Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Method
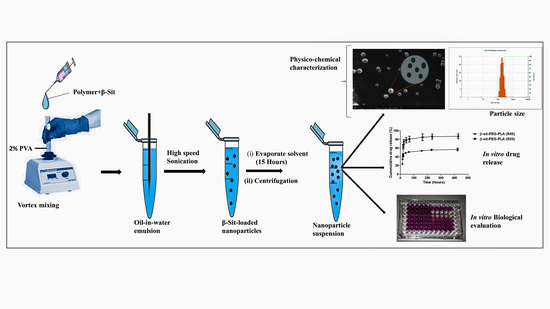
2.3. Preparation of Nanoparticles
2.3.1. Physico-Chemical Characterization of Nanoparticles
2.3.2. Nanoparticle Surface Morphology
2.3.3. Encapsulation Efficiency and Percent Drug Loading
2.4. In Vitro Release Profile
2.5. Stability Study
2.6. Biological Evaluation of Nanoparticles
2.6.1. Cell Culture
2.6.2. Cellular Uptake by Flow Cytometry
2.6.3. Confocal Microscopy
2.6.4. In Vitro Antiproliferative Activity
2.7. Statistical Analysis
3. Results and Discussions
3.1. Analytical Methods
3.2. Formulation Studies
3.3. Optimization Study for the Encapsulation of β-Sit
3.4. Encapsulation Efficiency and Drug-Loading Capacity
3.5. In Vitro Drug Release Profile
3.6. Stability
3.7. Cellular Uptake by Flow Cytometry
3.8. Confocal Microscopy
3.9. In Vitro Antiproliferative Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Awad, A.; Chinnam, M.; Fink, C.; Bradford, P. β-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine 2007, 14, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Vundru, S.S.; Kale, R.K.; Singh, R.P. β-sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement. Altern. Med. 2013, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Downie, C.A.; Fink, C.S. Inhibition of growth and stimulation of apoptosis by ß-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int. J. Mol. Med. 2000, 5, 541–545. [Google Scholar] [PubMed]
- Awad, A.B.; Roy, R.; Fink, C.S. β-Sitosterol, a plant sterol induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol. Rep. 2003, 10, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Mandadi, K.K.; Poulose, S.M.; Jadegoud, Y.; Nagana Gowda, G.A.; Patil, B.S. Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorg. Med. Chem. 2007, 15, 4923–4932. [Google Scholar] [CrossRef] [PubMed]
- Raicht, R.F.; Cohen, B.I.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective Effect of Plant Sterols against Chemically Induced Colon Tumors in Rats. Cancer Res. 1980, 40, 403–405. [Google Scholar] [PubMed]
- Park, C.; Moon, D.O.; Rhu, C.H.; Choi, B.T.; Lee, W.H.; Kim, G.Y.; Choi, Y.H. Beta-sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol. Pharm. Bull. 2007, 30, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Von Holtz, R.L.; Fink, C.S.; Awad, A.B. β-sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr. Cancer 1998, 32, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.S.B.; Ameen, S.S. Beta-Sitosterol: A Promising but Orphan Nutraceutical to Fight Against Cancer. Nutr. Cancer 2015, 67, 1214–1220. [Google Scholar]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, E.; Schubert, R.; Zelkó, R. Effect of β-sitosterol concentration and high pressure homogenization on the chlorhexidine release from vesicular gels. Int. J. Pharm. 2006, 307, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachari, Y.; Madan, P.; Lin, S. Development of pH-and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. Int. J. Pharm. 2007, 338, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, N.; Stan, R.; Meghea, A. Novel bio-active lipid nanocarriers for the stabilization and sustained release of sitosterol. Nanotechnology 2012, 23, 455702. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Jarowski, C.I. Oral absorption efficiency of acid-labile antibiotics from lipid-drug delivery systems. J. Pharm. Sci. 1975, 64, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, B.; Bergenståhl, B.; Kronberg, B. A method for the preparation of submicron particles of sparingly water-soluble drugs by precipitation in oil-in-water emulsions. II: Influence of the emulsifier, the solvent, and the drug substance. J. Pharm. Sci. 1993, 82, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, H.; Koide, H.; Shimizu, K.; Asai, T.; Shimizu, N.K.; Ishikado, A.; Makino, T.; Oku, N. Chemoprevention of tumor metastasis by liposomal β-sitosterol intake. Biol. Pharm. Bull. 2008, 31, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Williams, H.; Fink, C.S. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast cancer cells. Nutr. Cancer 2001, 40, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lakkakula, J.R.; Maçedo Krause, R.W. A vision for cyclodextrin nanoparticles in drug delivery systems and pharmaceutical applications. Nanomedicine 2014, 9, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wischke, C.; Mittal, S.; Mitra, A.; Schwendeman, S.P. Design of controlled release PLGA microspheres for hydrophobic fenretinide. Mol. Pharm. 2016, 13, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagam, D.; Novak, K.; Oyewumi, M.O. Therapeutic Delivery of Simvastatin Loaded in PLA-PEG Polymersomes Resulted in Amplification of Anti-inflammatory Effects in Activated Microglia. AAPS J. 2018, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Cella, C.; Gerges, I.; Milani, P.; Lenardi, C.; Argentiere, S. Calcium Stearate as an Effective Alternative to Poly (vinyl alcohol) in Poly-Lactic-co-Glycolic Acid Nanoparticles Synthesis. Biomacromolecules 2017, 18, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.B.; Silva, S.L.; Galvão, M.A.; Ferreira, M.R.; Araújo, E.L.; Randau, K.P.; Soares, L.A. Total phytosterol content in drug materials and extracts from roots of Acanthospermum hispidum by UV-VIS spectrophotometry. Revista Brasileira de Farmacognosia 2013, 23, 736–742. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Kenny, A.P. The determination of cholesterol by the Liebermann-Burchard reaction. Biochem. J. 1952, 52, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nath, M.C.; Chakravorty, M.K.; Chowdhury, S.R. Liebermann-Burchard Reaction for Steroids. Nature 1946, 157, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, L.; Liu, C.; Wang, B. β-Sitosterol solubility in selected organic solvents. J. Chem. Eng. Data 2010, 55, 2917–2919. [Google Scholar] [CrossRef]
- Xiong, Q.; Ruan, B.; Whitby, F.G.; Tuohy, R.P.; Belanger, T.L.; Kelley, R.I.; Wilson, W.K.; Schroepfer, G.J. A colorimetric assay for 7-dehydrocholesterol with potential application to screening for Smith–Lemli–Opitz syndrome. Chem. Phys. Lipids 2002, 115, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Firempong, C.K.; Wang, Y.-W.; Xu, W.-Q.; Wang, M.-M.; Cao, X.; Zhu, Y.; Tong, S.-S.; Yu, J.-N.; Xu, X.-M. Ergosterol-loaded poly(lactide-co-glycolide) nanoparticles with enhanced in vitro antitumor activity and oral bioavailability. Acta Pharmacol. Sin. 2016, 37, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval, A.; Bahadur, P.; Raval, A. Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating. J. Pharm. Anal. 2017, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-S.; Kim, B.-S.; Puri, A.; Kumar, K.; Cho, C.-W. Stability of paclitaxel-loaded solid lipid nanoparticles in the presence of 2-hydoxypropyl-β-cyclodextrin. Arch. Pharm. Res. 2016, 39, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-P.; Wong, W.-P.; Chen, L.-C.; Su, C.-Y.; Chen, L.-G.; Liu, D.-Z.; Ho, H.-O.; Sheu, M.-T. Physical and Pharmacokinetic Characterizations of trans-Resveratrol (t-Rev) Encapsulated with Self-Assembling Lecithin-based Mixed Polymeric Micelles (sa LMPMs). Sci. Rep. 2017, 7, 10674. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lee, Y.B.; Oh, I.J. Cellular uptake of poly (DL-lactide-co-glycolide) nanoparticles: Effects of drugs and surface characteristics of Nanoparticles. J. Pharm. Investig. 2015, 45, 659–667. [Google Scholar] [CrossRef]
- McCall, R.L.; Sirianni, R.W. PLGA Nanoparticles Formed by Single- or Double-emulsion with Vitamin E-TPGS. J. Vis. Exp. JoVE 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.C.; Lee, H.S.; Choung, I.Y.; Cho, K.I.; Ahn, Y.; Choi, E.J. The effect of type of organic phase solvents on the particle size of poly (d, l-lactide-co-glycolide) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Shan, X.; Yuan, Y.; Liu, C.; Xu, F.; Sheng, Y. Comparison of the PLA-mPEG and mPEG-PLA-mPEG copolymers nanoparticles on the plasma protein adsorption and in vivo biodistribution. Soft Matter 2009, 5, 2875–2883. [Google Scholar] [CrossRef]
- Chen, W.; Palazzo, A.; Hennink, W.E.; Kok, R.J. Effect of particle size on drug loading and release kinetics of gefitinib-loaded PLGA microspheres. Mol. Pharm. 2016, 14, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Khosroushahi, A.Y. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell. Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| (a) | ||||||
| Formulation | Weight of β-sitosterol Used for Formulation | |||||
| 2 mg/mL | 4 mg/mL | |||||
| Z-average (nm) | PDI | ζ (mV) | Z-average (nm) | PDI | ζ (mV) | |
| β-Sit-PLGA-EtAC | 215.1 ± 29.7 | 0.10 ± 0.003 | −13.9 ± 1.61 | 231.2 ± 0.60 | 0.126 ± 0.02 | −16.1 ± 7.09 |
| β-Sit-PLGA-DCM | 311.1 ± 94.4 | 0.242 ± 0.03 | −14.0 ± 6.01 | 261.6 ± 4.74 a | 0.113 ± 0.03 a | −16.5 ± 0.21 a |
| β-Sit-PEG-PLA (R45) | 240.6 ± 23.3 | 0.18 ± 0.05 | −23.5 ± 0.27 | 276.7 ± 1.01 | 0.197 ± 0.01 | −21.5 ± 6.56 |
| β-Sit-PEG-PLA (R25) | 239.5 ± 7.9 | 0.17 ± 0.05 | −24.5 ± 0.99 | 279.4 ± 0.85 | 0.112 ± 0.04 | −21.2 ± 7.01 |
| (b) | ||||||
| Formulation | Physicochemical Properties of Coumarin 6-Labeled Nanoparticles | |||||
| Z-average (nm) | PDI | ζ (mV) | ||||
| β-Sit-PLGA-EtAC | 228 ± 0.45 | 0.10 ± 0.01 | −16.8 ± 0.33 | |||
| β-Sit-PEG-PLA (R45) | 245 ± 0.66 | 0.09 ± 0.02 | −19.6 ± 0.22 | |||
| Formulation | Weight of β-sitosterol Used for Formulation | |||||
|---|---|---|---|---|---|---|
| 2 mg/mL | 4 mg/mL | |||||
| EE (%) | EDL % | ADL (%) | EE (%) | EDL % | ADL (%) | |
| β-Sit-PLGA-EtAC | 62.89 ± 4.66 | 4.76 | 3.00 ± 0.22 | 48.41 ± 23.82 | 9.09 | 4.4 ± 2.17 |
| β-Sit-PLGA-DCM | 85.13 ± 6.35 | 4.76 | 4.05 ± 0.30 | 88.48 ± 0.01 a | 9.09 | 8.04 ± 0.01 a |
| β-Sit-PEG-PLA (R45) | 51.83 ± 19.72 | 4.76 | 2.47 ± 0.94 | 71.02 ± 22.48 | 9.09 | 6.46 ± 2.04 |
| β-Sit-PEG-PLA (R25) | 34.84 ± 1.71 | 4.76 | 1.67 ± 0.08 | 66.85 ± 8.13 | 9.09 | 6.08 ± 0.74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andima, M.; Costabile, G.; Isert, L.; Ndakala, A.J.; Derese, S.; Merkel, O.M. Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity. Pharmaceutics 2018, 10, 232. https://doi.org/10.3390/pharmaceutics10040232
Andima M, Costabile G, Isert L, Ndakala AJ, Derese S, Merkel OM. Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity. Pharmaceutics. 2018; 10(4):232. https://doi.org/10.3390/pharmaceutics10040232
Chicago/Turabian StyleAndima, Moses, Gabriella Costabile, Lorenz Isert, Albert J. Ndakala, Solomon Derese, and Olivia M. Merkel. 2018. "Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity" Pharmaceutics 10, no. 4: 232. https://doi.org/10.3390/pharmaceutics10040232
APA StyleAndima, M., Costabile, G., Isert, L., Ndakala, A. J., Derese, S., & Merkel, O. M. (2018). Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity. Pharmaceutics, 10(4), 232. https://doi.org/10.3390/pharmaceutics10040232