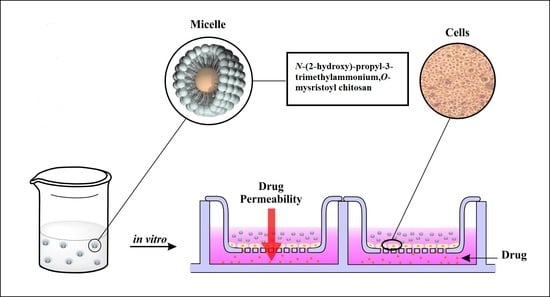
N-(2-Hydroxy)-propyl-3-trimethylammonium, O-Mysristoyl Chitosan Enhances the Solubility and Intestinal Permeability of Anticancer Curcumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 3,6-O,O’-Myristoyl Chitosan
2.3. Synthesis of N-(2-Hydroxy)-Propyl-3-Trimethylammonium Chitosan Chloride
2.4. Preparation of CUR-Loaded DMCh and DMCat Micelles
2.5. Characterization
2.5.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.2. 1H NMR Spectroscopy
2.5.3. Conductometric Titration
2.5.4. Critical Aggregation Concentration (CAC)
2.5.5. Particle Size and Surface Charge
2.5.6. Drug Encapsulation Efficiency
2.6. In Vitro Studies
2.6.1. Cell Culturing Reagents
2.6.2. Cytotoxicity Assay
2.6.3. Permeability Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Spectroscopic Characterizatio
3.2. Critical Aggregation Concentration
3.3. Drug Encapsulation Efficiency
3.4. Particle Size and Surface Charge
3.5. Cytotoxicity Assay
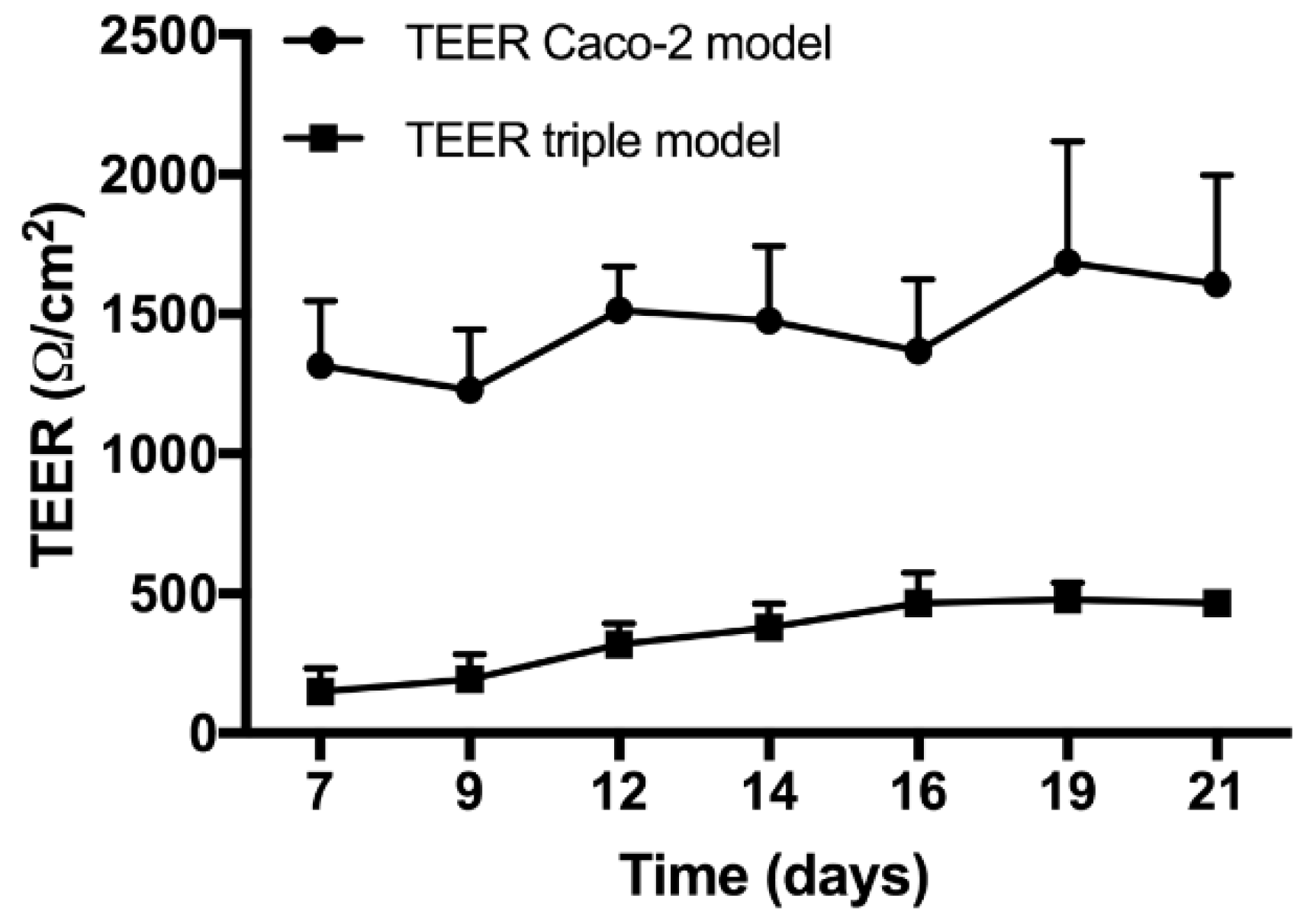
3.6. Permeability Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.R.; Qiao, P. Drug Delivery in Cancer Therapy, Quo Vadis? Mol. Pharm. 2018, 15, 3603–3616. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Saesoo, S.; Ruktanonchai, U.R.; Srinuanchai, W.; Puttipipatkhachorn, S. Synthesis and anticervical cancer activity of novel pH responsive micelles for oral curcumin delivery. Int. J. Pharm. 2014, 477, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-applied curcumin for different diseases therapy. Biomed. Res. Int. 2014, 2014, 39426. [Google Scholar] [CrossRef] [PubMed]
- Sarisozen, C.; Abouzeid, A.H.; Torchilin, V.P. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur. J. Pharm. Biopharm. 2014, 88, 539–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popat, A.; Karmaka, S.R.; Jambhrunkar, S.; Xu, C.; Yu, C. Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity. Colloids Surf. B Biointerfaces 2014, 117, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Imran, M.; Butt, T.T.; Ali Shah, S.W.; Sohail, M.; Malik, A.; Das, S.; Thu, H.E.; Adam, A.; Hussain, Z. Curcumin based nanomedicines as efficient Nanoplatform for treatment of cancer: New developments in reversing cancer drug resistance, rapid internalization, and improved anticancer efficacy. Trends Food Sci. Technol. 2018, 80, 8–22. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Orhan, I.E.; Bawazeer, S. Health perspectives of a bioactive compound curcumin: A review. Trends Food Sci. Technol. 2018, 74, 33–45. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Angeles, L.; Angeles, L.; Haven, N. Phase 1 study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef]
- Kyung, H.; Minkyu, A.; Sun, J.; Sym, J. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2014, 74, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Cortez, A.J.; Tudre, P.J.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.L.; Lacroix, M.; Ispas-Szabo, P.; Mateescu, M.A. N-acylated chitosan: Hydrophobic matrices for controlled drug release. J. Control. Release 2003, 93, 1–13. [Google Scholar] [CrossRef]
- Ishihar, M.A.; Kishimoto, M.; Fujita, S.; Hattori, H.; Kanatani, Y. Biological, Chemical and Physical Compatibility of Chitosan and Biopharmaceuticals. Chitosan-Based Syst. Biopharm. Deliv. Target. Polym. Ther. 2012, 93–106. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Li, J. Formation, microstructure, biodistribution and absence of toxicity of polymeric micelles formed by N-octyl-N,O-carboxymethyl chitosan. Carbohydr. Polym. 2011, 83, 1959–1969. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ming, J.; He, B.; Fan, Y.; Gu, Z.; Zhang, X. Preparation and characterization of novel polymeric micelles for 9-nitro-20(S)-camptothecin delivery. Eur. J. Pharm. Sci. 2008, 34, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Muley, P.; Kumar, S.; El Kourati, F.; Kesharwani, S.S.; Tummala, H. Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route. Int. J. Pharm. 2016, 500, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshida, C.; Murakami, Y. Design of novel sheet-shaped chitosan hydrogel for wound healing: A hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater. Sci. Eng. C 2013, 33, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cai, L.; Liu, P.; You, J.; Yuan, H.; Hu, F. Biomaterials Tumor cells-speci fi c targeting delivery achieved by A54 peptide functionalized polymeric micelles. Biomaterials 2012, 33, 8858–8867. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Li, X.; Zhang, S.; Liu, J.; Di, D.; Zhang, Y.; Zhao, Q.; Wang, S. Redox and pH dual-responsive PEG and chitosan-conjugated hollow mesoporous silica for controlled drug release. Mater. Sci. Eng. C 2016, 67, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, Z.; Taylor, K.M.G.; Puri, S.; Cath, A.; Al-jamal, K.; Bai, J.; Klippstein, R.; Wang, T.; Forbes, B.; Chana, J. Mixed micelles of lipoic acid-chitosan-poly(ethylene glycol) and distearoylphosphatidylethanolamine-poly(ethylene glycol) for tumor delivery. Eur. J. Pharm. Sci. 2017, 101, 228–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Li, N.; Ju, C.; Sun, M.; Zhang, C.; Pin, Q.G. Biomaterials The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials 2011, 32, 4609–4620. [Google Scholar] [CrossRef]
- Huo, M.; Liu, Y.; Wang, L.; Yin, T.; Qin, C.; Xiao, Y.; Yin, L.; Liu, J.; Zhou, J. Redox-sensitive micelles based on O,N-hydroxyethyl chitosan-octylamine conjugates for triggered intracellular delivery of paclitaxel Redox-sensitive micelles based on O,N-hydroxyethyl chitosan-octylamine conjugates for triggered intracellular deliver. Mol. Pharm. 2016, 13, 1750–1762. [Google Scholar] [CrossRef] [PubMed]
- Attia, E.A.B.; Ong, Z.Y.; Hedrick, J.L.; Lee, P.P.; Lee, P.L.R.; Hammond, P.T.; Yang, Y.-Y. Mixed micelles self-assembled from block copolymers for drug delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 182–194. [Google Scholar] [CrossRef]
- Jiang, G.B.B.; Quan, D.; Liao, K.; Wang, H. Novel polymer micelles prepared from chitosan grafted hydrophobic palmitoyl groups for drug delivery. Mol. Pharm. 2006, 3, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q.; et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.Q.; Zhao, M.D.D.; Yuan, H.; You, J.; Du, Y.Z.Z.; Zeng, S. A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: Properties and in vitro transfection studies. Int. J. Pharm. 2006, 315, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Chenguang, L.I.U.; Desai, K.G.; Xiguang, C.; Hyun-jin, P. Preparation and Charaterization of Self-assembled Nanoparticles Based on Linolenic-acid Modified Chitosan. J. Ocean Univ. China 2005, 4, 234–239. [Google Scholar] [CrossRef]
- Hu, F.Q.; Ren, G.F.; Yuan, H.; Du, Y.Z.; Zeng, S. Shell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxel. Colloids Surf. B Biointerfaces 2006, 50, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Yu, D.; Wu, Y.; Li, J.; Li, H. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int. J. Pharm. 2010, 394, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Z.Z.; Wang, L.; Yuan, H.; Wei, X.H.H.; Hu, F.Q.Q. Preparation and characteristics of linoleic acid-grafted chitosan oligosaccharide micelles as a carrier for doxorubicin. Colloids Surf. B Biointerfaces 2009, 69, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.S.; Almeida, A.; Prezotti, F.; Cury, B.; Campana-Filho, S.P.; Sarmento, B. Synthesis and characterization of 3,6-O,O’-dimyristoyl chitosan micelles for oral delivery of paclitaxel. Colloids Surf. B Biointerfaces 2017, 152, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Gao, Y.; Heng, L.; Liu, Y.; Yao, G.; Wang, Y.; Liu, Y. Amphiphilic N-(2,3-dihydroxypropyl)-chitosan-cholic acid micelles for paclitaxel delivery. Carbohydr. Polym. 2013, 94, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sedlarik, V. Amphiphilic chitosan-grafted-functionalized polylactic acid based nanoparticles as a delivery system for doxorubicin and temozolomide co-therapy. Int. J. Pharm. 2014, 474, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, Y.; Lucy, L.; Ping, Q. Polymeric micelle systems of hydroxycamptothecin based on amphiphilic N-alkyl-N-trimethyl chitosan derivatives. Colloids Surf. B Biointerfaces 2007, 55, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, A.; Debbaudt, A.; Albertengo, L.; Rodríguez, M.S. Synthesis and characterization of N-propyl-N-methylene phosphonic chitosan derivative. Carbohydr. Polym. 2010, 79, 475–480. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Gonil, P.; Treetong, A.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Opanasopit, P. pH-Responsive polymeric micelles based on amphiphilic chitosan derivatives: Effect of hydrophobic cores on oral meloxicam delivery. Int. J. Pharm. 2016, 497, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, W.; Wang, L.Y.; Su, Z.G.; Ma, G.H. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials 2007, 28, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Sonia, T.A.; Sharma, C.P. In vitro evaluation of N-(2-hydroxy) propyl-3-trimethyl ammonium chitosan for oral insulin delivery. Carbohydr. Polym. 2011, 84, 103–109. [Google Scholar] [CrossRef]
- Ruihua, H.; Bingchao, Y.; Zheng, D.; Wang, B. Preparation and characterization of a quaternized chitosan. J. Mater. Sci. 2012, 47, 845–851. [Google Scholar] [CrossRef]
- Santos, D.M.; Bukzem, A.L.; Campana-Filho, S.P. Response surface methodology applied to the study of the microwave-assisted synthesis of quaternized chitosan. Carbohydr. Polym. 2016, 138, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Milas, M.; Le Dung, P. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Almeida, A.; Silva, D.S.; Gonçalves, V.; Sarmento, B. Synthesis and characterization of chitosan-grafted-polycaprolactone micelles for modulate intestinal paclitaxel delivery. Drug Deliv. Transl. Res. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.S.; Almeida, A.; Prezotti, F.G.; Facchinatto, W.M.; Colnago, L.A.; Campana-Filho, S.P.; Sarmento, B. Self-aggregates of 3,6-O,O’-dimyristoylchitosan derivative are effective in enhancing the solubility and intestinal permeability of camptothecin. Carbohydr. Polym. 2017, 177, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajim, A.A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 94, 87–94. [Google Scholar] [CrossRef]
- Fiamingo, A.; Delezuk, J.A.D.M.; Trombotto, S.; David, L.; Campana-Filho, S.P. Extensively deacetylated high molecular weight chitosan from the multistep ultrasound-assisted deacetylation of beta-chitin. Ultrason. Sonochem. 2016, 32, 79–85. [Google Scholar] [CrossRef] [PubMed]
- ISO/EN10993-5. International Standard ISO 10993-5 Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods; International Organization for Standardization: Geneva, Switzerland, 2009; Volume 42.
- Araújo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Andrade, F.; Araújo, F.; Ferreira, D.; Sarmento, B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur. J. Pharm. Biopharm. 2013, 83, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Cho, J.; Grant, J.; Piquette-miller, M.; Allen, C. Synthesis and Physicochemical and Dynamic Mechanical Properties of a Water-Soluble Chitosan Derivative as a Biomaterial. Biomacromolecules 2006, 10, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wan, Y.; Wang, X.; Zha, Q.; Liu, H.; Qiu, Z.; Zhang, S. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf. B Biointerfaces 2012, 91, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Dong, H.; Cai, X.; Ren, T. Pluronic F127 nanomicelles engineered with nuclear localized functionality for targeted drug delivery. Mater. Sci. Eng. C 2013, 33, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Ji, Q.; Qiu, L. Targeted delivery of anticancer drugs by aptamer AS1411 mediated Pluronic F127/cyclodextrin-linked polymer composite micelles. Nanomedicine 2014, 1, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, L.; Du, Y.; Hu, F. Stearic acid-g-chitosan polymeric micelle for oral drug delivery: In intro transport and in vivo absorption. Mol. Pharm. 2010, 8, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ping, Q.; Zhang, H.; Shen, J. Preparation of N-alkyl-O-sulfate chitosan derivatives and micellar solubilization of taxol. Carbohydr. Polym. 2003, 54, 137–141. [Google Scholar] [CrossRef]
- Zhang, C.; Qineng, P.; Zhang, H. Self-assembly and characterization of paclitaxel-loaded N-octyl-O-sulfate chitosan micellar system. Colloids Surf. B Biointerfaces 2004, 39, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, A.; Almeida, A.; Sarmento, B. Elucidation of the impact of cell culture conditions of Caco-2 cell monolayer on barrier integrity and intestinal permeability. Eur. J. Pharm. Biopharm. 2017, 119, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Tozaki, H.; Komoike, J.; Tada, C.; Maruyama, T.; Terabe, A.; Suzuki, T.; Yamamoto, A.; Muranishi, S. Chitosan capsules for colon-specific drug delivery: Improvement of insulin absorption from the rat colon. J. Pharm. Sci. 1997, 86, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Chauvierre, C.; Leclerc, L.; Labarre, D.; Appel, M.; Marden, M.C.; Couvreur, P.; Vauthier, C. Enhancing the tolerance of poly(isobutylcyanoacrylate) nanoparticles with a modular surface design. Int. J. Pharm. 2007, 338, 327–332. [Google Scholar] [CrossRef] [PubMed]







| Drug (μg) | Micelles–CUR | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample DMCh | Sample DMCat | |||||||
| Size (nm) 1 | PDI 2 | Zeta (mV) 3 | EE (%) 4 | Size (nm) 1 | PDI 2 | Zeta (mV) 3 | EE (%) 4 | |
| 0 | 356 ± 21 | 0.53 ± 0.03 | 32.1 ± 4.4 | - | 343 ± 19 | 0.21 ± 0.02 | 34.0 ± 0.8 | - |
| 50 | 289 ± 17 | 0.49 ± 0.04 | 42.5 ± 2.1 | 92.7 ± 0.8 | 268 ± 43 | 0.62 ± 0.01 | 30.5 ± 1.2 | 100 ± 0.5 |
| 100 | 281 ± 38 | 0.44 ± 0.04 | 49.1 ± 0.2 | 81.8 ± 1.0 | 231 ± 15 | 0.66 ± 0.02 | 28.8 ± 1.0 | 73.8 ± 1.0 |
| 150 | 386 ± 168 | 0.44 ± 0.16 | 46.6 ± 4.12 | 80.9 ± 0.9 | 372 ± 23 | 0.49 ± 0.03 | 33.0 ± 1.5 | 68.6 ± 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.S.; M. dos Santos, D.; Almeida, A.; Marchiori, L.; Campana-Filho, S.P.; Ribeiro, S.J.L.; Sarmento, B. N-(2-Hydroxy)-propyl-3-trimethylammonium, O-Mysristoyl Chitosan Enhances the Solubility and Intestinal Permeability of Anticancer Curcumin. Pharmaceutics 2018, 10, 245. https://doi.org/10.3390/pharmaceutics10040245
Silva DS, M. dos Santos D, Almeida A, Marchiori L, Campana-Filho SP, Ribeiro SJL, Sarmento B. N-(2-Hydroxy)-propyl-3-trimethylammonium, O-Mysristoyl Chitosan Enhances the Solubility and Intestinal Permeability of Anticancer Curcumin. Pharmaceutics. 2018; 10(4):245. https://doi.org/10.3390/pharmaceutics10040245
Chicago/Turabian StyleSilva, Daniella S., Danilo M. dos Santos, Andreia Almeida, Leonardo Marchiori, Sérgio P. Campana-Filho, Sidney J. L. Ribeiro, and Bruno Sarmento. 2018. "N-(2-Hydroxy)-propyl-3-trimethylammonium, O-Mysristoyl Chitosan Enhances the Solubility and Intestinal Permeability of Anticancer Curcumin" Pharmaceutics 10, no. 4: 245. https://doi.org/10.3390/pharmaceutics10040245
APA StyleSilva, D. S., M. dos Santos, D., Almeida, A., Marchiori, L., Campana-Filho, S. P., Ribeiro, S. J. L., & Sarmento, B. (2018). N-(2-Hydroxy)-propyl-3-trimethylammonium, O-Mysristoyl Chitosan Enhances the Solubility and Intestinal Permeability of Anticancer Curcumin. Pharmaceutics, 10(4), 245. https://doi.org/10.3390/pharmaceutics10040245