An Intrinsic Mitochondrial Pathway Is Required for Phytic Acid-Chitosan-Iron Oxide Nanocomposite (Phy-CS-MNP) to Induce G0/G1 Cell Cycle Arrest and Apoptosis in the Human Colorectal Cancer (HT-29) Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Iron Oxide Nanoparticles
2.3. Synthesis of Chitosan-Iron Oxide Nanoparticles
2.4. Synthesis of Phytic Acid-Chitosan-Iron Oxide Nanocomposite
2.5. Cell Lines
2.6. Determination of Cytotoxicity Using MTT Assay
2.7. Determination of Cell Cycle Arrest
2.8. Determination of Apoptosis by Annexin V-Propidium Iodide Staining
2.9. Determination of Bax and Bcl-2 Activities
2.10. Caspase-3 and Caspase-8 Activities
2.11. Total RNA Extraction and cDNA Synthesis
2.12. Quantitative Real-Time Polymerase Chain Reaction
2.13. Protein Extraction
2.14. Western Blotting Analysis
2.15. Statistical Analysis
3. Results
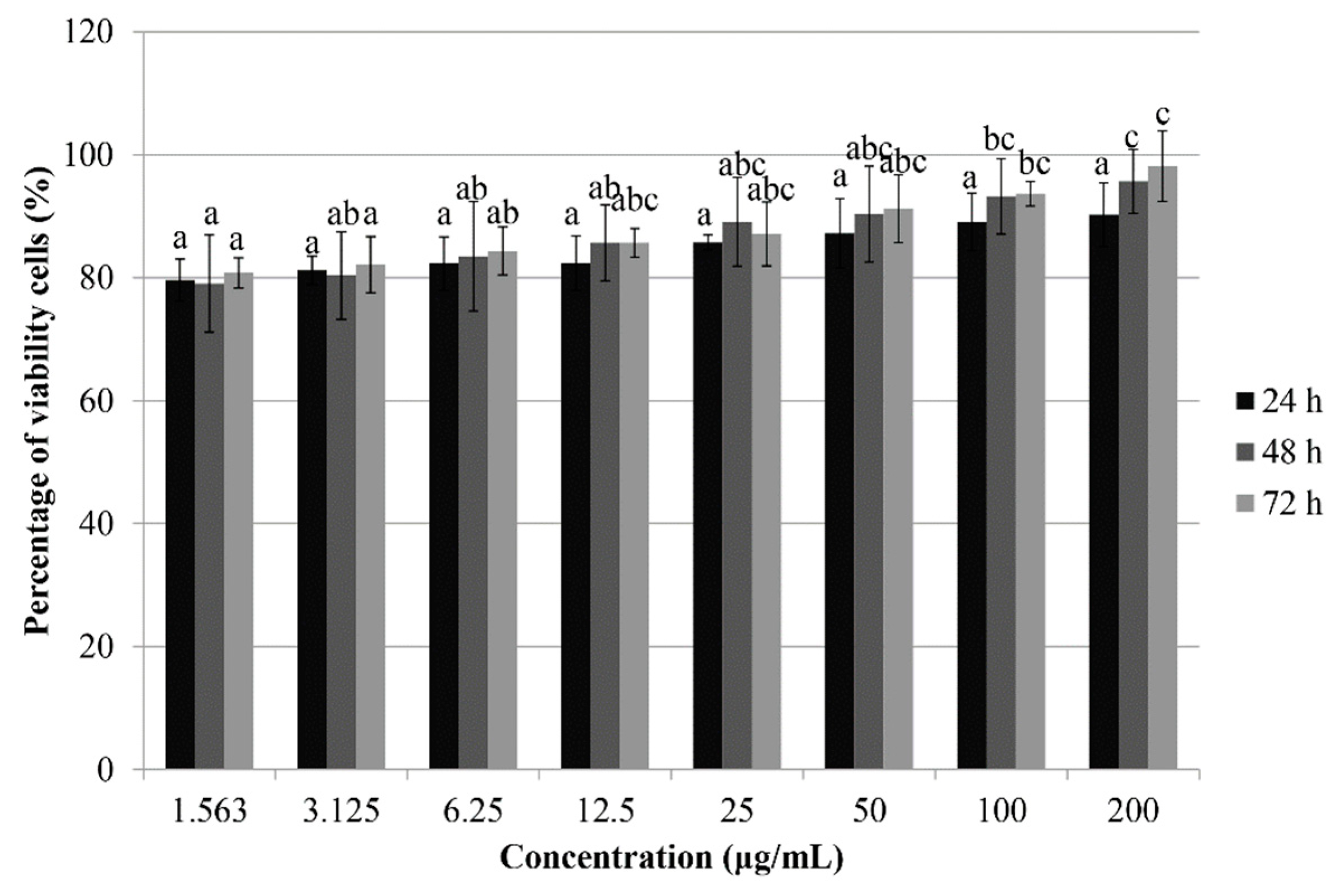
3.1. Treatment with Phy-CS-MNP Nanocomposite Decreases the Viability of HT-29 Cells
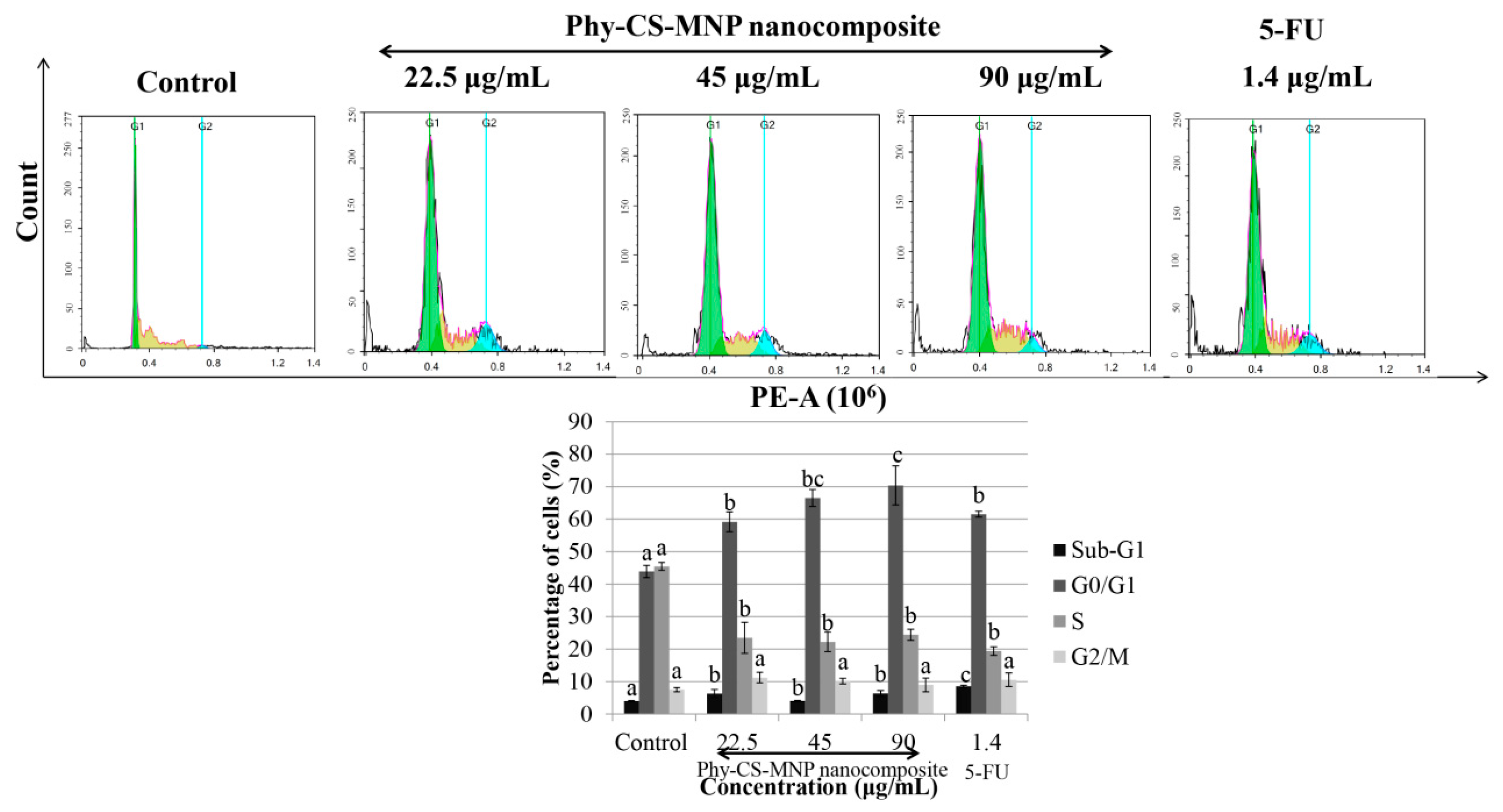
3.2. Treatment with Phy-CS-MNP Nanocomposite Triggers G0/G1 Cell Cycle Arrest in HT-29 Cells
3.3. Treatment with Phy-CS-MNP Nanocomposite Triggers Apoptosis in HT-29 Cells
3.4. Treatment with Phy-CS-MNP Nanocomposite Triggers Bax and Downregulates Bcl-2 Protein Expression in HT-29 Cells
3.5. Treatment with Phy-CS-MNP Nanocomposite Does Not Activate Caspase-3 and -8 Activities in HT-29 Cells
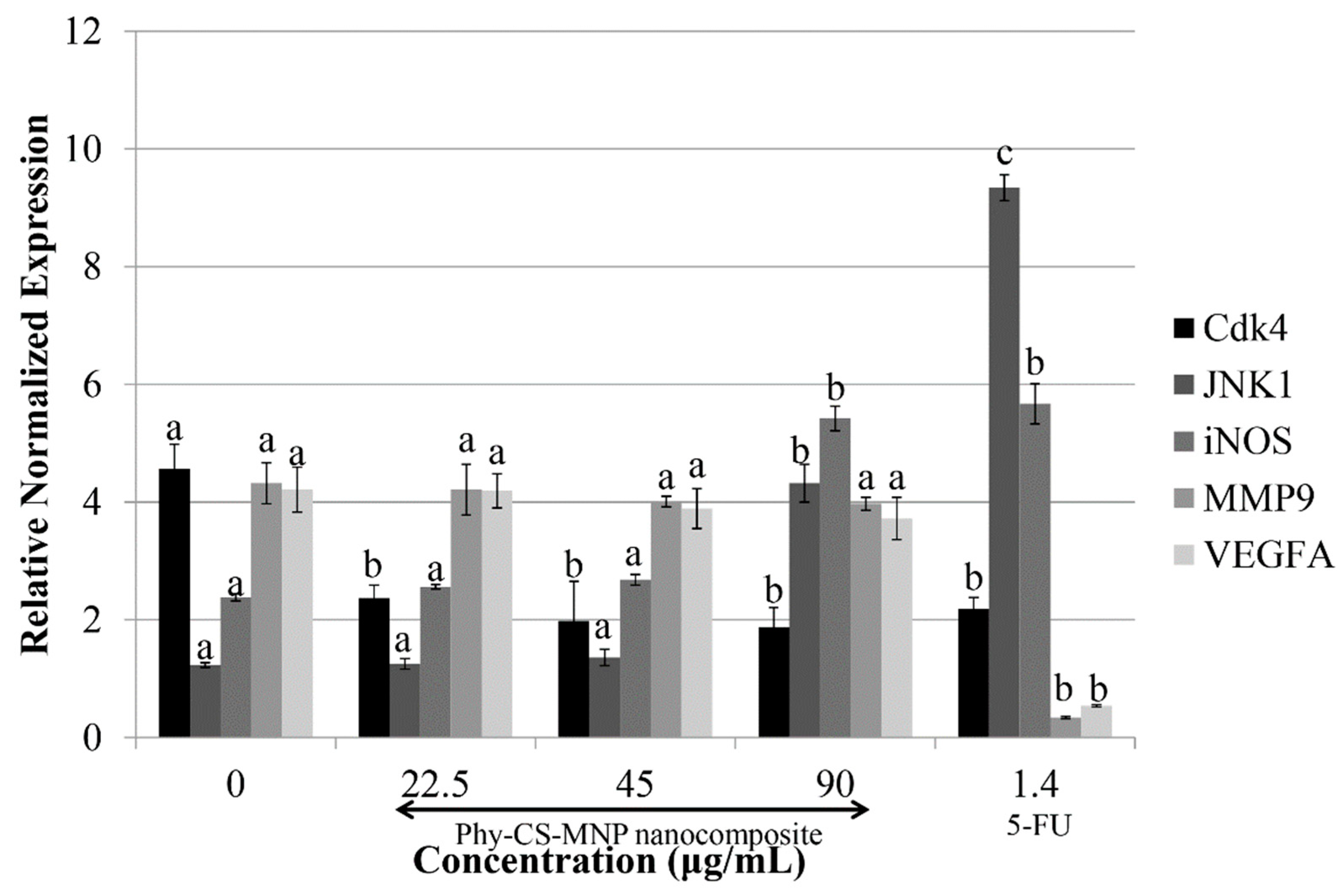
3.6. Treatment with Phy-CS-MNP Nanocomposite Downregulates Cdk4 and Upregulates JNK1 and iNOS mRNA Expression in HT-29 Cells
3.7. Treatment with Phy-CS-MNP Nanocomposite Upregulates the Protein Levels of Cytochrome c and NF-κB
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Cancer Research Fund International. Colorectal Cancer Statistics. 2018. Available online: https://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/colorectal-cancer-statistics (accessed on 17 April 2018).
- Kapral, M.; Wawszczyk, J.; Sośnicki, S.; Jesse, K.; Węglarz, L. Modulating effect of inositol hexaphosphate on arachidonic acid-dependent pathways in colon cancer cells. Prostag. Other Lipid Mediat. 2017, 131, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Ojha, D.; Goswami, D.; Das, R.; Chandra, N.S.; Chatterjee, T.K.; Chakravarty, A.; Chakravarty, S.; Chattopadhyay, D. Inositol hexa phosphoric acid (phytic acid), a nutraceuticals, attenuates iron-induced oxidative stress and alleviates liver injury in iron overloaded mice. Biomed. Pharmacother. 2017, 87, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, C.; Li, X.; Yang, F.; Cheng, L.; Liu, C.; Song, Y. Inositol hexaphosphate suppresses colorectal cancer cell proliferation via the Akt/GSK-3β/β-catenin signaling cascade in a 1,2-dimethylhydrazine-induced rat model. Eur. J. Pharmacol. 2017, 805, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wakaskar, R.R. Promising effects of nanomedicine in cancer drug delivery. J. Drug Target 2018, 26, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Dehghanizade, S.; Arasteh, J.; Mirzaie, A. Green synthesis of silver nanoparticle using Anthemis atropatana extract: Characterization and in vitro biological activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Calavia, P.G.; Chambrier, I.; Cook, M.J.; Haines, A.H.; Field, R.A.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J. Colloid Interface Sci. 2018, 512, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-B.; Shao, H.-H.; Jin, G.-L.; Huang, F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: Preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2013, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Nie, L.; Li, F.; Aguilar, Z.P.; Xu, H.; Xiong, Y.; Fu, F.; Xu, H. Folic acid conjugated magnetic iron oxide nanoparticles for nondestructive separation and detection of ovarian cancer cells from whole blood. Biomater. Sci. 2016, 4, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Dorniani, D.; Saifullah, B.; Gothai, S.; Hussein, M.Z.; Pandurangan, A.K.; Arulselvan, P.; Norhaizan, M.E. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017, 12, 2361–2372. [Google Scholar] [CrossRef]
- Poller, W.C.; Löwa, N.; Wiekhorst, F.; Taupitz, M.; Wagner, S.; Möller, K.; Baumann, G.; Stangl, V.; Trahms, L.; Ludwig, A. Magnetic particle spectroscopy reveals dynamic changes in the magnetic behavior of very small superparamagnetic iron oxide nanoparticles during cellular uptake and enables determination of cell-labeling efficacy. J. Biomed. Nanotechnol. 2016, 12, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Sano, K.; Kanazaki, K.; Ohashi, M.; Deguchi, J.; Kanada, Y.; Ono, M.; Saji, H. In vivo HER2-targeted magnetic resonance tumor imaging using iron oxide nanoparticles conjugated with anti-HER2 fragment antibody. Mol. Imaging Biol. 2016, 18, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.; An, Q.; Li, W.; Wu, P. Synthesis of graphene@Fe3O4@C core-shell nanosheets for high-performance lithium ion batteries. J. Mater. Chem. A 2015, 3, 7036–7043. [Google Scholar] [CrossRef]
- Xianbo, M.; Zeeshan, A.; Song, L.; Nongyue, H. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar]
- Haldorai, Y.; Shim, J.-J. Chitosan-zinc oxide hybrid composite for enhanced dye degradation and antibacterial activity. Compos. Interfaces 2013, 20, 365–377. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015, 122, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shao, H.; Huang, Y.; Kwak, B. Synthesis of MRI contrast agent by coating superparamagnetic iron oxide with chitosan. IEEE Trans. Magn. 2005, 41, 4102–4104. [Google Scholar]
- Qu, J.; Liu, G.; Wang, Y.; Hong, R. Preparation of Fe3O4-chitosan nanoparticles used for hyperthermia. Adv. Powder Technol. 2010, 21, 461–467. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, H.; Xia, M.; Deng, B.; Shen, H.; Ji, G.; Li, G.; Xie, Y. The effect of phytic acid on tight junctions in the human intestinal Caco-2 cell line and its mechanism. Eur. J. Pharm. Sci. 2015, 80, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, Z.; Hou, L.; Zhang, L.; Zhang, J.; Wang, Y.; Liu, C.; Xu, P.; Liu, L.; Gai, X.; et al. Phytic acid attenuates inflammatory responses and the levels of NF-κB and p-ERK in MPTP-induced Parkinson’s disease model of mice. Neurosci. Lett. 2015, 597, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Dodi, G.; Hritcu, D.; Lisa, G.; Popa, M.I. Core-shell magnetic chitosan particles functionalized by grafting: Synthesis and characterization. Chem. Eng. J. 2012, 203, 130–141. [Google Scholar] [CrossRef]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nanopart. Res. 2012, 14, 964. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Ravenelle, F.; Zhu, X.X. Polysaccharides for Drug Delivery and Pharmaceutical Applications; American Chemical Society: Washington, DC, USA, 2006; ISBN 9780841239609. [Google Scholar]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Ismail, M.; Webster, T.J. Synthesis, characterization, controlled release, and antibacterial studies of a novel streptomycin chitosan magnetic nanoantibiotic. Int. J. Nanomed. 2014, 9, 549–557. [Google Scholar] [Green Version]
- Ho, Y.S.; Ofomaja, A.E. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J. Hazard. Mater. 2006, 129, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yan, L.; Hou, W.-G.; Liu, S.-J. Synthesis and release behavior of composites of camptothecin and layered double hydroxide. J. Solid State Chem. 2010, 183, 1811–1816. [Google Scholar] [CrossRef]
- Barahuie, F.; Hussein, M.Z.; Arulselvan, P.; Fakurazi, S.; Zainal, Z. Development of the anticancer potential of a chlorogenate-zinc layered hydroxide nanohybrid with controlled release property against various cancer cells. Sci. Adv. Mater. 2013, 5, 1983–1993. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Manilkara zapota (L.) P. Royen leaf water extract triggered apoptosis, activated caspase-dependent pathway in HT-29 human colorectal cancer cell line. Biomed. Pharmacother. 2018. submitted. [Google Scholar]
- Zhang, N.; Bing, T.; Liu, X.; Qi, C.; Shen, L.; Wang, L.; Shangguan, D. Cytotoxicity of guanine-based degradation products contributes to the antiproliferative activity of guanine-rich oligonucleotides. Chem. Sci. 2015, 6, 3831–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Yao, Y.; Zhu, X.; Zhang, K.; Zhou, F.; Zhu, L. Amyloid β induces NLRP3 inflammasome activation in retinal pigment epithelial cells via NADPH oxidase and mitochondria-dependent ROS production. J. Biochem. Mol. Toxicol. 2017, 31, e21887. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef] [PubMed]
- Tor, Y.S.; Yazan, L.S.; Foo, J.B.; Wibowo, A.; Cheah, Y.K.; Abdullah, R.; Ismail, M.; Ismail, I.S.; Yeap, S.K. Induction of apoptosis in MCF-7 cells via oxidative stress generation, mitochondria-dependent and caspase-independent pathway by ethyl acetate extract of Dillenia suffruticosa and its chemical profile. PLoS ONE 2015, 10, e0127441. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell death beyond apoptosis. Leukemia 2000, 14, 1502–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bröker, L.E.; Kruyt, F.A.E.; Giaccone, G. Cell death independent of caspases: A review. Clin. Cancer Res. 2005, 11, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Nylandsted, J.; Rohde, M.; Brand, K. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl. Acad. Sci. USA 2000, 97, 7871–7876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyanarayana, C.; Deevi, D.S.; Rajagopalan, R.; Srinivas, N.; Rajagopal, S. DRF 3188 a novel semi-synthetic analog of andrographolide: Cellular response to MCF 7 breast cancer cells. BMC Cancer 2004, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.T.; Chen, Y.T.; Hsieh, Y.H.; Weng, C.J.; Yeh, J.C.; Yang, S.F.; Lin, C.W.; Yang, J.S. Glabridin induces apoptosis and cell cycle arrest in oral cancer cells through the JNK1/2 signaling pathway. Environ. Toxicol. 2018, 33, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, L.; Ma, H.; Li, H.; Li, Y.; Lu, Y.; Wang, Q.; Li, W. Dihydroartemisinin induces apoptosis in human gastric cancer cell line BGC-823 through activation of JNK1/2 and p38 MAPK signaling pathways. J. Recept. Signal Transduct. Res. 2017, 37, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Huynh, K.; Yeap, S.K.; Hazilawati, H.; Roselina, K. Brewers’ rice modulates oxidative stress in azoxymethane mediated colon carcinogenesis in rats. World J. Gastroenterol. 2015, 21, 8826–8835. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Alabasi, G.; Sandaltzopoulos, R.; Marcu, K.B.; Kolettas, E. Roles of NF-ĸB signaling in the regulation of miRNAs impacting on inflammation in cancer. Biomedicines 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; van Loo, G. Inflammation and the metabolic syndrome: The tissue-specific functions of NF-κB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Karl, S.; Pritschow, Y.; Volcic, M.; Häcker, S.; Baumann, B.; Wiesmüller, L.; Debatin, K.M.; Fulda, S. Identification of a novel pro-apoptotic function of NF-κB in the DNA damage response. J. Cell Mol. Med. 2009, 13, 4239–4256. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bardhan, K.; Yang, D.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Liles, G.B.; Lee, J.R.; Liu, K. NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J. Biol. Chem. 2012, 287, 25530–25540. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Genestier, L.; Green, D.R. Regulation of Fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor κB. J. Biol. Chem. 1999, 274, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Nata, T.; Morishita, R.; Matsushita, H.; Nakagami, H.; Yamamoto, K.; Yamazaki, K.; Nakabayashi, M.; Ogihara, T.; Kaneda, Y. Endothelial apoptosis induced by oxidative stress through activation of NF-κB: Antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension 2001, 38, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.G. NFkB anti-apoptotic or pro-apoptotic, maybe both. Cell Cycle 2010, 9, 3131–3132. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, K.; Yin, N.; Ma, X.; Zhao, G.; Qiu, C.; Deng, G. Thymol mitigates lipopolysaccharide-induced endometritis by regulating the TLR4- and ROS-mediated NF-κB signaling pathways. Oncotarget 2017, 8, 20042–20055. [Google Scholar] [PubMed]



| Primer Name [Accession Number] | Oligonucleotides (5′-3′) Sequence |
|---|---|
| Cdk4 [NM_000075.3] | F–GAAACTCTGAAGCCGACCAG R–AGGCAGAGATTCGCTTGTGT |
| JNK1 [NM_139046.3] | F–GTGATCAATGGCTCTCAGCA R–TGACTAACCGACTCCCCATC |
| iNOS [AF049656.1] | F–GTGGTGACAAGCACATTTGG R–GTCATGAGCAAAGGCACAGA |
| MMP9 [NM_004994.2] | F–GACAAGAAGTGGGGCTTCTG R–GCCATTCACGTCGTCCTTAT |
| VEGFA [NM_001287044.1] | F–CCCACTGAGGAGTCCAACAT R–AAATGCTTTCTCCGCTCTGA |
| ACTBa [NM_001101.3] | F–AGAGCTACGAGCTGCCTGAC R–AGCACTGTGTTGGCGTACAG |
| GAPDHa [NM_002046.4] | F–GGATTTGGTCGTATTGGGC R–TGGAAGATGGTGATGGGATT |
| 18S rRNA a [HQ387008.1] | F–GTAACCCGTTGAACCCCATT R–CCATCCAATCGGTAGTAGCG |
| Cancer Cell Lines | 24 h | 48 h | 72 h |
|---|---|---|---|
| HT-29 | 112.71 ± 18.72 a | 79.33 ± 9.02 b | 45.63 ± 5.77 c |
| HCT-116 | 157.13 ± 9.03 a | 120.24 ± 6.75 b | 101.34 ± 12.23 b |
| HeLa | 134.96 ± 10.22 a | 130.77 ± 9.05 a | 127.01 ± 5.11 a |
| HGT-1 | 176.11 ± 13.19 a | 154.07 ± 12.33 a | 76.92 ± 15.09 b |
| HepG2 | 98.05 ± 5.23 a | 82.13 ± 6.56 b | 80.11 ± 2.36 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, B.L.; Norhaizan, M.E.; Chan, L.C. An Intrinsic Mitochondrial Pathway Is Required for Phytic Acid-Chitosan-Iron Oxide Nanocomposite (Phy-CS-MNP) to Induce G0/G1 Cell Cycle Arrest and Apoptosis in the Human Colorectal Cancer (HT-29) Cell Line. Pharmaceutics 2018, 10, 198. https://doi.org/10.3390/pharmaceutics10040198
Tan BL, Norhaizan ME, Chan LC. An Intrinsic Mitochondrial Pathway Is Required for Phytic Acid-Chitosan-Iron Oxide Nanocomposite (Phy-CS-MNP) to Induce G0/G1 Cell Cycle Arrest and Apoptosis in the Human Colorectal Cancer (HT-29) Cell Line. Pharmaceutics. 2018; 10(4):198. https://doi.org/10.3390/pharmaceutics10040198
Chicago/Turabian StyleTan, Bee Ling, Mohd Esa Norhaizan, and Lee Chin Chan. 2018. "An Intrinsic Mitochondrial Pathway Is Required for Phytic Acid-Chitosan-Iron Oxide Nanocomposite (Phy-CS-MNP) to Induce G0/G1 Cell Cycle Arrest and Apoptosis in the Human Colorectal Cancer (HT-29) Cell Line" Pharmaceutics 10, no. 4: 198. https://doi.org/10.3390/pharmaceutics10040198
APA StyleTan, B. L., Norhaizan, M. E., & Chan, L. C. (2018). An Intrinsic Mitochondrial Pathway Is Required for Phytic Acid-Chitosan-Iron Oxide Nanocomposite (Phy-CS-MNP) to Induce G0/G1 Cell Cycle Arrest and Apoptosis in the Human Colorectal Cancer (HT-29) Cell Line. Pharmaceutics, 10(4), 198. https://doi.org/10.3390/pharmaceutics10040198






