A Comprehensive Map of FDA-Approved Pharmaceutical Products
Abstract
:1. Introduction
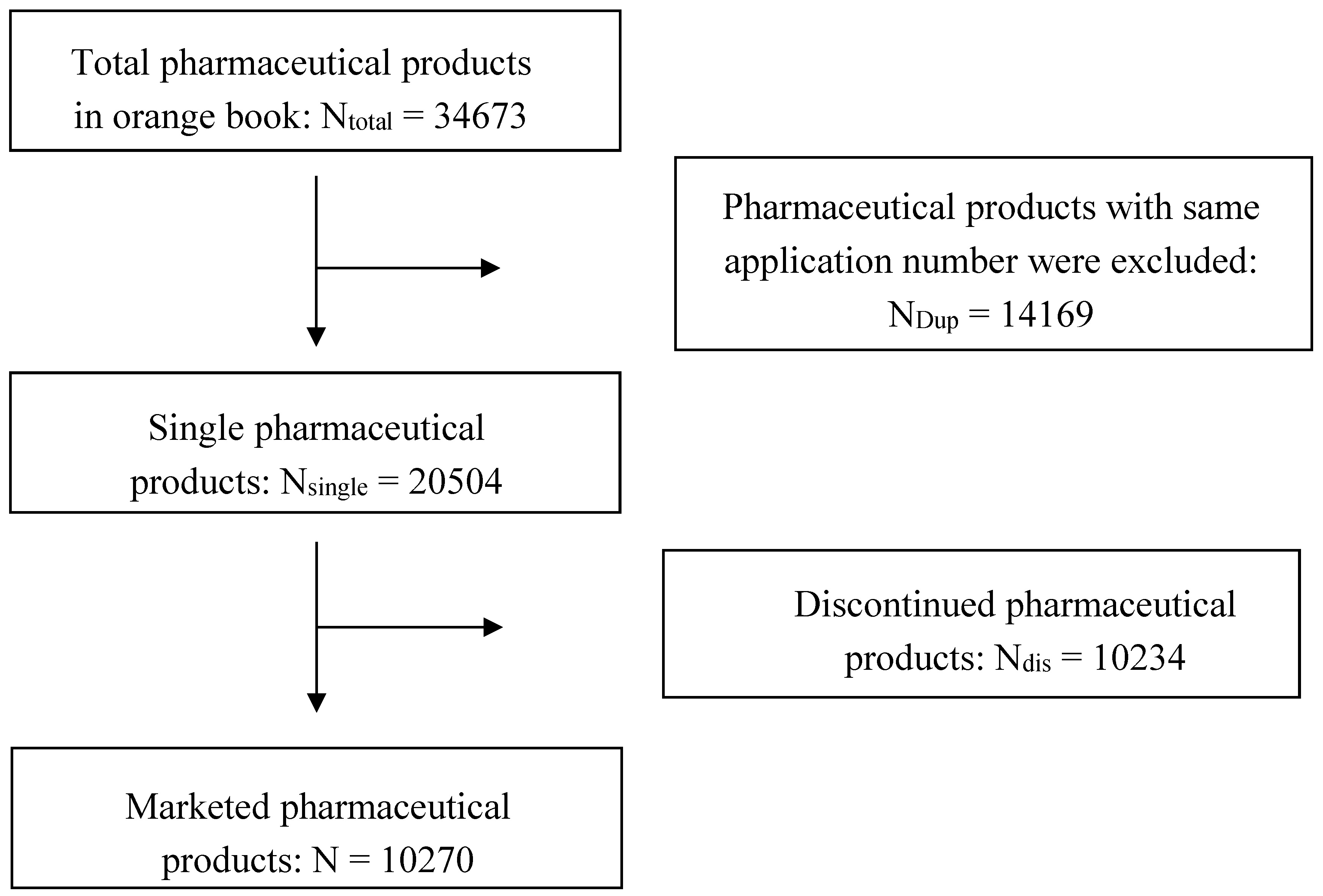
2. Data Collection and Analysis
3. The Overview of Pharmaceutical Products
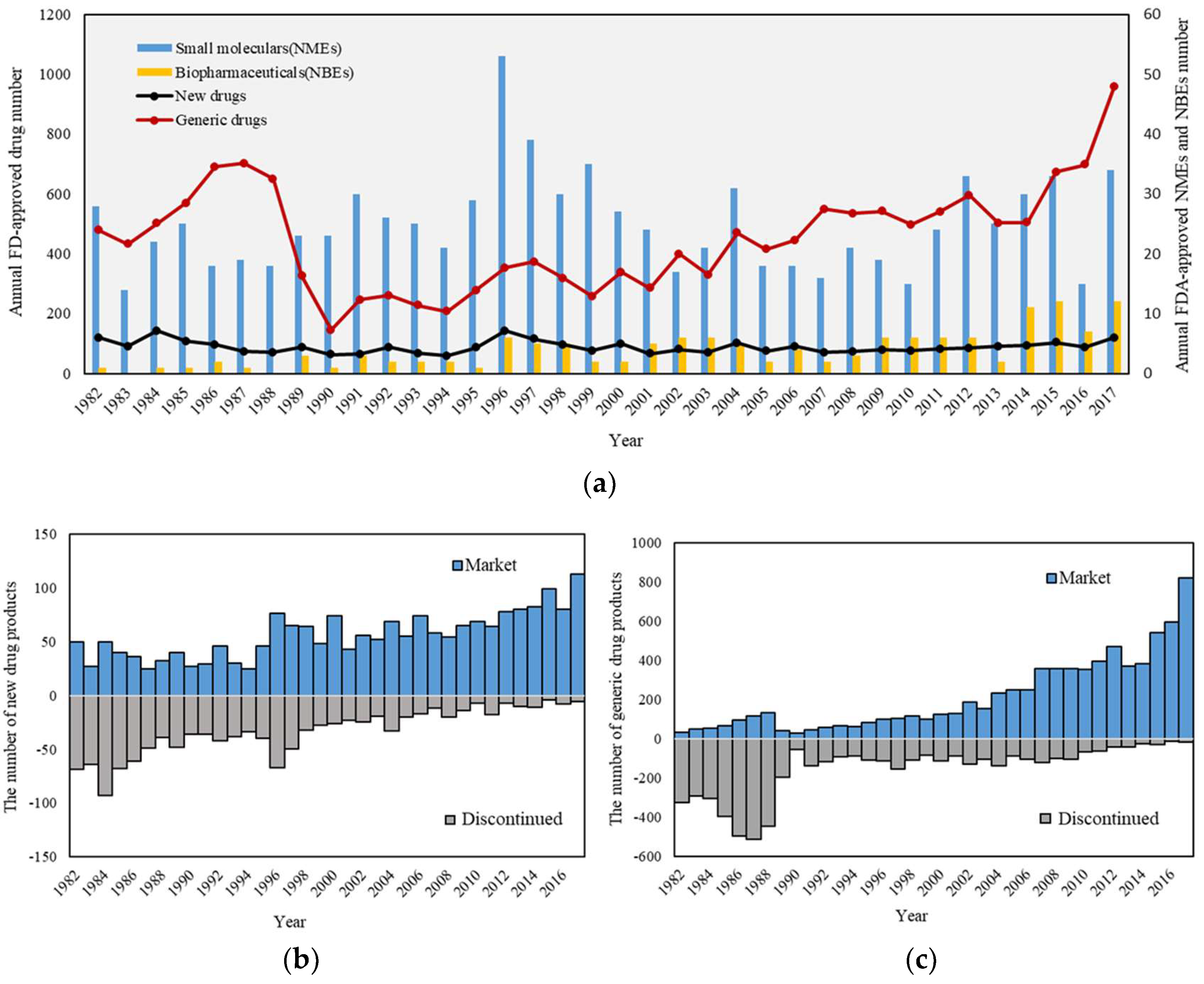
3.1. Outline of Annual Approval and Discontinued Number
3.2. Proportion of Route of Administration
3.3. Distribution of Formulations
4. The Analysis of Advanced DDSs
4.1. Oral Sustained Release Preparations
4.2. Inhalation Systems
4.3. Transdermal Patch
4.4. Complex Injections
4.4.1. Liposome Injections
4.4.2. Nanoparticle and Suspension Injections
4.4.3. Microsphere Injections
4.4.4. Emulsion Injections
5. Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 2009, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Smietana, K.; Siatkowski, M.; Møller, M. Trends in clinical success rates. Nat. Rev. Drug Discov. 2016, 15, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Asher, M. 2017 FDA drug approvals. Nat. Rev. Drug Discov. 2018, 17, 81–85. [Google Scholar]
- Verma, R.K.; Garg, S. Drug delivery technologies and future directions. Pharm. Technol. 2001, 25, 1–14. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K. Drug delivery of the future: Chasing the invisible gorilla. J. Control. Release 2016, 240, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled drug delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Lee, P.I.; Li, J.X. Evolution of oral controlled release dosage forms. In Oral Controlled Release Formulation Design and Drug Delivery; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 21–31. [Google Scholar]
- Clark, A.R. Medical aerosol inhalers: Past, present, and future. Aerosol Sci. Technol. 1995, 22, 374–391. [Google Scholar] [CrossRef]
- Pastore, M.N. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABi-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Kelly, C. The balance between innovation and competition: The hatch-waxman act, the 2003 amendments, and beyond. Food Drug Law J. 2011, 66, 417. [Google Scholar] [PubMed]
- Danzis, S.D. The Hatch-Waxman act: History, structure, and legacy. Antitrust Law J. 2003, 71, 585–608. [Google Scholar]
- Rumore, M.M. The Hatch-Waxman act-25 years later: Keeping the pharmaceutical scale balanced. Pharm. Times 2009, 20, 4–7. [Google Scholar]
- The 38th Edition Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM071436.pdf (accessed on 31 December 2017).
- FDA Data Standards of Manual(monographs). Available online: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/DataStandardsManualmonographs/ucm071667.htm (accessed on 11 January 2006).
- FDA Data Standards of Manual(monographs). Available online: http://wayback.archiveit.org/7993/20171115111312/https://www.fda.gov/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/DataStandardsManualmonographs/ucm071666.htm (accessed on 15 December 2006).
- Ken., M. Transdermal patches: Past, present and future. Ther. Deliv. 2015, 6, 639–641. [Google Scholar]
- Garg, S. Pre-meal insulin analogue insulin lispro vs humulin® R insulin treatment in young subjects with type 1 diabetes. Diabet. Med. 1996, 13, 47–52. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Ujoodha, R.; Jhugroo, A.; Hulse, G.K.; Jhurry, D. Naltrexone: A review of existing sustained drug delivery systems and emerging nano-based systems. J. Control. Release 2014, 183, 154–166. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W. Freeze-drying of drug-free and drug-loaded solid lipid nanoparticles (SLN). Int. J. Pharm. 1997, 157, 171–179. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Scott, R.L.; Mulloy, J.P. Small bowel procardia XL tablet bezoar mimicking cystic pneumatosis intestinalis. Abdom. Imaging 1996, 21, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Warts, G. Veregen: A botanical for treatment of genital warts. Obstet. Gynecol. 2008, 112, 693–694. [Google Scholar]
- Fitzgerald, S. Fda approves first 3D-printed epilepsy drug experts assess the benefits and caveats. Neurol. Today 2015, 15, 26–27. [Google Scholar] [CrossRef]
- FDA. Approved Products-Kymriah (Tisagenlecleucel). 2018. Available online: https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm573706.htm (accessed on 13 August 2017).
- Plowman, R.S.; Peters-Strickland, T.; Savage, G.M. Digital medicines: Clinical review on the safety of tablets with sensors. Expert Opin. Drug Saf. 2018, 17, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Shulman, S.R.; Kaitin, K.I. The prescription drug user fee act of 1992. PharmacoEconomics 1996, 9, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.-P.; Kros, J.M. Approved car T cell therapies: Ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov. Today 2018, 23, 1175–1182. [Google Scholar] [CrossRef]
- Zuñiga, L.; Calvo Hernáez, M.B. Biosimilars—The way forward. Hosp. Pharm. Eur. 2010, 50, 1–2. [Google Scholar]
- Siramshetty, V.B.; Nickel, J. Withdrawn—A resource for withdrawn and discontinued drugs. Nucleic Acids Res. 2015, 44, D1080–D1086. [Google Scholar] [CrossRef]
- Scharschmidt, B.; Mokhtarani, M. Methods of Therapeutic Monitoring of Nitrogen Scavenging Drugs. U.S. Patent 9,095,559, 4 August 2015. [Google Scholar]
- Leichs, C.; Breitenbach, A.; Lehrke, I.; Galfetti, P. Non-Mucoadhesive Film Dosage Forms. U.S. Patent 8,580,830, 12 November 2013. [Google Scholar]
- Myers, G.L.; Hariharan, M.S.; Davidson, K.; Sanghvi, P. Stabilized Amine-Containing Actives in Oral Film compositions. U.S. Patent 9,095,577, 4 August 2015. [Google Scholar]
- Rosen, C. Effervescent Compositions Comprising Bisphosphonates and Methods Related Thereto. U.S. Patent 7,488,496, 10 February 2009. [Google Scholar]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Rowland, M.; Noe, C.R. Impact of the pharmaceutical sciences on health care: A reflection over the past 50 years. J. Pharm. Sci. 2012, 101, 4075–4099. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Q. Big data analysis of global advances in pharmaceutics and drug delivery 1980–2014. Drug Discov. Today 2017, 22, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Malaterre, V.; Ogorka, J.; Loggia, N.; Gurny, R. Oral osmotically driven systems: 30 years of development and clinical use. Eur. J. Pharm. Biopharm. 2009, 73, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Conte, U.; Maggi, L. Multi-layered hydrophilic matrices as constant release devices (geomatrixtm systems). J. Control. Release 1993, 26, 39–47. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, P. Gastroretentive drug delivery systems: A promising approach. Am. J. Pharm. Res. 2016, 6, 5225–5235. [Google Scholar]
- Patton, J.S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996, 19, 3–36. [Google Scholar] [CrossRef]
- Groneberg, D.; Witt, C.; Wagner, U.; Chung, K.F.; Fischer, A. Fundamentals of pulmonary drug delivery. Respir. Med. 2003, 97, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir. Care 2005, 50, 1313–1322. [Google Scholar]
- Newman, S.P. Principles of metered-dose inhaler design. Respir. Care 2005, 50, 1177–1190. [Google Scholar]
- Peguin, R.P.; da Rocha, S. Solvent–solute interactions in hydrofluoroalkane propellants. J. Phys. Chem. B 2008, 112, 8084–8094. [Google Scholar] [CrossRef]
- Anderson, P.J. History of aerosol therapy: Liquid nebulization to MDIs to DPIs. Respir. Care 2005, 50, 1139–1150. [Google Scholar]
- Brunaugh, A.D.; Smyth, H.D. Formulation techniques for high dose dry powders. Int. J. Pharm. 2018, 547, 489–498. [Google Scholar] [CrossRef] [PubMed]
- FDA Phase Out of Combivent Inhalation Aerosol. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm371901.htm (accessed on 31 December 2013).
- Agu, R.U.; Ugwoke, M.I.; Armand, M.; Kinget, R.; Verbeke, N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir. Res. 2001, 2, 198. [Google Scholar] [Green Version]
- Dunn, C.; Curran, M.P. Inhaled human insulin (exubera®). Drugs 2006, 66, 1013–1032. [Google Scholar] [CrossRef] [PubMed]
- Nuffer, W.; Trujillo, J.M.; Ellis, S.L. Technosphere insulin (afrezza) a new, inhaled prandial insulin. Ann. Pharmacother. 2015, 49, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.C., Jr.; Soter, N.A. The structure of normal skin and the morphology of atopic eczema. J. Investig. Dermatol. 1976, 67, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Gale, R. Skin adhesives and skin adhesion: 1. Transdermal drug delivery systems. Biomaterials 1998, 19, 1119–1136. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Chen, J.; Gao, C.; Zhang, Y.; Wang, T.; Qian, Y.; Yang, B.; Dong, P.; Zhang, Y. Inorganic nano-targeted drugs delivery system and its application of platinum-based anticancer drugs. J. Nanosci. Nanotechnol. 2017, 17, 1–17. [Google Scholar] [CrossRef]
- Adler-Moore, J.; Proffitt, R.T. Ambisome: Lipsomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 2002, 49, 21–30. [Google Scholar] [CrossRef]
- Hartrick, C.T.; Hartrick, K.A. Extended-release epidural morphine (Depodur™): Review and safety analysis. Expert Rev. Neurother. 2008, 8, 1641–1648. [Google Scholar] [CrossRef]
- Chahar, P.; Cummings, K.C., III. Liposomal bupivacaine: A review of a new bupivacaine formulation. J. Pain Res. 2012, 5, 257–264. [Google Scholar] [PubMed]
- Phuphanich, S.; Maria, B. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt®) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase iii study. J. Neuro-Oncol. 2007, 81, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. OncoTargets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.S.; Lux, S.E. Degradation of membrane phospholipids and thiols in peroxide hemolysis: Studies in vitamin E deficiency. Blood 1968, 32, 549–568. [Google Scholar] [PubMed]
- Daghistani, N.; Rey, J.A. Invega trinza: The first four-times-a-year, long-acting injectable antipsychotic agent. Pharm. Ther. 2016, 41, 222–227. [Google Scholar]
- Blackmer, J. Benralizumab for severe eosinophilic asthma. Clinical RX Forum. 2018, 6, 1–5. [Google Scholar]
- Sinha, V.; Trehan, A. Biodegradable microspheres for protein delivery. J. Control. Release 2003, 90, 261–280. [Google Scholar] [CrossRef]
- Parker, K.L.; Baens-Bailon, R.G. Depot leuprolide acetate dosage for sexual precocity. J. Clin. Endocrinol. Metab. 1991, 73, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Fløgstad, A.K.; Halse, J. Sandostatin lar in acromegalic patients: Long term treatment. J. Clin. Endocrinol. Metab. 1997, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bobo, W.V.; Shelton, R.C. Risperidone long-acting injectable (risperdal consta®) for maintenance treatment in patients with bipolar disorder. Expert Rev. Neurother. 2010, 10, 1637–1658. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Wilson, S. Diminished bacterial defences with intralipid. Lancet 1980, 316, 819–820. [Google Scholar] [CrossRef]
- Wolin, E.M.; Manon, A. Lanreotide depot: An antineoplastic treatment of carcinoid or neuroendocrine tumors. J. Gastrointest. Cancer 2016, 47, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Smith, S.C. (Eds.) Computational Pharmaceutics: Application of Molecular Modeling in Drug Delivery; John Wiley & Sons Ltd.: Chichester, West Sussex, UK, 2015. [Google Scholar]
- Yang, Y.; Ye, Z.; Su, Y.; Zhao, Q.; Li, X.; Ouyang, D. Deep learning for in vitro prediction of pharmaceutical formulations. Acta Pharm. Sin. B 2018. [Google Scholar] [CrossRef]
- Han, R.; Yang, Y.; Li, X.; Ouyang, D. Predicting oral disintegrating tablet formulations by neural network techniques. Asian J. Pharm. Sci. 2018, 13, 336–342. [Google Scholar] [CrossRef]
- Zhao, Q.; Miriyala, N.; Su, Y.; Chen, W.; Gao, X.; Shao, L.; Yan, R.; Li, H.; Yao, X.; Cao, D.; et al. Computer-aided formulation design for a highly soluble lutein–cyclodextrin multiple-component delivery system. Mol. Pharm. 2018, 15, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Grady, D. FDA Approves First Gene-Altering Leukemia Treatment, Costing $475,000. New York Times. Available online: https://www.nytimes.com/2017/08/30/health/gene-therapy-cancer.html?mcubz=3 (accessed on 30 August 2017).
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Belluck, P. First Digital Pill Approved to Worries about Biomedical ‘Big Brother’. New York Times. Available online: https://www.nytimes.com/2017/11/13/health/digital-pill-fda.html (accessed on 13 November 2017).




| Year | Drug Delivery System | |
|---|---|---|
| 1952 | The first sustained-release technology Spansule® | [10] |
| 1956 | The first pressurized metered dose inhaler (MDI) | |
| 1969 | The first dry powder inhalation (DPI) | [11] |
| 1979 | The first transdermal patch Transdermal Scop® | [21] |
| 1982 | The first recombinant human insulin Humulin R® | [22] |
| 1984 | The first Biodegradable microsphere Vivitrol® | [23] |
| 1986 | The first injection microsphere Decapeptyl® | [24] |
| 1989 | The first Push-Pull Osmotic Pump product Procardia XL® | [25] |
| 1995 | The first FDA-approved liposome Doxil® | [13] |
| 2005 | The first FDA-approved nanoparticle Abraxane® | [14] |
| 2006 | The first FDA-approved botanical medicine VeregenTM | [26] |
| 2015 | The first FDA-approved 3D print drug Spritam® | [27] |
| 2017 | The first FDA-approved gene therapy Kymriah® | [28] |
| 2017 | The first FDA-approved digital drug Abilify MyCite® | [29] |
| Route | Number (New Drugs) | Number (Generic Drugs) | Ratio | Ranking |
|---|---|---|---|---|
| Oral | 1119 | 5252 | 4.69 | 1 |
| Injection | 702 | 1609 | 2.29 | 2 |
| Cutaneous | 295 | 599 | 2.03 | 3 |
| Mucosal | 205 | 331 | 1.61 | 4 |
| Inhalation | 66 | 61 | 0.92 | 5 |
| Key Drug Delivery Technologies | Number of Global Publications | Number of Global Clinical Trials | Ratio of Clinical Trials to Publications (%) | Number of Clinical Trials in US | Number of Marketed Products in the US | Ratio of Products to Clinical Trials in the US (%) |
|---|---|---|---|---|---|---|
| Oral sustained release preparations | 7150 | 1798 | 25.15 | 859 | 205 | 24.55 |
| Transdermal patch | 4161 | 570 | 13.70 | 323 | 54 | 17.09 |
| Aerosol inhalation * | 3204 | 342 | 10.67 | 171 | 16 | 9.36 |
| Powder inhalation * | 5227 | 878 | 16.80 | 383 | 25 | 6.53 |
| Spray inhalation * | 2284 | 194 | 8.49 | 82 | 4 | 4.88 |
| Liposome injection # | 3885 | 342 | 8.80 | 232 | 8 | 3.45 |
| Emulsion injection # | 5033 | 269 | 5.34 | 119 | 5 | 4.20 |
| Microsphere injection # | 1329 | 92 | 6.92 | 45 | 11 | 7.32 |
| Suspension injection # | 3558 | 58 | 1.63 | 40 | 2 | 5.00 |
| Nanoparticle injection # | 5468 | 601 | 10.99 | 276 | 11 | 5.07 |
| Drug Name | Active Ingredient | Composition/Type | Company | Indication | Approval Date |
|---|---|---|---|---|---|
| Liposome | - | - | - | - | - |
| New drugs | - | - | - | - | - |
| Doxil® | Doxorubicin hydrochloride | HSPC, cholesterol and PEG | Janssen | Ovarian Cancer; Sarcoma; Myeloma | 1995 |
| Ambisome® | Amphotericin B | HSPC, DSPG, cholesterol and amphotericin B | Astellas | Fungal infection | 1997 |
| Depocyt® | Cytarabine | Cholesterol, Triolein, DOPC and DPPG | Pacira | Lymphomatous | 1999 |
| Exparel® | Bupivacaine | DOPC and DOPE | Pacira | Local anesthetic | 2011 |
| Marqibo kit® | Vincrinstine Sulfate | Cholesterol and eggs sphingomyelin | Talon | Acute lymphoblastic leukemia | 2012 |
| Onivyde® | Irinotecan hydrochlorine | DSPC, MPEG-2000-DSPE | Ipsen | Adenocarcinoma of the pancreas | 2015 |
| Generic drugs | - | - | - | - | - |
| Doxorubicin hydrochloride | Doxorubicin hydrochloride | DSPC and cholesterol | Sun pharma | Ovarian cancer; sarcoma | 2013 |
| Doxorubicin hydrochloride | Doxorubicin hydrochloride | DSPC and cholesterol | Dr Reddys | Ovarian cancer; sarcoma | 2017 |
| Microsphere | - | - | - | - | - |
| Lupron Depot® | Leuprolide Acetate | PLGA | Abbvie | Advanced prostatic cancer | 1989 |
| Sandostatin Lar® | Octreotide acetate | PLGA | Novartis | Acromegaly | 1998 |
| Trelstar® | Triptorelin pamoate | PLGA | Allergen | Advanced prostate cancer | 2000 |
| Definity® | Perflutren | DPPA, DPPC and MPEG-5000-DPPE | Lantheus | Ultrasound contrast agent | 2001 |
| Risperdal Consta® | Risperidone | PLG | Janssen | Schizophrenia; Bipolar I Disorder | 2003 |
| Vivitrol® | Naltrexone | PLG | Alkermes | Alcohol dependence | 2006 |
| Bydureon® | Exenatide synthetic | PLGA | Astrazeneca AB | Type 2 diabetes | 2012 |
| Signifor Lar® | Pasireotide pamoate | PLGA | Novartis | Acromegaly | 2014 |
| Lumason® | Sulfur hexafluoride lipid-type microspheres | DSPC and DPPG-Na | Bracco | Ultrasound contrast agent | 2014 |
| Bydureon Bcise® | Exenatide | PLGA | Astrazeneca AB | Type 2 diabetes | 2017 |
| Triptodur Kit® | Triptorelin pamoate | PLGA | Arbor | Central precocious puberty | 2017 |
| Suspension and nanoparticle | - | - | - | - | |
| Atridox® | Doxycycline hyclate | PLA | Tolmar | Chronic adult periodontitis | 1998 |
| Eligard® | Leuprolide acetate | PLGA(Atrigel®) | Tolmar | Advanced prostate cancer | 2002 |
| Abraxane® | Paclitaxel | Protein nanoparticle | Abraxis | Metastatic Breast Cancer; Non-Small Cell Lung Cancer | 2005 |
| Somatuline Depot® | Lanreotide acetate | Nanotube [74] | Ipsen | Acromegaly | 2007 |
| Zyprexa Relprevv® | Olanzapine pamoate | Microcrystal | Eli lilly | Schizophrenia | 2009 |
| Invega Sustenna® | Paliperidone palmitate | Nanocrystal | Janssen | Schizophrenia | 2009 |
| Feraheme® | Ferumoxytol | carbohydrate-coated iron-oxide nanoparticle | Amag | Iron deficiency anemia | 2009 |
| Sustol® | Granisetron | Ortho ester (Biochronomer™) | Heron | Nausea and vomiting | 2012 |
| Abilify Maintena® | Aripiprazole | Nanocrystal | Otsuka | Schizophrenia | 2013 |
| Ryanodex® | Dantrolene sodium | Nanocrystal | Eagle | Malignant hyperthermia | 2014 |
| Invega Trinza® | Paliperidone palmitate | Nanocrystal | Janssen | Schizophrenia | 2015 |
| Aristada® | Aripiprazole Lauroxil | Nanocrystal | Alkermes | Schizophrenia | 2015 |
| Sublocade® | Buprenorphine | PLGA | Indivior | Moderate to severe opioid use disorder | 2017 |
| Emulsion | - | - | - | - | - |
| Intralipid® | Soybean Oil | Fat Emulsion | Fresenius | Parenteral nutrition | 1975 |
| Cleviprex® | Clevidipine | Lipid emulsion | Chiesi | Reduction of blood pressure | 2008 |
| Perikabiven® | Amino acids | Lipid emulsion | Fresenius | Parenteral nutrition | 2014 |
| Smoflipid® | Fish oil | Lipid emulsion | Fresenius | Parenteral nutrition | 2016 |
| Cinvanti® | Aprepitant | Lipid emulsion | Heron | Acute and delayed nausea and vomiting | 2017 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. https://doi.org/10.3390/pharmaceutics10040263
Zhong H, Chan G, Hu Y, Hu H, Ouyang D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics. 2018; 10(4):263. https://doi.org/10.3390/pharmaceutics10040263
Chicago/Turabian StyleZhong, Hao, Ging Chan, Yuanjia Hu, Hao Hu, and Defang Ouyang. 2018. "A Comprehensive Map of FDA-Approved Pharmaceutical Products" Pharmaceutics 10, no. 4: 263. https://doi.org/10.3390/pharmaceutics10040263
APA StyleZhong, H., Chan, G., Hu, Y., Hu, H., & Ouyang, D. (2018). A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics, 10(4), 263. https://doi.org/10.3390/pharmaceutics10040263






