Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1-34)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Fabrication of PTH-Loaded MNs
2.4. Determination of Drug Contents in PTH-Loaded MNs
2.5. Stability of PTH in MNs Fabricated from HA
2.6. Skin Piercing Ability of MNs
2.7. Effect of PTH-Loaded MNs on Transepidermal Water Loss (TEWL)
2.8. Dissolution of MNs Following Application in Rats
2.9. In Vivo Transdermal Delivery of PTH
2.10. The Determination of PTH Concentrations in Plasma
2.11. Skin Irritation Following Application of MNs in Rats
2.12. Therapeutic Potential of PTH-Loaded MNs for Treatment of Osteoporosis
3. Results
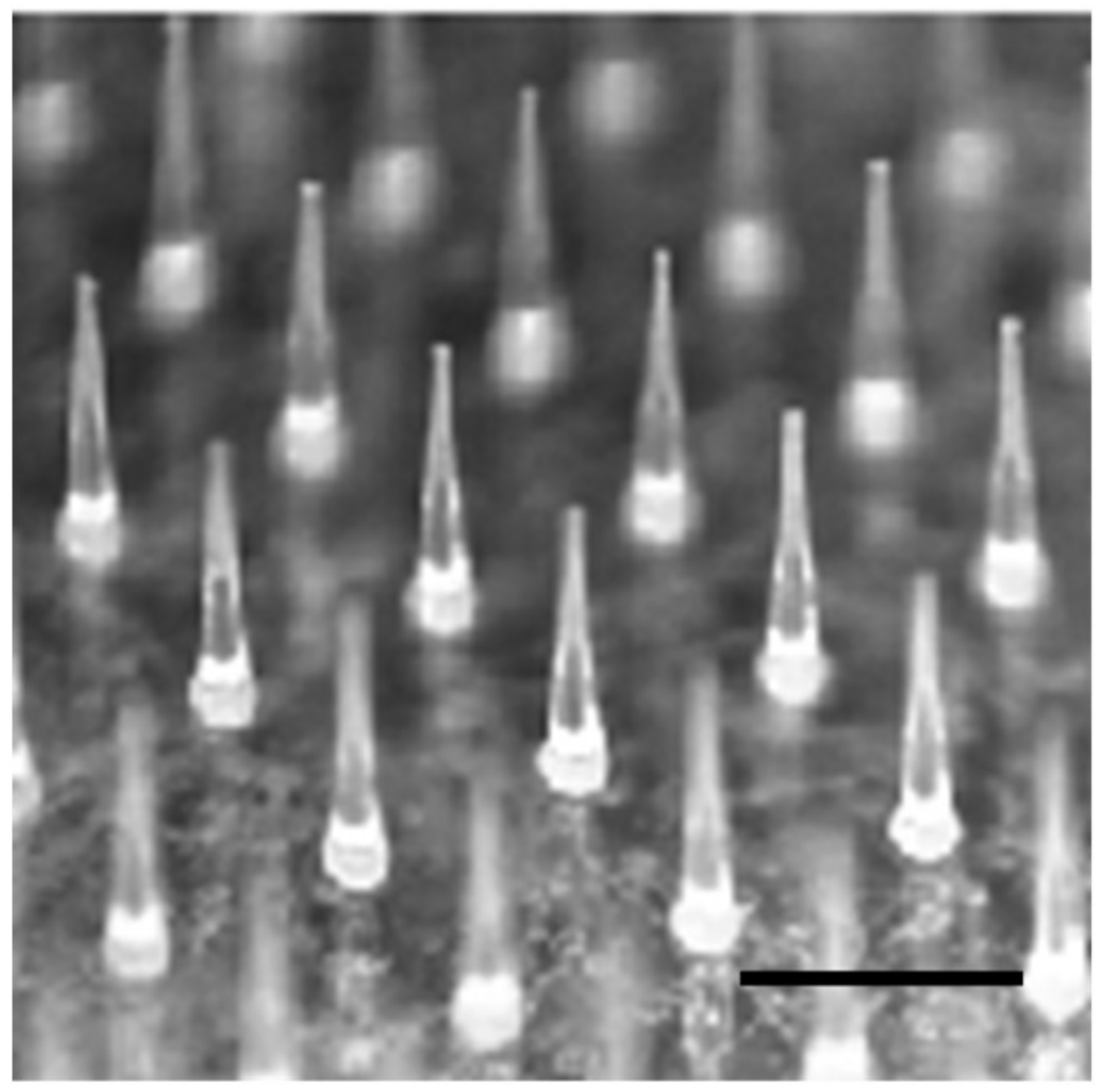
3.1. Fabrication of PTH-Loaded MNs
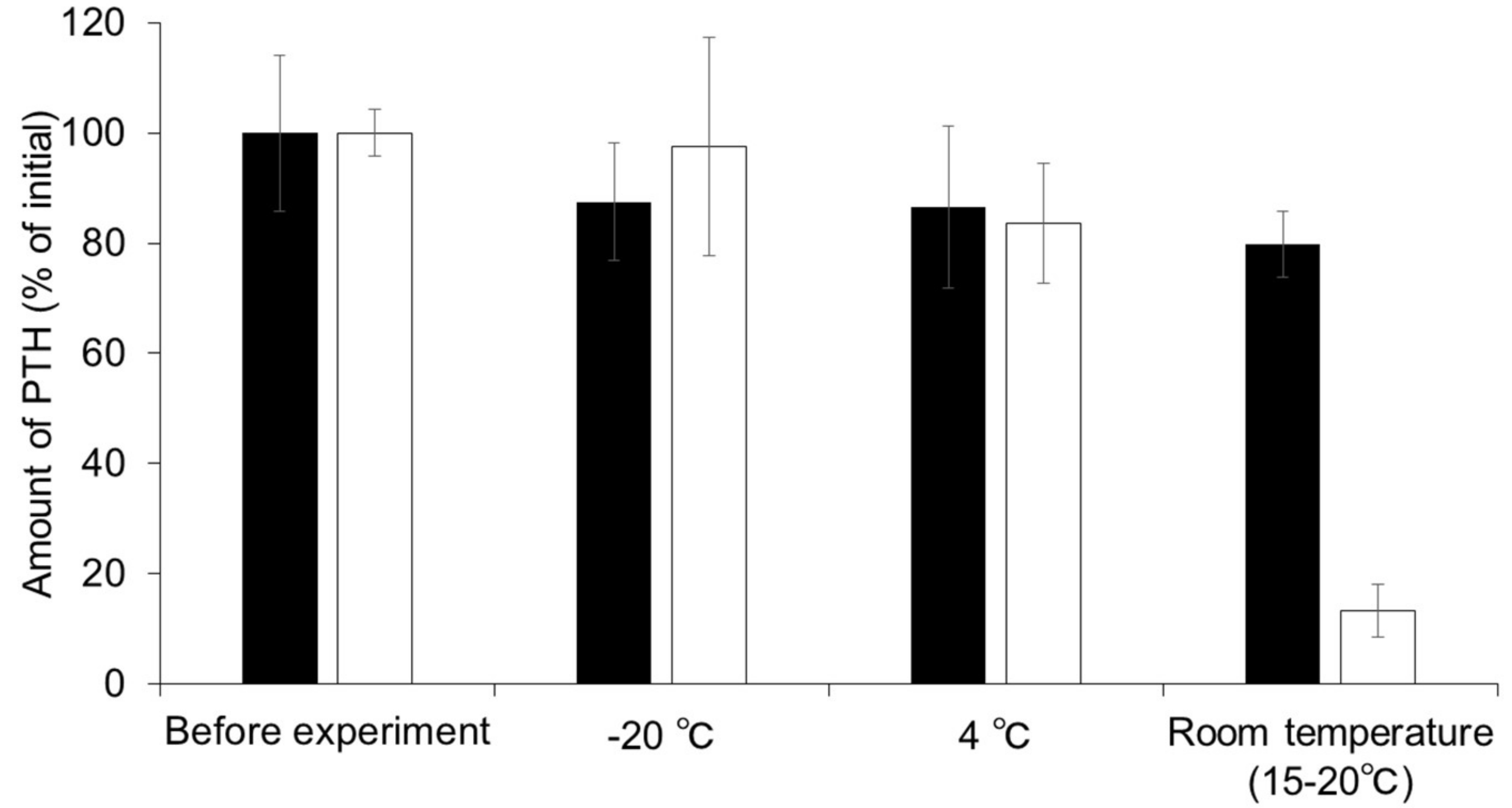
3.2. Stability of PTH in MNs Fabricated from HA
3.3. Skin Piercing Ability of PTH-Loaded MNs
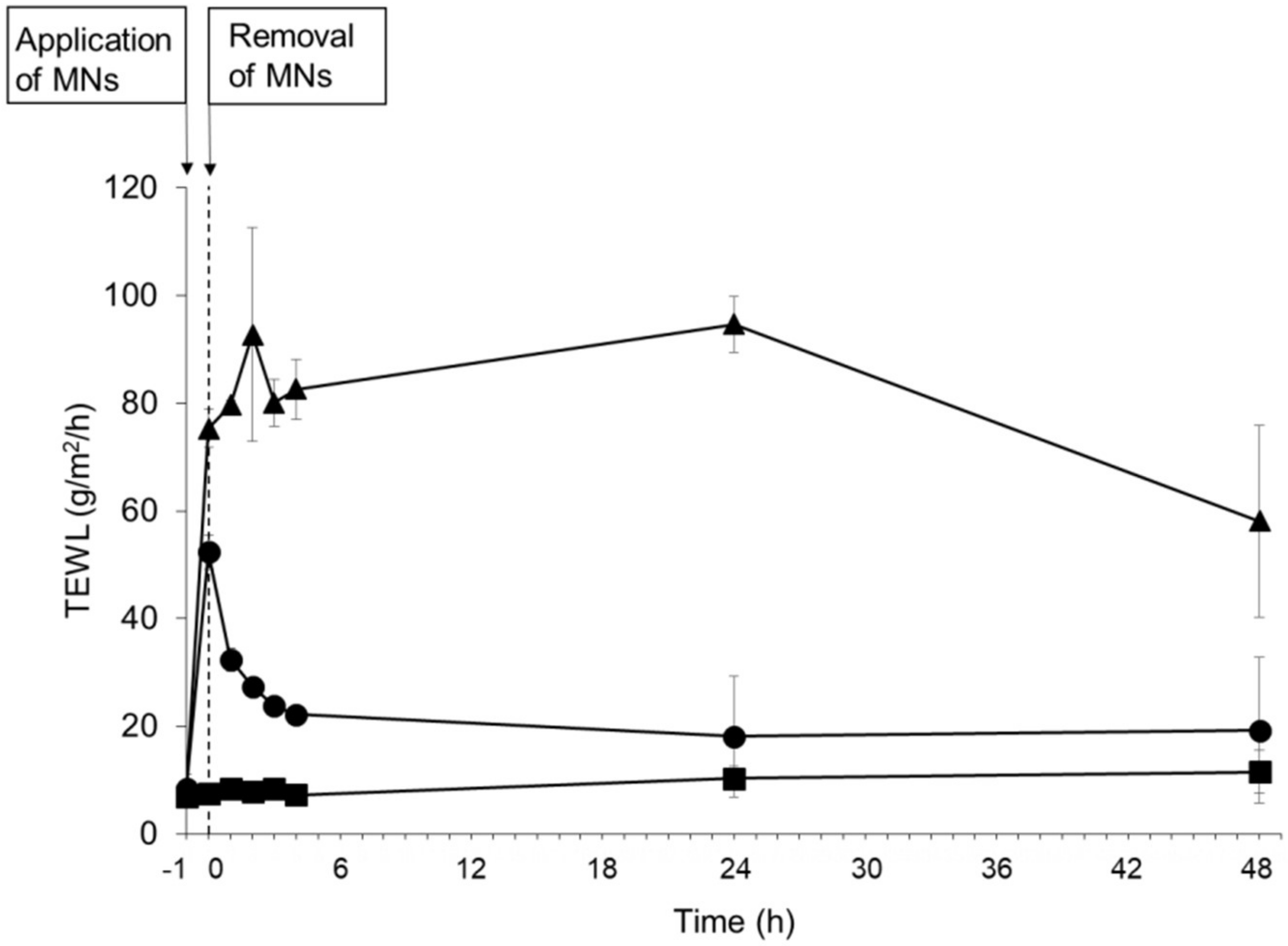
3.4. Evaluation of Skin Barrier Function Following Application of PTH-Loaded MNs
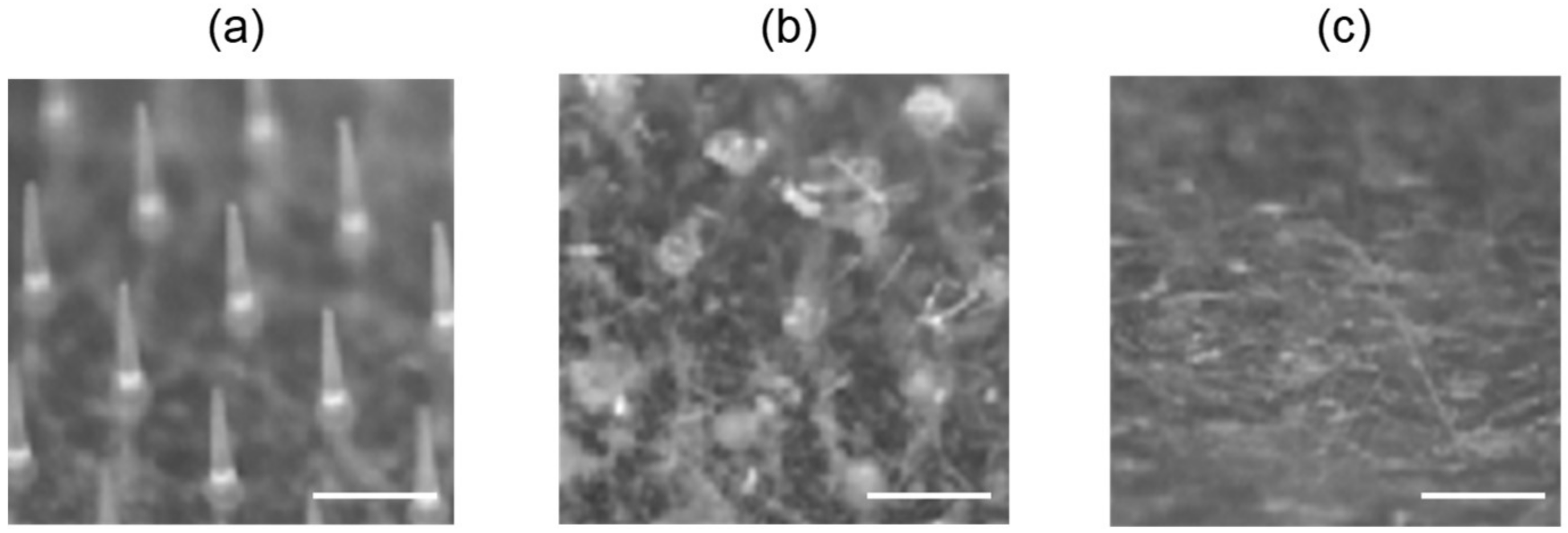
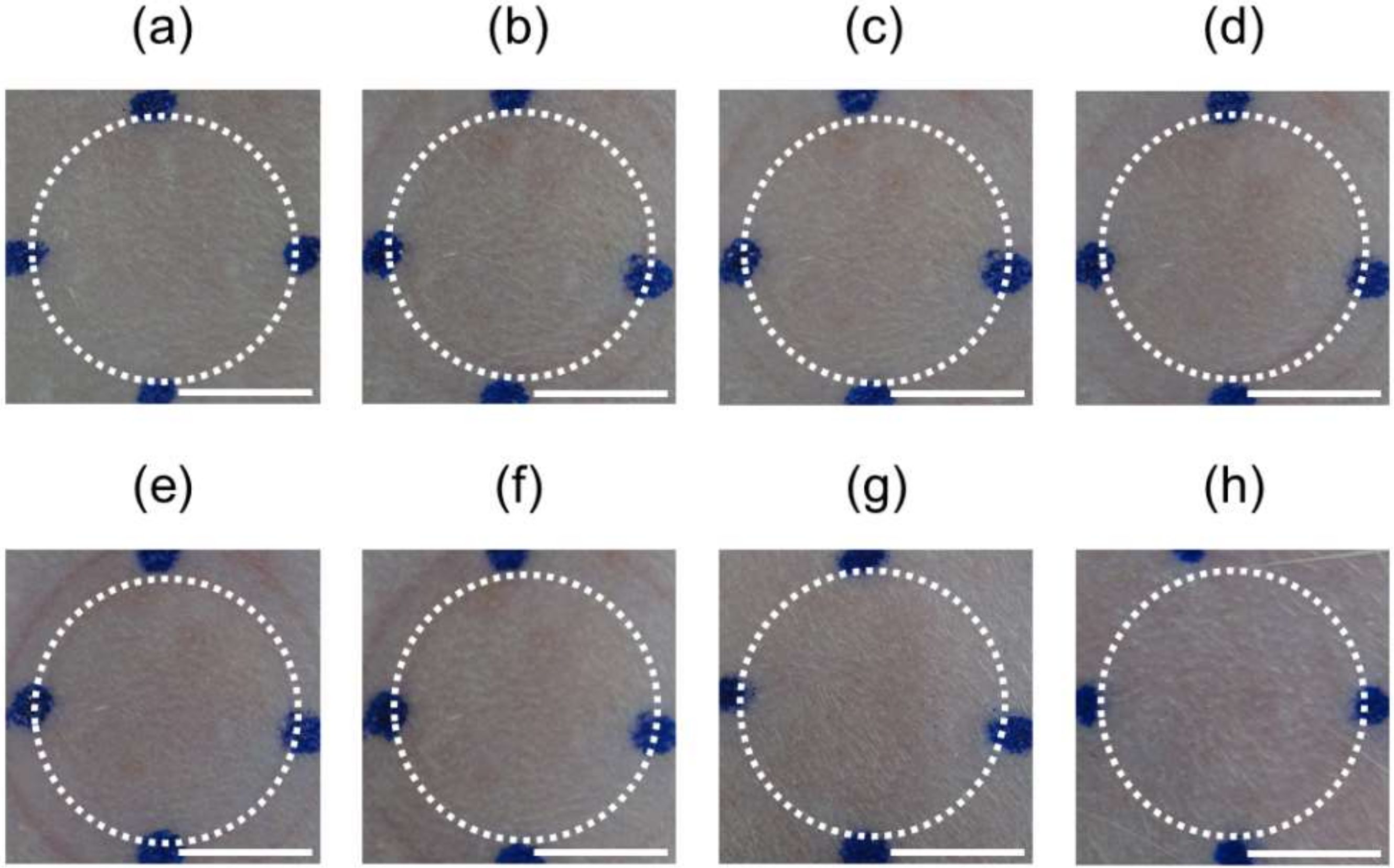
3.5. Dissolution of Needles Following Application of MNs
3.6. Pharmacokinetics after Application of PTH-Loaded MNs
3.7. Skin Irritation Following Application of MNs
3.8. Therapeutic Potential of PTH-Loaded MNs in a Rat Model of Osteoporosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silva, B.C.; Costa, A.G.; Cusano, N.E.; Kousteni, S.; Bilezikian, J.P. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J. Endocrinol. Investig. 2011, 34, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.L.; Yamamoto, A. Penetration and enzymatic barriers to peptide and protein absorption. Adv. Drug Deliv. Rev. 1989, 4, 171–207. [Google Scholar] [CrossRef]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.F. Parathyroid hormone (1-34)-coated microneedle patch system: Clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Shiraki, M.; Fukunaga, M.; Hagino, H.; Sone, T.; Nakano, T.; Kishimoto, H.; Ito, M.; Yoshikawa, H.; Kishida, M.; et al. 24-Month Open-Label Teriparatide Once-Weekly Efficacy Research Trial Examining Bone Mineral Density in Subjects with Primary Osteoporosis and High Fracture Risk. Adv. Ther. 2017, 34, 1727–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, Y.; Hatanaka, T.; Sugibayashi, K.; Omiya, Y. Prediction of Skin Permeability of Drugs: Comparison of Human and Hairless Rat Skin. J. Pharm. Pharmacol. 1992, 44, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Quan, Y.; Zang, L.; Jin, M. Trypsin as a novel potential absorption enhancer for improving the transdermal delivery of macromolecules. J. Pharm. Pharmacol. 2009, 61, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nagase, Y.; Iga, K.; Kawase, M.; Oka, M. Prevention of Bone Loss in Ovariectomized Rats by Pulsatile Transdermal Iontophoretic Administration of Human PTH (1-34). J. Pharm. Sci. 2002, 91, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Zorec, B.; Becker, S.; Reberšek, M.; Miklavčič, D.; Pavšelj, N. Skin electroporation for transdermal drug delivery: The influence of the order of different square wave electric pulses. Int. J. Pharm. 2013, 457, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Herwadkar, A.; Sachdeva, V.; Taylor, L.F.; Silver, H.; Banga, A.K. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int. J. Pharm. 2012, 423, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Walther, W.; Siegel, R.; Kobelt, D.; Knösel, T.; Dietel, M.; Bembenek, A.; Aumann, J.; Schleef, M.; Baier, R.; Stein, U.; et al. Novel jet-injection technology for nonviral intratumoral gene transfer in patients with melanoma and breast cancer. Clin. Cancer Res. 2008, 14, 7545–7553. [Google Scholar] [CrossRef] [PubMed]
- Migalska, K.; Morrow, D.I.J.; Garland, M.J.; Thakur, R.; Woolfson, A.D.; Donnelly, R.F. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm. Res. 2011, 28, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hirono, M.; Fukushima, K.; Sugioka, N.; Takada, K. Two-layered dissolving microneedles formulated with intermediate-acting insulin. Int. J. Pharm. 2012, 436, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiu, Y.; Gao, Y. Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharm. Sin. B 2014, 4, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Park, H.H.; Do, K.Y.; Han, M.; Hyun, D.H.; Kim, C.G.; Kim, C.H.; Lee, S.S.; Hwang, S.J.; Shin, S.C.; et al. Influence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calcein. Eur. J. Pharm. Biopharm. 2008, 69, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Arya, J.; Prausnitz, M.R. Microneedle patches for vaccination in developing countries. J. Control. Release 2016, 240, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur. J. Pharm. Biopharm. 2014, 86, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Liu, S.; Tanaka, Y.; Hitomi, K.; Hayashi, R.; Hirai, Y.; Kusamori, K.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; et al. Development of a novel self-dissolving microneedle array of alendronate, a nitrogen-containing bisphosphonate: Evaluation of transdermal absorption, safety, and pharmacological effects after application in rats. J. Pharm. Sci. 2012, 101, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of Transdermal Delivery of Exendin-4 Using Novel Tip-Loaded Microneedle Arrays Fabricated from Hyaluronic Acid. Mol. Pharm. 2016, 13, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Daddona, P.E.; Maa, Y.F. Demonstrated solid-state stability of parathyroid hormone PTH(1-34) coated on a novel transdermal microprojection delivery system. Pharm. Res. 2009, 26, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Tanaka, Y.; Hitomi, K.; Liu, S.; Quan, Y.-S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Efficient transdermal delivery of alendronate, a nitrogen-containing bisphosphonate, using tip-loaded self-dissolving microneedle arrays for the treatment of osteoporosis. Pharmaceutics 2017, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of Transdermal Delivery of Sumatriptan Succinate Using a Novel Self-dissolving Microneedle Array Fabricated from Sodium Hyaluronate in Rats. Biol. Pharm. Bull. 2015, 38, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Statistical moments in pharmacokinetics. J. Pharmacokinet. Biopharm. 1978, 6, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Shayan, F.L.; Kim, S.; Huh, I.; Ma, Y.; Yang, H.; Kang, G.; Jung, H. Physicochemical study of ascorbic acid 2-glucoside loaded hyaluronic acid dissolving microneedles irradiated by electron beam and gamma ray. Carbohydr. Polym. 2018, 180, 297–303. [Google Scholar] [CrossRef] [PubMed]
- González-Vázquez, P.; Larrañeta, E.; McCrudden, M.T.C.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kwon, H.J.; Ahn, G.R.; Ko, E.J.; Yoo, K.H.; Kim, B.J.; Lee, C.; Kim, D. Hyaluronic acid microneedle patch for the improvement crow’s feet wrincles. Dermatol. Ther. 2017, 30, e12546. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Aponte, M.; Esposito, R.; Bondi, M.; Palmieri, B. Comparison of the effects of hyaluronidase and hyaluronic acid on probiotics growth. BMC Microbiol. 2013, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Neglia, R.G.; Baschieri, M.C.; Cermelli, C.; Caratozzolo, M.; Righi, E.; Palmieri, B.; Blasi, E. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J. Mater. Sci. Mater. Med. 2011, 22, 2329–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Luo, H.; Lu, W.; Luan, H.; Wu, Y.; Luo, J.; Wang, Y.; Pi, J.; Lim, C.Y.; Wang, H. Rapidly Dissolvable Microneedle Patches for Transdermal Delivery of Exenatide. Pharm. Res. 2014, 31, 3348–3360. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Wang, X.; Maa, Y. Effect of irradiation on parathyroid hormone PTH(1–34) coated on a novel transdermal microprojection delivery system to produce a sterile product—Adhesive compatibility. J. Pharm. Sci. 2010, 99, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Sandby-Møller, J.; Poulsen, T.; Wulf, H.C. Epidermal Thickness at Different Body Sites: Relationship to Age, Gender, Pigmentation, Blood Content, Skin Type and Smoking Habits. Acta Derm. Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Katsumi, H.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Sakane, T.; Yamamoto, A. Permeation of sumatriptan succinate across human skin using multiple types of self-dissolving microneedle arrays fabricated from sodium hyaluronate. J. Drug Target. 2016, 24, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Roskos, K.V.; Guy, R.H. Assessment of skin barrier function using transepidermal water loss: Effect of age. Pharm. Res. 1989, 6, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, Y.A.; Morrow, D.I.J.; Garland, M.J.; Donnelly, R.F.; El-Khordagui, L.K.; Meidan, V.M. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: Assessments by transepidermal water loss. Toxicol. Vitr. 2010, 24, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Caffarel-Salvador, E.; Migalska, K.; Woolfson, A.D.; Donnelly, R.F. Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J. Control. Release 2012, 159, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharm. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; Van den Berg, D.J.; Groenink, W.H.H.; Verpoorten, H.; Lüttge, R.; Bouwstra, J.A. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Dobnig, H.; Turner, R.T. The Effects of Programmed Administration of Human Parathyroid Hormone Fragment (1–34) on Bone Histomorphometry and Serum Chemistry in Rats. Endocrinology 1997, 138, 4607–4612. [Google Scholar] [CrossRef] [PubMed]

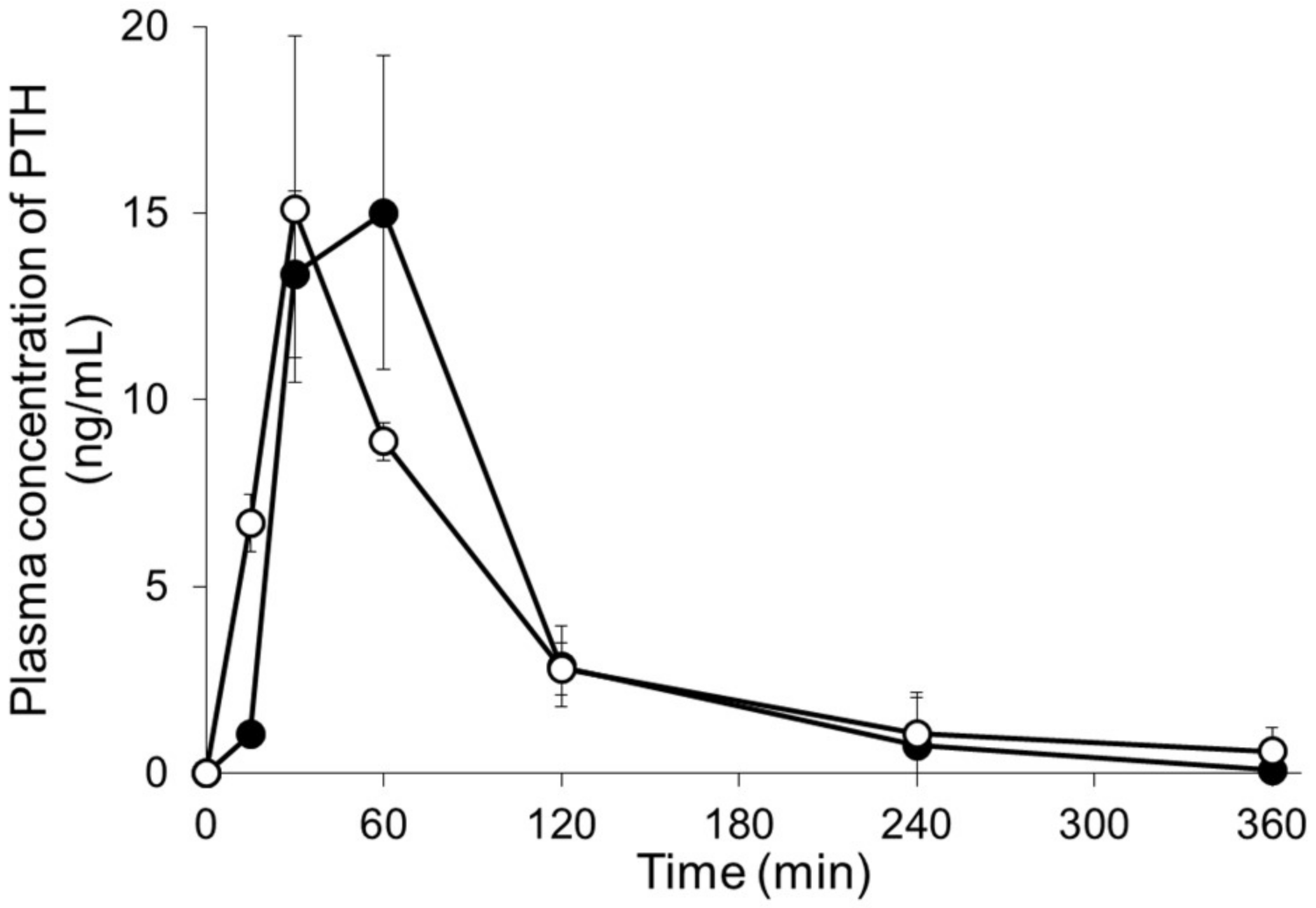

| Dose (µg/rat) | Cmax (ng/mL) | Tmax (min) | AUC0–∞ min (ng·mL/min) | BA (%) | |
|---|---|---|---|---|---|
| PTH s.c. | 20 | 15.1 ± 4.6 | 30 ± 0 | 1354 ± 484 | — |
| PTH-loaded MNs | 20 | 15.3 ± 3.6 | 50 ± 17 | 1355 ± 54 | 100 ± 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, C.; Katsumi, H.; Suzuki, T.; Quan, Y.-s.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1-34). Pharmaceutics 2018, 10, 215. https://doi.org/10.3390/pharmaceutics10040215
Naito C, Katsumi H, Suzuki T, Quan Y-s, Kamiyama F, Sakane T, Yamamoto A. Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1-34). Pharmaceutics. 2018; 10(4):215. https://doi.org/10.3390/pharmaceutics10040215
Chicago/Turabian StyleNaito, Chihiro, Hidemasa Katsumi, Tomoko Suzuki, Ying-shu Quan, Fumio Kamiyama, Toshiyasu Sakane, and Akira Yamamoto. 2018. "Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1-34)" Pharmaceutics 10, no. 4: 215. https://doi.org/10.3390/pharmaceutics10040215
APA StyleNaito, C., Katsumi, H., Suzuki, T., Quan, Y.-s., Kamiyama, F., Sakane, T., & Yamamoto, A. (2018). Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1-34). Pharmaceutics, 10(4), 215. https://doi.org/10.3390/pharmaceutics10040215




