo, L.; Zhang, F. Melt extrusion vs. spray drying: The effect of processing methods on crystalline content of naproxen-povidone formulations. Eur. J. Pharm. Sci. 2017, 102, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; McGinity, J.W. Influence of vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int. J. Pharm. 2000, 202, 63–70. [Google Scholar] [CrossRef]
- Wu, C.; McGinity, J.W. Influence of methylparaben as a solid-state plasticizer on the physicochemical properties of Eudragit® RS PO hot-melt extrudates. Eur. J. Pharm. Biopharm. 2003, 56, 95–100. [Google Scholar] [CrossRef]
- Lakshman, J.P.; Cao, Y.; Kowalski, J.; Serajuddin, A.T. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol. Pharm. 2008, 5, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Yablon, D.G. Scanning Probe Microscopy for Industrial Applications: Nanomechanical Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ruland, W. X-ray determination of crystallinity and diffuse disorder scattering. Acta Crystallogr. 1961, 14, 1180–1185. [Google Scholar] [CrossRef] [Green Version]
- Hermans, P.; Weidinger, A. Quantitative x-ray investigations on the crystallinity of cellulose fibers. A background analysis. J. Appl. Phys. 1948, 19, 491–506. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Bonnefille, M.; Aranyos, A.; Mitchell, J.C.; Douroumis, D. Taste masking of paracetamol by hot-melt extrusion: An in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Miller, D.A.; Peppas, N.A.; McGinity, J.W. Influence of sulfobutyl ether beta-cyclodextrin (Captisol) on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion. Int. J. Pharm. 2008, 350, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Lenz, E.; Jensen, K.T.; Blaabjerg, L.I.; Knop, K.; Grohganz, H.; Lobmann, K.; Rades, T.; Kleinebudde, P. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin-arginine. Eur. J. Pharm. Biopharm. 2015, 96, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Li, L.; Marsac, P.; Marks, B.; Liu, Z.; Brown, C. Impact of polymer type on bioperformance and physical stability of hot melt extruded formulations of a poorly water soluble drug. Int. J. Pharm. 2016, 505, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Reading, M.; Elliott, D.; Hill, V. A new approach to the calorimetric investigation of physical and chemical transitions. J. Therm. Anal. Calorim. 1993, 40, 949–955. [Google Scholar] [CrossRef]
- Lamm, M.S.; DiNunzio, J.; Khawaja, N.N.; Crocker, L.S.; Pecora, A. Assessing Mixing Quality of a Copovidone-TPGS Hot Melt Extrusion Process with Atomic Force Microscopy and Differential Scanning Calorimetry. AAPS PharmSciTech 2016, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Sarode, A.L.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H. Hot melt extrusion (HME) for amorphous solid dispersions: Predictive tools for processing and impact of drug-polymer interactions on supersaturation. Eur. J. Pharm. Sci. 2013, 48, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Morgan, D.J.; Mendham, A.P.; Pang, J.; Snowden, M.J.; Douroumis, D. Drug-polymer intermolecular interactions in hot-melt extruded solid dispersions. Int. J. Pharm. 2013, 443, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Scoutaris, N.; Maniruzzaman, M.; Moradiya, H.G.; Halsey, S.A.; Bradley, M.S.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. Implementation of transmission NIR as a PAT tool for monitoring drug transformation during HME processing. Eur. J. Pharm. Biopharm. 2015, 96, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saerens, L.; Dierickx, L.; Quinten, T.; Adriaensens, P.; Carleer, R.; Vervaet, C.; Remon, J.P.; De Beer, T. In-line NIR spectroscopy for the understanding of polymer-drug interaction during pharmaceutical hot-melt extrusion. Eur. J. Pharm. Biopharm. 2012, 81, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Renterghem, J.; Kumar, A.; Vervaet, C.; Remon, J.P.; Nopens, I.; Vander Heyden, Y.; De Beer, T. Elucidation and visualization of solid-state transformation and mixing in a pharmaceutical mini hot melt extrusion process using in-line Raman spectroscopy. Int. J. Pharm. 2017, 517, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrand, J.H.; Scott, R.S. The Solubility of Nonelectrolyte; Reinhold Publishing Corporation: New York, NY, USA, 1950. [Google Scholar]
- Hansen, C.M. The Universality of the solubility parameter. Ind. Eng. Chem. Prod. Res. Dev. 1969, 8, 1–11. [Google Scholar] [CrossRef]
- Barton, A.F.M. Solubility Parameters. Chem. Rev. 1975, 75, 731–753. [Google Scholar] [CrossRef]
- Desai, D.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H.; Phuapradit, W.; Vaka, S.R.K. Selection of Solid-State Plasticizers as Processing Aids for Hot-Melt Extrusion. J. Pharm. Sci. 2018, 107, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W. Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 1990; pp. 189–225. [Google Scholar]
- Fedors, R.F. A Method for Estimating Both the Solubility Parameters and Molar Volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Meena, A.; Parikh, T.; Gupta, S.S.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-II: Cellulosic polymers. J. Excip. Food Chem. 2014, 5, 46–55. [Google Scholar]
- Maru, S.M.; de Matas, M.; Kelly, A.; Paradkar, A. Characterization of thermal and rheological properties of zidovidine, lamivudine and plasticizer blends with ethyl cellulose to assess their suitability for hot melt extrusion. Eur. J. Pharm. Sci. 2011, 44, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Gerding, T.G.; Repka, S.L.; McGinity, J.W. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev. Ind. Pharm. 1999, 25, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Selmin, F.; Montanari, L. Fast dissolving films made of maltodextrins. Eur. J. Pharm. Biopharm. 2008, 70, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Fredersdorf, A.; Schroeder, B.; Kucera, S.; Prodduturi, S.; Repka, M.A.; McGinity, J.W. The influence of guaifenesin and ketoprofen on the properties of hot-melt extruded polyethylene oxide films. Eur. J. Pharm. Sci. 2004, 22, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Hulsmann, S.; Backensfeld, T.; Keitel, S.; Bodmeier, R. Melt extrusion—An alternative method for enhancing the dissolution rate of 17b-estradiol hemihydrate. Eur. J. Pharm. Biopharm. 2000, 49, 237–242. [Google Scholar] [CrossRef]
- Zhu, Y.; Shah, N.H.; Waseem Malick, A.; Infeld, M.H.; McGinity, J.W. McGinity, Controlled Release of a Poorly Water-Soluble Drug from Hot-Melt Extrudates Containing Acrylic Polymers. Drug Dev. Ind. Pharm. 2008, 32, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, P.; Zhang, X.; Shen, F.; Gogos, C.G. Effects of extrusion process parameters on the dissolution behavior of indomethacin in Eudragit EPO solid dispersiond. Int. J. Pharm. 2010, 383, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Abu-Diak, O.A.; Jones, D.S.; Andrews, G.P. Andrews, An investigation into the dissolution properties of celecoxib melt extrudates: Understanding the role of polymer type and concentration in stabilizing supersturated drug concentrations. Mol. Pharm. 2011, 8, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Abu-Diak, O.; Kusmanto, F.; Hornsby, P.; Hui, Z.; Jones, D.S. Physicochemical characterization and drug-release properties of celecoxib hot-melt extruded glass solutions. J. Pharm. Pharmacol. 2010, 62, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; AbuDiak, O.A.; Jones, D.S. Physicochemical Characterization of Hot Melt Extruded Bicalutamide-Polyvinylpyrrolidone Solid Dispersions. J. Pharm. Sci. 2010, 99, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Gryczke, A.; Schminke, S.; Maniruzzaman, M.; Beck, J.; Douroumis, D. Development and evaluation of orally disintegratingtablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surf. B Biointerfaces 2011, 86, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Coutts-Lendon, C.A.; Wright, N.A.; Mieso, E.V.; Koenig, J.L. The use of FT-IR imaging as an analytical tool for the characterization of drug delivery systems. J. Control. Release 2003, 93, 223–248. [Google Scholar] [CrossRef]
- Pudlas, M.; Kyeremateng, S.O.; Williams, L.A.; Kimber, J.A.; van Lishaut, H.; Kazarian, S.G.; Woehrle, G.H. Analyzing the impact of different excipients on drug release behavior in hot-melt extrusion formulations using FTIR spectroscopic imaging. Eur. J. Pharm. Sci. 2015, 67, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Maddineni, S.; Lu, J.; Repka, M.A. Melt extrusion with poorly soluble drugs. Int. J. Pharm. 2013, 453, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.; McConville, J.T.; Yang, W.; Williams, R.O., III; McGinity, J.W. Hot-melt extrusion for enhanced delivery of drug particles. J. Pharm. Sci. 2007, 96, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; Broze, G.; Pestieau, A.; Tatton, A.S.; Baumans, F.; Damblon, C.; Krier, F.; Evrard, B. Investigation of a suitable in vitro dissolution test for itraconazole-based solid dispersions. Eur. J. Pharm. Sci. 2016, 85, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Perdikoulias, J.; Dobbie, T. Pharmaceutical Extrusion Technology (Drugs and the Pharmaceutical Sciences); Marcel Dekker: New York, NY, USA, 2003; p. 133. [Google Scholar]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Quintavalle, U.; Voinovich, D.; Perissutti, B.; Serdoz, F.; Grassi, G.; Dal Col, A.; Grassi, M. Preparation of sustained release co-extrudates by hot-melt extrusion and mathematical modelling of in vitro/in vivo drug release profiles. Eur. J. Pharm. Sci. 2008, 33, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Injection molding and its application to drug delivery. J. Control. Release 2012, 159, 324–331. [Google Scholar] [CrossRef] [PubMed]
- FDA. Highlights of Prescribing Information; AbbVie Inc.: North Chicago, IL, USA, 2015. [Google Scholar]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kang, C.-Y.; Park, J.-B. Advances in hot-melt extrusion technology toward pharmaceutical objectives. J. Pharm. Investig. 2017, 47, 123–132. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Zhu, L.-M.; Branford-White, C.J.; Yang, X.L. Three-dimensional printing in pharmaceutics: Promises and problems. J. Pharm. Sci. 2008, 97, 3666–3690. [Google Scholar] [CrossRef] [PubMed]
- Dreiblatt, A. Technological Considerations Related to Scale-Up of Hot-Melt Extrusion Processes. Hot-Melt Extrus. Pharm. Appl. 2012, 285–300. [Google Scholar]
- Maniruzzaman, M.; Nokhodchi, A. Continuous manufacturing via hot-melt extrusion and scale up: Regulatory matters. Drug Discov. Today 2017, 22, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Wahl, P.R.; Menezes, J.C.; Koller, D.M.; Kavsek, B.; Francois, K.; Roblegg, E.; Khinast, J.G. Supervisory control system for monitoring a pharmaceutical hot melt extrusion process. AAPS PharmSciTech 2013, 14, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Baronsky-Probst, J.; Moltgen, C.V.; Kessler, W.; Kessler, R.W. Process design and control of a twin screw hot melt extrusion for continuous pharmaceutical tamper-resistant tablet production. Eur. J. Pharm. Sci. 2016, 87, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lenz, E.; Lobmann, K.; Rades, T.; Knop, K.; Kleinebudde, P. Hot Melt Extrusion and Spray Drying of Co-amorphous Indomethacin-Arginine with Polymers. J. Pharm. Sci. 2017, 106, 302–312. [Google Scholar] [CrossRef] [PubMed]

| Advantages | Explanation | References |
|---|---|---|
| Absence of solvents |
| [30,31,32] |
| Improved bioavailability |
| [13,14,15,16,30,31,32,33,34] |
| Modify drug release |
| [4,17,18,35,36,37,38,39,40,41] |
| Uniform dispersion of disperse solids in the molten mass |
| [42,43,44,45,46,47] |
| Few processing steps |
| [1,26,44,48,49] |
| Continuous operation and ease of scalability |
| [27,28,43,50] |
| No requirements for the compressibility of active ingredients |
| [4,51] |
| Wide range of dosage forms and delivery routes |
| [3,4,5,6,7,8,9,10] |
| High process temperatures are necessary |
| [1,52] |
| High energy input coming from the applied shear forces |
| [18,44,53] |
| Feedstock must have good flow properties |
| [23,43] |
| Need for excipients |
| [25,54,55] |
| Scope | Techniques | Technique Principle | Results | References |
|---|---|---|---|---|
| The chemical and thermal stability of extrudates | Chromatographic techniques
| Chromatographic techniques are based on the separation of different analytes from complex mixtures based upon several factors (solubility/miscibility, column affinity, volatility, molecular weight, etc.). Analytes can be detected by different detectors (i.e., mass spectrometer, photo diode array, etc.). |
| [4,14,18,30,31,44,56,57,58,59,60,61,62] |
Thermal analysis techniques
| Thermal analysis techniques are based on isothermal, scanning, and modulated temperature. A change in weight or heat flow of the samples is recorded. |
| [4,14,32,44,45,49,52,59,63,64] | |
| The solid physical state of extrudates |
| Microscopy techniques combine information from microscopy and physical state of the sample. HSM combines results from microscopy and thermal analysis. HS-PLM combines results from microscopy and thermal analysis under polarized light. AFM combines results from microscopy and solid state. |
| [14,38,44,59,65] |
| X-ray beams hitting crystalline solid materials are scattered in all directions, producing distinct scattering patterns, similar to fingerprints. A halo, that is the absence of diffraction peaks, corresponds to a completely amorphous sample. The crystallinity degree can be calculated by Ruland’s or Hermans and Weidinger’s methods. |
| [1,38,44,54,61,66,67,68,69,70,71] | |
Thermal analysis techniques
| See above in the table |
| [14,54,64,68,71,72,73] | |
| The drug–polymer interaction | Spectroscopic techniques
| Spectroscopic techniques are based on the exposure of molecules to different radiations (vibrational, magnetic, …). Information can be qualitative and quantitative. |
| [10,38,44,50,52,61,74,75,76,77,78] |
| The miscibility/solubility of drug–polymer systems |
| The miscibility drug–polymer can be deduced by solubility parameters. The solubility parameter can predict if one material will dissolve in another to form a solution. Thus, it can be used to predict miscibility of drugs and excipients. The miscibility/solubility of drug–polymer system has important repercussions on feasibility process and increase in dissolution rate. The glass transition temperature can be theoretically predicted from the Gordon Taylor equation. Deviation from the predicted values can be explained in term of immiscibility or in term of enhanced molecule-molecule interaction. |
| [39,54,55,59,74,79,80,81,82,83,84] |
| The rheological properties of extrudates |
| By applying a shear stress or a shear strain on a free flowing material, rheology permits to characterize the flowing properties of a material, according to the temperature. |
| [29,32,52,63,74,85,86] |
| Physicomechanical properties of films produced by hot melt extrusion |
| The tensile tester permits the characterization of films produced by the HME process. Several parameters can be determined such as the elastic modulus, the tensile strength and the elongation. | [33,53,87,88,89] | |
| The drug particle dissolution from extrudates |
| Well standardized apparatus and methods are described by different Pharmacopeias. The test is based on the dissolution of drug molecules in a dissolution medium during a specific time interval under validated conditions. The drug dissolved can be quantified by different methods (UV, MS). Results can be confirmed by different in vivo methods. |
| [21,34,38,39,40,41,90,91,92,93,94,95,96,97,98,99,100,101,102,103] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Censi, R.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P. Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics 2018, 10, 89. https://doi.org/10.3390/pharmaceutics10030089
Censi R, Gigliobianco MR, Casadidio C, Di Martino P. Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics. 2018; 10(3):89. https://doi.org/10.3390/pharmaceutics10030089
Chicago/Turabian StyleCensi, Roberta, Maria Rosa Gigliobianco, Cristina Casadidio, and Piera Di Martino. 2018. "Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process" Pharmaceutics 10, no. 3: 89. https://doi.org/10.3390/pharmaceutics10030089
APA StyleCensi, R., Gigliobianco, M. R., Casadidio, C., & Di Martino, P. (2018). Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics, 10(3), 89. https://doi.org/10.3390/pharmaceutics10030089