Blood-Brain Delivery Methods Using Nanotechnology
Abstract
:1. Introduction
2. The Blood-Brain Barrier
2.1. The Anatomical Structure of the Blood-Brain Barrier
2.2. The Physiology of the Blood-Brain Barrier
3. Nanotechnology Approaches for Crossing the Blood-Brain Barrier
3.1. Organic Nanomaterials
3.1.1. Polymeric Nanoparticles
3.1.2. Liposomes
3.1.3. Dendrimers
3.1.4. Micelles
3.2. Inorganic Nanomaterials
3.2.1. Gold Nanoparticles
3.2.2. Silica Nanoparticles
3.2.3. Carbon Nanotubes
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Barnabas:, W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Methods 2018, 311, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Nutt, D.; Oertel, W.; Boyer, P.; Jaarsma, J.; Destrebecq, F.; Esposito, G.; Quoidbach, V. Towards earlier diagnosis and treatment of disorders of the brain. Bull. World Health Organ. 2018, 96, 298A. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Liu, E.; Huang, Y. 16—The intra-brain distribution of brain targeting delivery systems. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 409–438. [Google Scholar]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-alzheimer drugs. J. Control. Release Off. J. Control. Release Soc. 2018, 281, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Shatzmiller, S.; Lapidot, I.; Zats, G. Blood brain barrier crossing for therapeutic and diagnostic agents. SM J. Neurol. Disord. Stroke 2016, 2, 1012. [Google Scholar]
- Alexander, J.J. Blood-brain barrier (bbb) and the complement landscape. Mol. Immunol. 2018, 102, 26–31. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the blood-brain barrier: Recent advances in drug delivery to the brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Çetin, M.; Aytekin, E.; Yavuz, B.; Bozdağ-Pehlivan, S. Chapter 7—Nanoscience in targeted brain drug delivery. In Nanotechnology Methods for Neurological Diseases and Brain Tumors; Gürsoy-Özdemir, Y., Bozdağ-Pehlivan, S., Sekerdag, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 117–147. [Google Scholar]
- Ayodele, A.T.; Valizadeh, A.; Adabi, M.; Esnaashari, S.S.; Madani, F.; Khosravani, M.; Adabi, M. Ultrasound nanobubbles and their applications as theranostic agents in cancer therapy: A review. Biointerface Res. Appl. Chem. 2017, 7, 2253–2262. [Google Scholar]
- Faisal, N.; Kumar, K. Polymer and metal nanocomposites in biomedical applications. Biointerface Res. Appl. Chem. 2017, 7, 2286–2294. [Google Scholar]
- Husain, Q. Nanosupport bound lipases their stability and applications. Biointerface Res. Appl. Chem. 2017, 7, 2194–2216. [Google Scholar]
- Kaur, M.; Singh, G.; Khanna, K.; Kaur, N. Nanotechnology: A review. In Proceedings of the Second National Conference on Advances in Manufacturing Systems, S B S State Technical Campus, Ferozepur, India, 23–24 December 2015. [Google Scholar]
- Abou el Ela, A.E.S.F.; El Khatib, M.M.; Salem-Bekhit, M.M. Design, characterization and microbiological evaluation of microemulsion based gel of griseofulvin for topical delivery system. Biointerface Res. Appl. Chem. 2017, 7, 2277–2285. [Google Scholar]
- Davoodi, S.D.; Saghavaz, B.H. Optimal synthesis and characterization of magnetic CuMnFe2O4 nanoparticles coated by peg for drug delivery. Biointerface Res. Appl. Chem. 2017, 7, 2249–2252. [Google Scholar]
- Pignatello, R.; Fuochi, V.; Petronio, G.P.; Greco, A.S.; Furneri, P.M. Formulation and characterization of erythromycin–loaded solid lipid nanoparticles. Biointerface Res. Appl. Chem. 2017, 7, 2145–2150. [Google Scholar]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Abbruscato, T. Chapter 6—The blood-brain. In Conn’s Translational Neuroscience; Conn, P.M., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 141–146. [Google Scholar]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. Chapter 3—The blood-brain barrier. In Handbook of Clinical Neurology; Pittock, S.J., Vincent, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, pp. 39–59. [Google Scholar]
- Khanna, A.K.; Farag, E. Chapter 3—Blood-brain barrier. In Essentials of Neuroanesthesia; Prabhakar, H., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 51–58. [Google Scholar]
- Quintana, F.J. Astrocytes to the rescue! Glia limitans astrocytic endfeet control cns inflammation. J. Clin. Investig. 2017, 127, 2897–2899. [Google Scholar] [CrossRef]
- Engelhard, H.H.; Arnone, G.D.; Mehta, A.I.; Nicholas, M.K. Chapter 8—Biology of the blood-brain and blood-brain tumor barriers. In Handbook of Brain Tumor Chemotherapy, Molecular Therapeutics, and Immunotherapy, 2nd ed.; Newton, H.B., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 113–125. [Google Scholar]
- Gürsoy-Özdemir, Y.; Tas, Y.C. Chapter 1—Anatomy and physiology of the blood-brain barrier. In Nanotechnology Methods for Neurological Diseases and Brain Tumors; Gürsoy-Özdemir, Y., Bozdağ-Pehlivan, S., Sekerdag, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 3–13. [Google Scholar]
- Gupta, S.; Dhanda, S.; Sandhir, R. 2—Anatomy and physiology of blood-brain barrier. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 7–31. [Google Scholar]
- Lindauer, U. Chapter 1—Physiology of cerebral blood vessels. In Brain Edema; Badaut, J., Plesnila, N., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 3–27. [Google Scholar]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Attwell, D.; Hall, C.; O’Farrell, F.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar]
- Chen, Z.-Y.; Gu, X.-D.; Zang, Y.-W. Chapter 16—Brain metastasis of colorectal cancer: Microenvironment and molecular mechanism. In Brain Metastases from Primary Tumors; Hayat, M.A., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 191–202. [Google Scholar]
- Tajes, M.; Ramos-Fernandez, E.; Weng-Jiang, X.; Bosch-Morato, M.; Guivernau, B.; Eraso-Pichot, A.; Salvador, B.; Fernandez-Busquets, X.; Roquer, J.; Munoz, F.J. The blood-brain barrier: Structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014, 31, 152–167. [Google Scholar] [CrossRef] [Green Version]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts BI 2016, 6, 225–248. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Shao, K.; Umeshappa, C.S. 3—Recent progress in blood-brain barrier transportation research. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 33–51. [Google Scholar]
- Erickson, M.A.; Banks, W.A. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: Bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards improvements for penetrating the blood-brain barrier—Recent progress from a material and pharmaceutical perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H. Chapter 10—Blood-brain barrier. In Drug-Like Properties, 2nd ed.; Di, L., Kerns, E.H., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 141–159. [Google Scholar]
- O’Keeffe, E.; Campbell, M. Modulating the paracellular pathway at the blood-brain barrier: Current and future approaches for drug delivery to the cns. Drug Discov. Today Technol. 2016, 20, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.U.; Chen, X. Penetrating the blood-brain barrier: Promise of novel nanoplatforms and delivery vehicles. ACS Nano 2015, 9, 9470–9474. [Google Scholar] [CrossRef]
- Takechi, R.; Fakhoury, M.; Al-Salami, H. Drug Permeation across the Blood-Brain Barrier: Applications of Nanotechnology. Br. J. Med. Med. Res. 2015, 6, 547–556. [Google Scholar]
- Kumar, M.; Sharma, P.; Maheshwari, R.; Tekade, M.; Shrivastava, S.K.; Tekade, R.K. Chapter 15—Beyond the blood-brain barrier: Facing new challenges and prospects of nanotechnology-mediated targeted delivery to the brain. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Kesharwani, P., Gupta, U., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 397–437. [Google Scholar]
- Zhang, T.T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel curcumin loaded nanoparticles engineered for blood-brain barrier crossing and able to disrupt abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V.; et al. Delivery of doxorubicin-loaded plga nanoparticles into u87 human glioblastoma cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Patra, M.; Panigrahi, A.K.; Khuda-Bukhsh, A.R. Boldine-loaded plga nanoparticles have improved efficiency of drug carriage and protective potential against cisplatin-induced toxicity. J. Ayurveda Integr. Med. 2018, in press. [Google Scholar] [CrossRef]
- Englert, C.; Trützschler, A.-K.; Raasch, M.; Bus, T.; Borchers, P.; Mosig, A.S.; Traeger, A.; Schubert, U.S. Crossing the blood-brain barrier: Glutathione-conjugated poly(ethylene imine) for gene delivery. J. Control. Release 2016, 241, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tian, H.; Xing, Z.; Zhang, D.; Guo, Y.; Guo, Z.; Zhu, X.; Chen, X. A non-viral suicide gene delivery system traversing the blood brain barrier for non-invasive glioma targeting treatment. J. Control. Release 2016, 243, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.; Csapó, E.; Majláth, Z.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I.; Knapp, L.; Toldi, J.; Vécsei, L.; Dékány, I. Targeting of the kynurenic acid across the blood-brain barrier by core-shell nanoparticles. Eur. J. Pharm. Sci. 2016, 86, 67–74. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cai, P.; Li, J.; Zhang, T.; Lin, L.; Abbasi, A.Z.; Henderson, J.T.; Rauth, A.M.; Wu, X.Y. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J. Control. Release 2017, 246, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Wang, C.-H. Convection enhanced delivery of liposome encapsulated doxorubicin for brain tumour therapy. J. Control. Release 2018, 285, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Chen, I.Y.; Rajesh, R. Use of functionalized liposomes loaded with antioxidants to permeate the blood-brain barrier and inhibit β-amyloid-induced neurodegeneration in the brain. J. Taiwan Inst. Chem. Eng. 2018, 87, 1–14. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, B.; Oue, H.; Banerjee, A.; Kanekiyo, T.; Singh, J. Dual functionalized liposome-mediated gene delivery across triple co-culture blood brain barrier model and specific in vivo neuronal transfection. J. Control. Release 2018, 286, 264–278. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.D.V.; Prieto, M.J. Pamam dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.D.; Xavier, M.; Leite, D.M.; Moreira, D.A.; Custódio, B.; Torrado, M.; Castro, R.; Leiro, V.; Rodrigues, J.; Tomás, H.; et al. Pamam dendrimers: Blood-brain barrier transport and neuronal uptake after focal brain ischemia. J. Control. Release 2018, 291, 65–79. [Google Scholar] [CrossRef]
- Desai, P.P.; Patravale, V.B. Curcumin cocrystal micelles—Multifunctional nanocomposites for management of neurodegenerative ailments. J. Pharm. Sci. 2018, 107, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Garello, F.; Pagoto, A.; Arena, F.; Buffo, A.; Blasi, F.; Alberti, D.; Terreno, E. MRI visualization of neuroinflammation using VCAM-1 targeted paramagnetic micelles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Wang, Z.; Kokuryo, D.; Aoki, I.; Yokoyama, M. A polymeric micelle magnetic resonance imaging (MRI) contrast agent reveals blood-brain barrier (BBB) permeability for macromolecules in cerebral ischemia-reperfusion injury. J. Control. Release 2017, 253, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Zaruba, K.; Oravec, M.; Kunes, M.; Babula, P.; Ulbrich, P.; Brezaniova, I.; Opatrilova, R.; Triska, J.; Suchy, P. Preparation of silica nanoparticles loaded with nootropics and their in vivo permeation through blood-brain barrier. BioMed Res. Int. 2015, 2015, 812673. [Google Scholar] [CrossRef] [PubMed]
- Kafa, H.; Wang, J.T.-W.; Rubio, N.; Klippstein, R.; Costa, P.M.; Hassan, H.A.F.M.; Sosabowski, J.K.; Bansal, S.S.; Preston, J.E.; Abbott, N.J.; et al. Translocation of lrp1 targeted carbon nanotubes of different diameters across the blood-brain barrier in vitro and in vivo. J. Control. Release 2016, 225, 217–229. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Qian, M.; Jiang, H.; Shi, W.; Chen, J.; Lächelt, U.; Wagner, E.; Lu, W.; Wang, Y.; et al. Augmented glioma-targeted theranostics using multifunctional polymer-coated carbon nanodots. Biomaterials 2017, 141, 29–39. [Google Scholar] [CrossRef]
- Guccione, C.; Oufir, M.; Piazzini, V.; Eigenmann, D.E.; Jähne, E.A.; Zabela, V.; Faleschini, M.T.; Bergonzi, M.C.; Smiesko, M.; Hamburger, M.; et al. Andrographolide-loaded nanoparticles for brain delivery: Formulation, characterisation and in vitro permeability using hCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2017, 119, 253–263. [Google Scholar] [CrossRef]
- Fernandes, J.; Ghate, M.V.; Basu Mallik, S.; Lewis, S.A. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm. 2018, 547, 563–571. [Google Scholar] [CrossRef]
- Abbina, S.; Parambath, A. 14—Pegylation and its alternatives: A summary. In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 363–376. [Google Scholar]
- Hu, Y.; Rip, J.; Gaillard, P.J.; de Lange, E.C.M.; Hammarlund-Udenaes, M. The impact of liposomal formulations on the release and brain delivery of methotrexate: An in vivo microdialysis study. J. Pharm. Sci. 2017, 106, 2606–2613. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Singh, J. Dual functionalized 5-fluorouracil liposomes as highly efficient nanomedicine for glioblastoma treatment as assessed in an in vitro brain tumor model. J. Pharm. Sci. 2018, 107, 2902–2913. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Chen, Y.; Yang, Z.; Zhang, L.; Xiao, W.; Yang, J.; Guo, L.; Wu, Y. Dual-targeting for brain-specific liposomes drug delivery system: Synthesis and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 4677–4686. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-L.; Huang, M.; Wang, X.-R.; Fu, J.; Han, M.; Shen, Y.-Q.; Xia, Z.; Gao, J.-Q. Transferrin-modified liposome promotes α-mangostin to penetrate the blood-brain barrier. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.M.D.C.; Henninger, N.; Bouley, J.; Alterman, J.F.; Haraszti, R.A.; Gilbert, J.W.; Sapp, E.; Coles, A.H.; Biscans, A.; Nikan, M.; et al. Transvascular delivery of hydrophobically modified sirnas: Gene silencing in the rat brain upon disruption of the blood-brain barrier. Mol. Ther. 2018, 26, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Priya, L.B.; Baskaran, R.; Padma, V.V. Chapter 21—Phytonanoconjugates in oral medicine. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 639–668. [Google Scholar]
- Jiang, Y.; Lv, L.; Shi, H.; Hua, Y.; Lv, W.; Wang, X.; Xin, H.; Xu, Q. Pegylated polyamidoamine dendrimer conjugated with tumor homing peptide as a potential targeted delivery system for glioma. Colloids Surf. B Biointerfaces 2016, 147, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Gajbhiye, V.; Kesharwani, P.; Jain, N.K. Ligand anchored poly(propyleneimine) dendrimers for brain targeting: Comparative in vitro and in vivo assessment. J. Colloid Interface Sci. 2016, 482, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Trent Magruder, J.; Lin, Y.-A.; Crawford, T.C.; Grimm, J.C.; Sciortino, C.M.; Wilson, M.A.; Blue, M.E.; Kannan, S.; Johnston, M.V.; et al. Generation-6 hydroxyl pamam dendrimers improve cns penetration from intravenous administration in a large animal brain injury model. J. Control. Release 2017, 249, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.; Zhang, F.; Mishra, M.K.; Zhang, Z.; Kambhampati, S.P.; Kannan, R.M.; Kannan, S. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials 2016, 101, 96–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellepeddi, V.K.; Mohammadpour, R.; Kambhampati, S.P.; Sayre, C.; Mishra, M.K.; Kannan, R.M.; Ghandehari, H. Pediatric oral formulation of dendrimer-N-acetyl-l-cysteine conjugates for the treatment of neuroinflammation. Int. J. Pharm. 2018, 545, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bhattacharjee, J.; Barick, K.C.; Verma, G.; Hassan, P.A.; Yakhmi, J.V. Chapter 7—Interfacial engineering of nanoparticles for cancer therapeutics. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–209. [Google Scholar]
- Joseph, M.; Trinh, H.M.; Mitra, A.K. Chapter 7—Peptide and protein-based therapeutic agents. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 145–167. [Google Scholar]
- Sezgin-bayindir, Z.; Ergin, A.D.; Parmaksiz, M.; Elcin, A.E.; Elcin, Y.M.; Yuksel, N. Evaluation of various block copolymers for micelle formation and brain drug delivery: In vitro characterization and cellular uptake studies. J. Drug Deliv. Sci. Technol. 2016, 36, 120–129. [Google Scholar] [CrossRef]
- Sydow, K.; Nikolenko, H.; Lorenz, D.; Müller, R.H.; Dathe, M. Lipopeptide-based micellar and liposomal carriers: Influence of surface charge and particle size on cellular uptake into blood brain barrier cells. Eur. J. Pharm. Biopharm. 2016, 109, 130–139. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Xu, Y.; Chen, Z.; Zhang, J.; Ping, Q.; Xiao, Y. Tween 80-modified hyaluronic acid-ss-curcumin micelles for targeting glioma: Synthesis, characterization and their in vitro evaluation. Int. J. Biol. Macromol. 2018, 120, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Hüwel, S.; Kogan, M.J.; Simon, U.; Galla, H.-J. The effects of gold nanoparticles functionalized with ß-amyloid specific peptides on an in vitro model of blood-brain barrier. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carter, D.A.; Ong, Z.Y.; McGilvery, C.M.; Dunlop, I.E.; Dexter, D.T.; Porter, A.E. L-dopa functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood-brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of nanoparticles through the blood-brain barrier for imaging and therapeutic applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.A.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L.; et al. Blood-brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-J.; Lee, E.-S.; Kang, M.; Jeong, Y.; Park, J.-H. In vivo multi-photon luminescence imaging of cerebral vasculature and blood-brain barrier integrity using gold nanoparticles. J. Mater. Chem. B 2015, 3, 2935–2938. [Google Scholar] [CrossRef]
- Tamba, B.I.; Streinu, V.; Foltea, G.; Neagu, A.N.; Dodi, G.; Zlei, M.; Tijani, A.; Stefanescu, C. Tailored surface silica nanoparticles for blood-brain barrier penetration: Preparation and in vivo investigation. Arab. J. Chem. 2018, 11, 981–990. [Google Scholar] [CrossRef]
- Song, Y.; Du, D.; Li, L.; Xu, J.; Dutta, P.; Lin, Y. In vitro study of receptor-mediated silica nanoparticles delivery across blood-brain barrier. ACS Appl. Mater. Interfaces 2017, 9, 20410–20416. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; de Lange Davies, C.; Sahlgren, C.; et al. Feasibility study of the permeability and uptake of mesoporous silica nanoparticles across the blood-brain barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. Ros responsive resveratrol delivery from ldlr peptide conjugated pla-coated mesoporous silica nanoparticles across the blood-brain barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Mehta, M. Chapter 19—Nanodiagnostics in microbiology and dentistry. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 391–419. [Google Scholar]
- Ahmed, W.; Elhissi, A.; Dhanak, V.; Subramani, K. Chapter 18—Carbon nanotubes: Applications in cancer therapy and drug delivery research. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 371–389. [Google Scholar]
- Lohan, S.; Raza, K.; Mehta, S.K.; Bhatti, G.K.; Saini, S.; Singh, B. Anti-Alzheimer’s potential of berberine using surface decorated multi-walled carbon nanotubes: A preclinical evidence. Int. J. Pharm. 2017, 530, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Kafa, H.; Wang, J.T.; Rubio, N.; Venner, K.; Anderson, G.; Pach, E.; Ballesteros, B.; Preston, J.E.; Abbott, N.J.; Al-Jamal, K.T. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials 2015, 53, 437–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
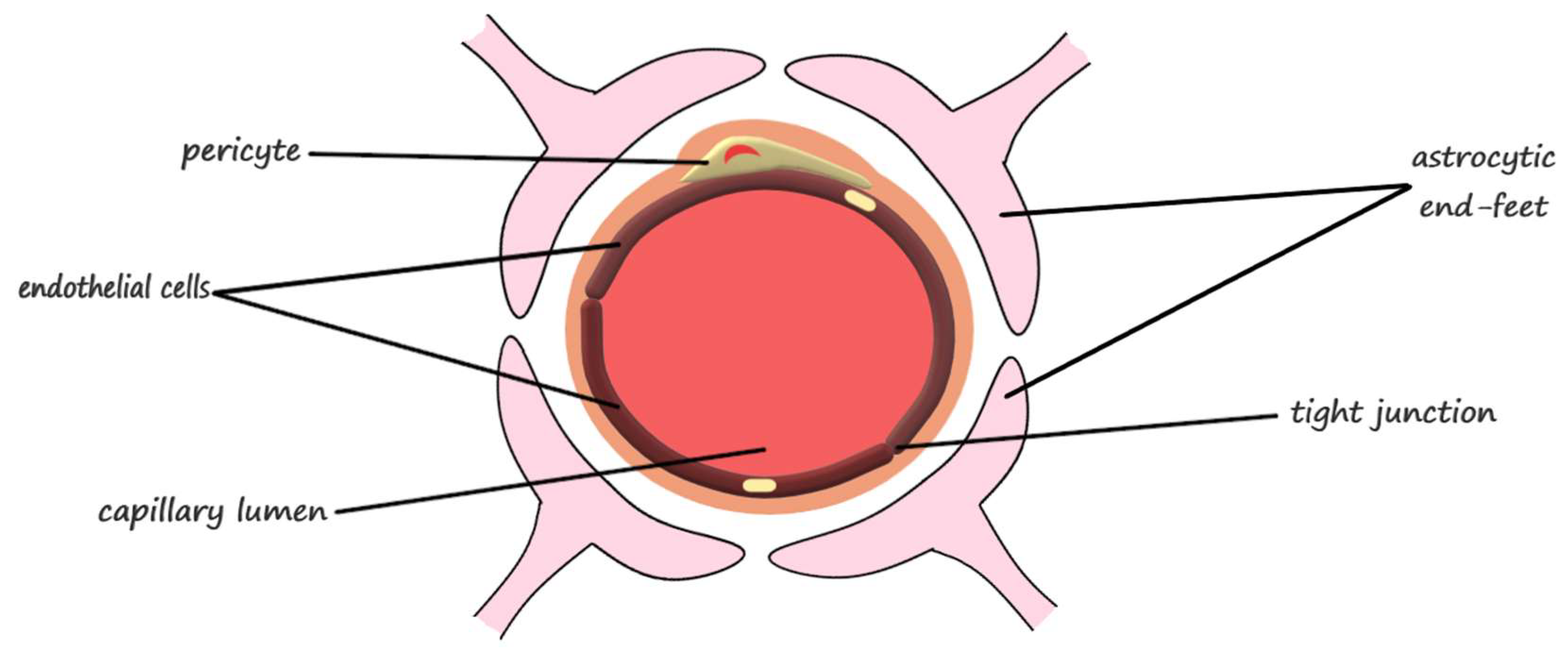
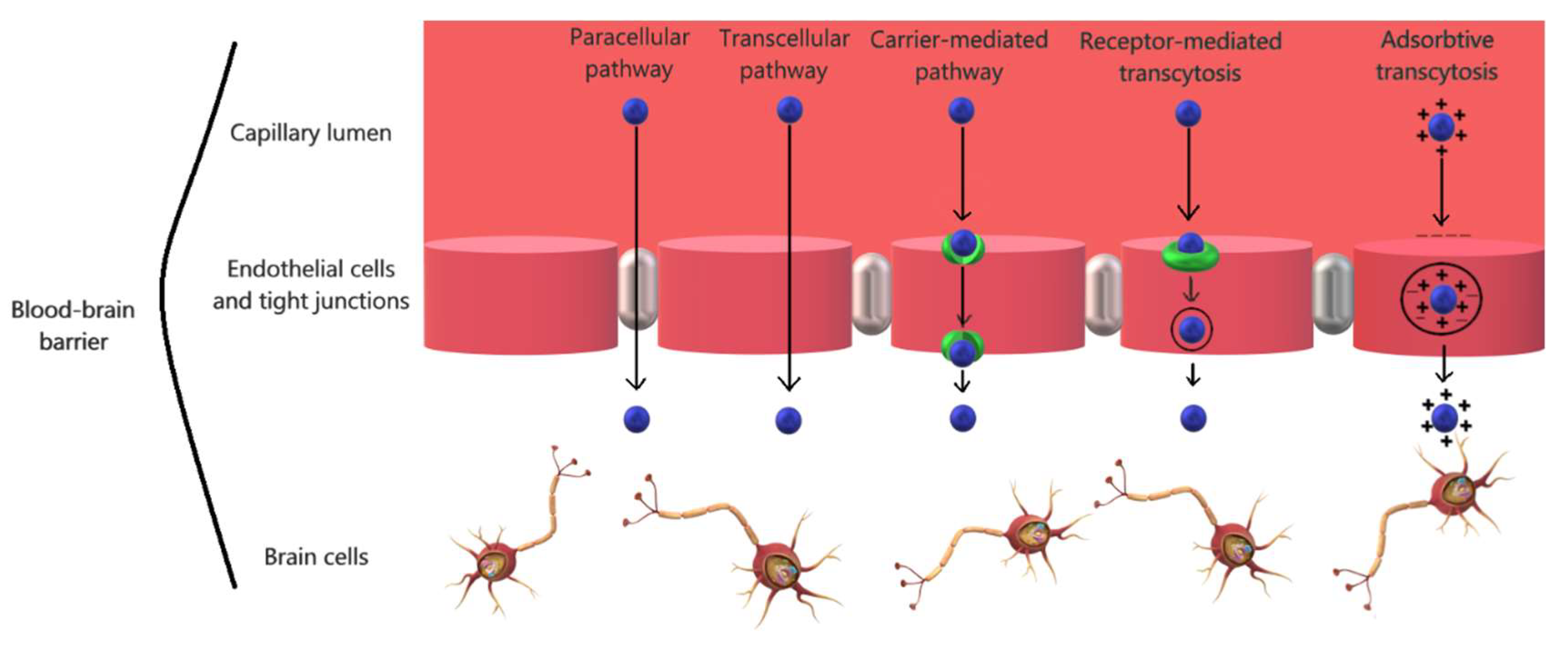
| Blood-Brain Barrier Component | Function | |
|---|---|---|
| Neurovascular unit | endothelial cells | barrier function, transport of micronutrients and macronutrients, receptor-mediated signaling, leukocyte trafficking, and osmoregulation [31] |
| astrocytes | responsible for proper neuron and neurovascular unit functions and modulation of the blood-brain barrier phenotype [31] regulation of metabolism, the modulation of neuronal transmission, and brain development and repair [22] | |
| pericytes | regulation of endothelial cell proliferation, survival, migration, differentiation, and vascular branching [31] involved in angiogenesis, maintenance of the blood-brain barrier, regulation of immune cell entry to the brain, control of the cerebral blood flow, and constriction of capillaries in stroke [28] | |
| neurons | modulation of the blood-brain barrier permeability through neuronal-microvascular communications [31] | |
| extracellular matrix | modulation of the blood-brain barrier permeability and maintenance of tight junctions [31] | |
| Junctional complexes | occludin | ensures a high electrical resistance (tightness) of the tight junctions [31] |
| claudins | primary barrier function of the tight junctions [31] | |
| junctional adhesion molecules | mediation of the early attachment of adjacent cell membranes, involved in developmental processes [31] | |
| membrane-associated guanylate kinase-like proteins | modulation of the blood-brain barrier permeability [31] |
| Nano-Carrier | Diameter (nm) | Surface Charge (mV) | Cellular Uptake (%) | ||
|---|---|---|---|---|---|
| Organic nanomaterials | Polymeric nanoparticles | poly(lactide-co-glycolic) acid | 200–250 | (−22)–(−13) | 75 [41] |
| 120 114 143 | −11.6 −14.9 −30.8 | n.r. 90 90 [42] | |||
| 115 | −17.4 | 17.46 [43,44] | |||
| poly(ethylene imine) | 104–160 277–287 116–118 | 28.4 −6.9 33.2 | ~100 <10 ~100 [43,44] | ||
| poly(ethylene imine)-poly(l-lysine) copolymer | 136 | 30 | n.r. [45] | ||
| poly(allylamine) hydrochloride | 106.5–113.5 | n.r. | n.r. [46] | ||
| human serum albumin | 221.9–228.3 | −12.3 | n.r. [47] | ||
| polyethylcyanoacrylate | 218–241.1 | (−3.85)–(−2.78) | n.r. [47] | ||
| chitosan | 300–324 | 0.584 | n.r. [48] | ||
| Liposomes | 173.45–182.79 | 0.56−3.68 | 60–70 [49] | ||
| 105–110 | (−5)–(−2) | 70–90 [50] | |||
| 158.7–165.05 | 7.66 | 65–70 [51] | |||
| 189.21–203.39 | −22.23 | n.r. [52] | |||
| Dendrimers | polyamidoamine | 5.1–8.2 | 2.07–3.15 | n.r. [53] | |
| poly(propyleneimine) | 37.8–47.6 | 18.2 | n.r. [54] | ||
| Micelles | 11.7–24.9 | −30–20 | n.r. [55] | ||
| 74.2 | −30.25 | n.r. [56] | |||
| 28.79 | −6.46 | n.r. [57] | |||
| Inorganic nanomaterials | Gold nanoparticles | 1.4–60.2 | −64–56 | n.r. [50] | |
| Silica nanoparticles | 120–128 | n.r. | n.r. [58] | ||
| Carbon nanotubes | 125–296 | n.r. | n.r. [59,60] | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. https://doi.org/10.3390/pharmaceutics10040269
Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics. 2018; 10(4):269. https://doi.org/10.3390/pharmaceutics10040269
Chicago/Turabian StyleTeleanu, Daniel Mihai, Cristina Chircov, Alexandru Mihai Grumezescu, Adrian Volceanov, and Raluca Ioana Teleanu. 2018. "Blood-Brain Delivery Methods Using Nanotechnology" Pharmaceutics 10, no. 4: 269. https://doi.org/10.3390/pharmaceutics10040269
APA StyleTeleanu, D. M., Chircov, C., Grumezescu, A. M., Volceanov, A., & Teleanu, R. I. (2018). Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics, 10(4), 269. https://doi.org/10.3390/pharmaceutics10040269








