A Systematic Review and Critical Analysis of the Role of Graphene-Based Nanomaterials in Cancer Theranostics
Abstract
:1. Introduction
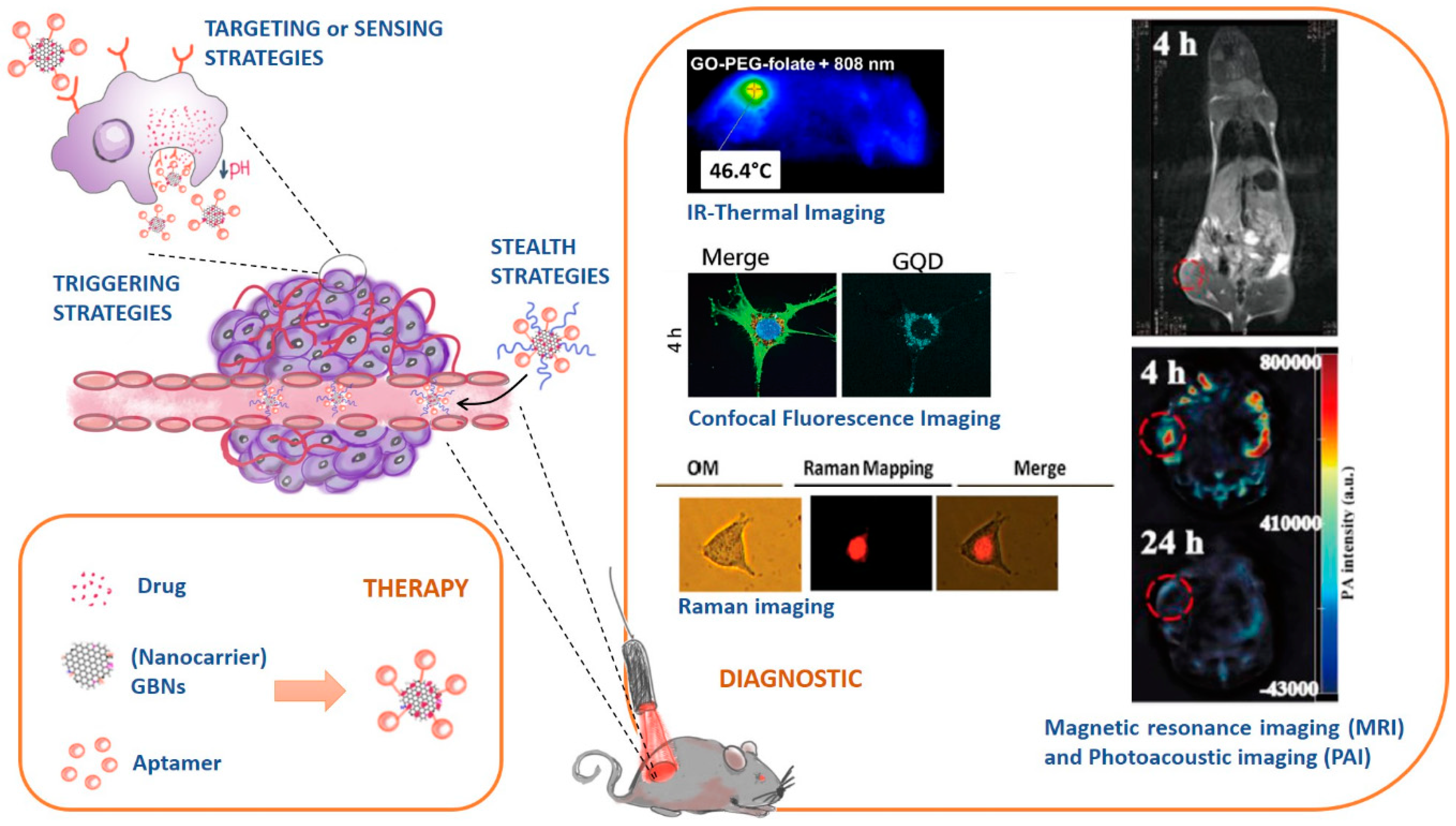
- Stealth strategies: nanocarriers can be coated with polymers (e.g. Polyethylene glycol, PEG) to keep them invisible to the immunity system and increase their circulation time;
- Targeting or sensing strategies: nanocarriers can be functionalized with ligands that are recognized by receptors overexpressed in cancer cell tissues;
- Triggering strategies: nanocarriers’ composition can be sensible to stimulus (e.g. pH, temperature changes) and releasing their cargo accordingly.
2. Methods
2.1. Eligibility Criteria
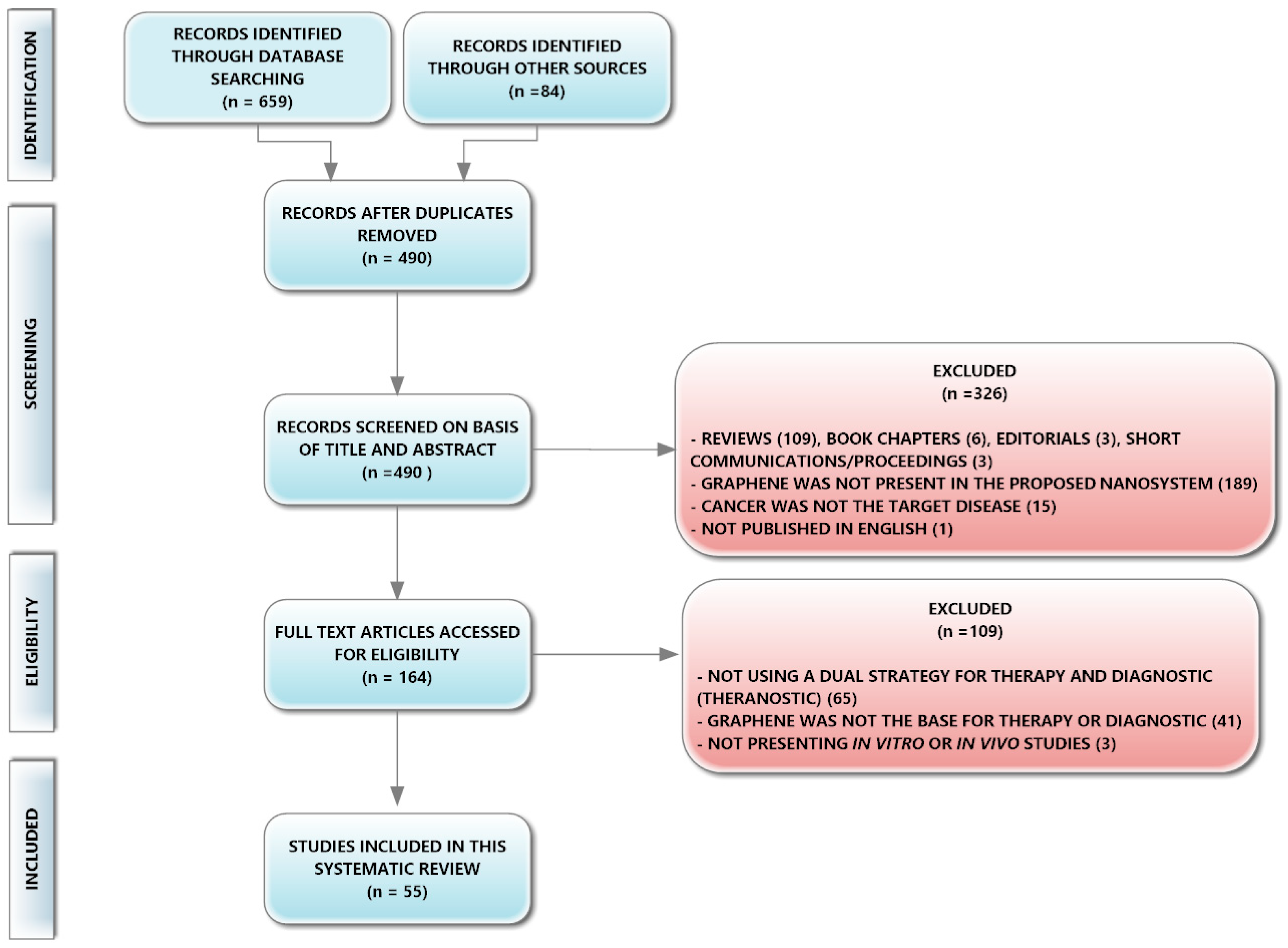
2.2. Information Sources, Search Strategy, Study Selection, and Data Collection Process
2.3. Data Items and Quality Assessment
3. Results
4. Discussion
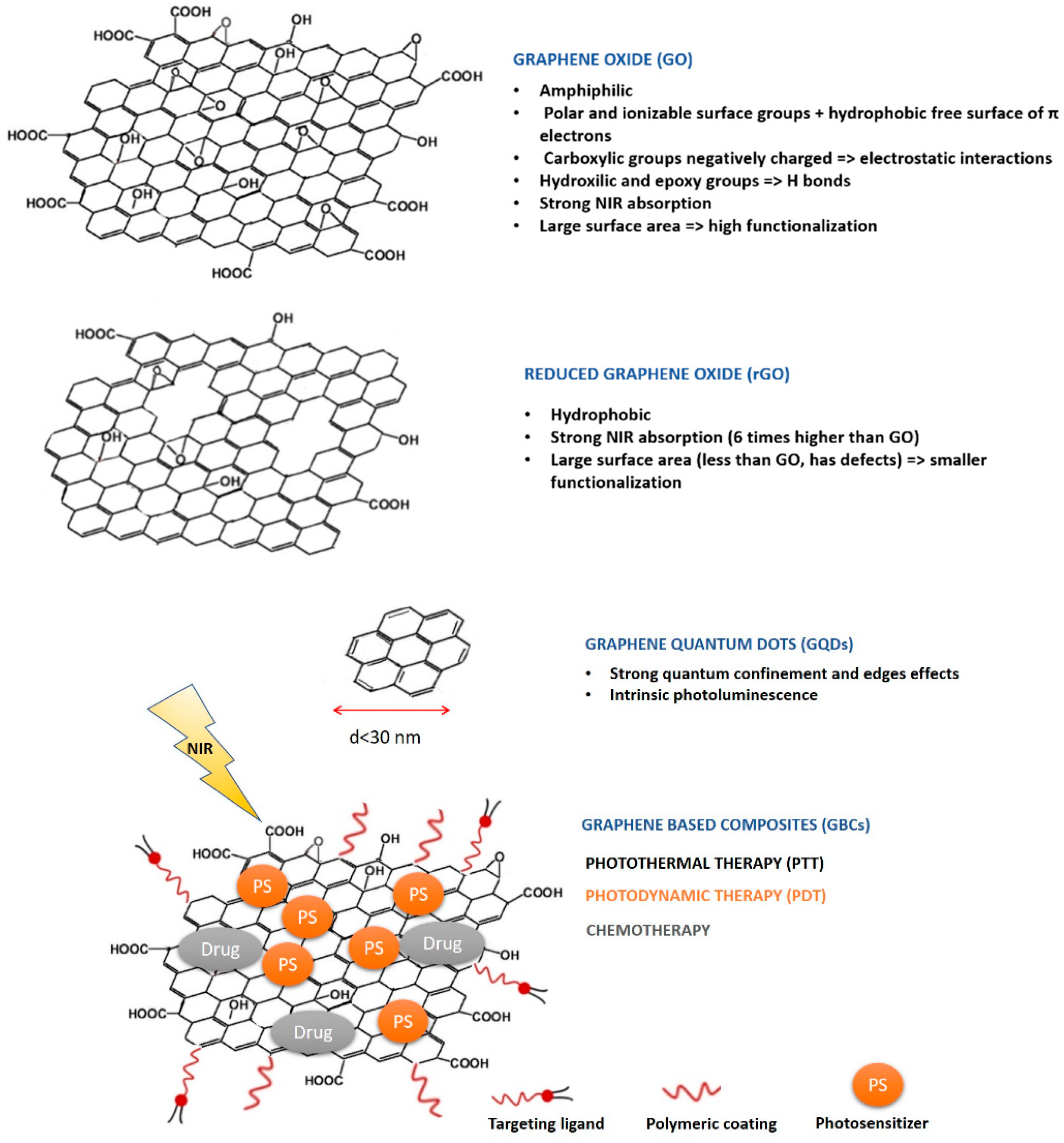
4.1. Role of Graphene-Based Nanomaterials in Therapy
4.1.1. Overview of the Different Therapeutic Strategies
4.1.2. Critical Comparison of Therapeutic Outcomes
4.2. Role of Graphene-Based Nanomaterials in Diagnostic
4.3. Role of Graphene-Based Nanomaterials in Toxicity
4.4. Pharmaceutical Applications of Graphene-Based Nanomaterials and Clinical Translation Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- WHO. Cancer Prevention and Control in the Context of an Integrated Approach; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, Y.; Xu, P.; Zhang, M.; Wu, H.; Yang, S. Graphene oxide/MnWO4 nanocomposite for magnetic resonance/photoacoustic dual-model imaging and tumor photothermo-chemotherapy. Carbon 2018, 138, 397–409. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Liu, T.-Y.; Chen, P.-J.; Chang, P.-H.; Chen, S.-Y. A High-Sensitivity and Low-Power Theranostic Nanosystem for Cell SERS Imaging and Selectively Photothermal Therapy Using Anti-EGFR-Conjugated Reduced Graphene Oxide/Mesoporous Silica/AuNPs Nanosheets. Small 2016, 12, 1458–1468. [Google Scholar] [CrossRef]
- Kalluru, P.; Vankayala, R.; Chiang, C.-S.; Hwang, K.C. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Su, Y.-L.; Yu, T.-W.; Chiang, W.-H.; Chiu, H.-C.; Chang, C.-H.; Chiang, C.-S.; Hu, S.-H. Hierarchically Targeted and Penetrated Delivery of Drugs to Tumors by Size-Changeable Graphene Quantum Dot Nanoaircrafts for Photolytic Therapy. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Ali, I.; Rahis, U.; Salim, K.; Rather, M.A.; Wani, W.A.; Haque, A. Advances in nano drugs for cancer chemotherapy. Curr. Cancer Drug Targets 2011, 11, 135–146. [Google Scholar] [CrossRef]
- Amer, M.H. Gene therapy for cancer: Present status and future perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef] [Green Version]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Liu, J.; Zhai, G. Recent developments of phototherapy based on graphene family nanomaterials. Curr. Med. Chem. 2017, 24, 268–291. [Google Scholar] [CrossRef]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice (†). Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef]
- Xia, Y.; Matham, M.V.; Su, H.; Padmanabhan, P.; Gulyás, B. Nanoparticulate contrast agents for multimodality molecular imaging. J. Biomed. Nanotechnol. 2016, 12, 1553–1584. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef]
- Lahooti, A.; Sarkar, S.; Laurent, S.; Shanehsazzadeh, S. Dual nano-sized contrast agents in PET/MRI: A systematic review. Contrast Med. Mol. Imaging 2016, 11, 428–447. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.X. Upconversion nanomaterials: Synthesis, mechanism, and applications in sensing. Sensors 2012, 12, 2414–2435. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.; Shen, J.; Li, Z.; Zhang, Y.; Han, G. Upconversion Nanoparticles: A Versatile Solution to Multiscale Biological Imaging. Bioconjug. Chem. 2015, 26, 166–175. [Google Scholar] [CrossRef]
- Kateb, B.; Yamamoto, V.; Yu, C.; Grundfest, W.; Gruen, J.P. Infrared thermal imaging: A review of the literature and case report. NeuroImage 2009, 47, T154–T162. [Google Scholar] [CrossRef]
- Kylili, A.; Fokaides, P.A.; Christou, P.; Kalogirou, S.A. Infrared thermography (IRT) applications for building diagnostics: A review. Appl. Energy 2014, 134, 531–549. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Yue, S. Raman Spectroscopy and Imaging for Cancer Diagnosis. J. Healthc. Eng. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Gu, Z. Photoacoustic Drug Delivery. Sensors 2017, 17. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [Green Version]
- Dasari Shareena, T.P.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Dekaliuk, M.O. Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl. Fluoresc. 2013, 1. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Garg, B.; Sung, C.H.; Ling, Y.C. Graphene-based nanomaterials as molecular imaging agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 737–758. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Mokdad, A.; Dimos, K.; Zoppellaro, G.; Tucek, J.; Perman, J.A.; Malina, O.; Andersson, K.K.; Ramanatha Datta, K.K.; Froning, J.P.; Zboril, R. The non-innocent nature of graphene oxide as a theranostic platform for biomedical applications and its reactivity towards metal-based anticancer drugs. RSC Adv. 2015, 5, 76556–76566. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The, P.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kmet, L.; Cook, L.; Lee, R. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. Available online: https://www.ihe.ca/advanced-search/standard-quality-assessment-criteria-for-evaluating-primary-research-papers-from-a-variety-of-fields (accessed on 17 October 2018).
- Mehra, N.K.; Jain, A.K.; Nahar, M. Carbon nanomaterials in oncology: An expanding horizon. Drug Discov. Today 2018, 23, 1016–1025. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Ninan, N.; Muthoosamy, K.; Manickam, S. Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [Green Version]
- Nurunnabi, M.; Khatun, Z.; Reeck, G.R.; Lee, D.Y.; Lee, Y.-K. Photoluminescent Graphene Nanoparticles for Cancer Phototherapy and Imaging. ACS Appl. Mater. Interfaces 2014, 6, 12413–12421. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Graphene Nanomesh Promises Extremely Efficient In Vivo Photothermal Therapy. Small 2013, 9, 3593–3601. [Google Scholar] [CrossRef]
- Battogtokh, G.; Ko, Y.T. Graphene oxide-incorporated pH-responsive folate-albumin-photosensitizer nanocomplex as image-guided dual therapeutics. J. Control. Release 2016, 234, 10–20. [Google Scholar] [CrossRef]
- Bi, H.; He, F.; Dai, Y.; Xu, J.; Dong, Y.; Yang, D.; Gai, S.; Li, L.; Li, C.; Yang, P. Quad-Model Imaging-Guided High-Efficiency Phototherapy Based on Upconversion Nanoparticles and ZnFe2O4 Integrated Graphene Oxide. Inorg. Chem. 2018, 57, 9988–9998. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef]
- Chen, H.; Liu, F.; Lei, Z.; Ma, L.; Wang, Z. Fe2O3@Au core@shell nanoparticle-graphene nanocomposites as theranostic agents for bioimaging and chemo-photothermal synergistic therapy. RSC Adv. 2015, 5, 84980–84987. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, X.; Yi, X.; Huang, M.; Ning, P.; Liu, T.; Ge, C.; Chai, Z.; Liu, Z.; Yang, K. Radionuclide I-131 labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials 2015, 66, 21–28. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.; Choi, Y. A graphene oxide-photosensitizer complex as an enzyme-activatable theranostic agent. Chem. Commun. 2013, 49, 1202–1204. [Google Scholar] [CrossRef]
- Dinda, S.; Kakran, M.; Zeng, J.; Sudhaharan, T.; Ahmed, S.; Das, D.; Selvan, S.T. Grafting of ZnS:Mn-doped nanocrystals and an anticancer drug onto graphene oxide for delivery and cell labeling. ChemPlusChem 2016, 81, 100–107. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, F.; Zhao, C.; Lv, Y.; Ma, G.; Wei, W.; Tian, Z. Beyond a Carrier: Graphene Quantum Dots as a Probe for Programmatically Monitoring Anti-Cancer Drug Delivery, Release, and Response. ACS Appl. Mater. Interfaces 2017, 9, 27396–27401. [Google Scholar] [CrossRef]
- Dong, H.; Dai, W.; Ju, H.; Lu, H.; Wang, S.; Xu, L.; Zhou, S.-F.; Zhang, Y.; Zhang, X. Multifunctional Poly(L-lactide)-Polyethylene Glycol-Grafted Graphene Quantum Dots for Intracellular MicroRNA Imaging and Combined Specific-Gene-Targeting Agents Delivery for Improved Therapeutics. Acs Appl. Mater. Interfaces 2015, 7, 11015–11023. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, L.; Wang, G.; Yang, K.; Chen, M.; Tian, R.; Ma, Q.; Zhu, L. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials 2016, 79, 36–45. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Yang, D.; Xu, L.; He, F.; Gai, S.; Yang, P. Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 2018, 47, 3931–3939. [Google Scholar] [CrossRef]
- Hai, L.; He, D.; He, X.; Wang, K.; Yang, X.; Liu, J.; Cheng, H.; Huang, X.; Shangguan, J. Facile fabrication of a resveratrol loaded phospholipid@reduced graphene oxide nanoassembly for targeted and near-infrared laser-triggered chemo/photothermal synergistic therapy of cancer in vivo. J. Mater. Chem. B 2017, 5, 5783–5792. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, J.; Gao, G.; Sheng, Z.; Cui, H.; Cai, L. Indocyanine Green-Loaded Polydopamine-Reduced Graphene Oxide Nanocomposites with Amplifying Photoacoustic and Photothermal Effects for Cancer Theranostics. Theranostics 2016, 6, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Zhu, X.; Li, H.; Wang, L.; Chi, X.; Chen, J.; Wang, X.; Chen, Z.; Gao, J. Facile integration of multiple magnetite nanoparticles for theranostics combining efficient MRI and thermal therapy. Nanoscale 2015, 7, 2667–2675. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, J.; Ke, H.; Wang, S.; Dai, Z. Graphene oxide modified PLA microcapsules containing gold nanoparticles for ultrasonic/CT bimodal imaging guided photothermal tumor therapy. Biomaterials 2013, 34, 4794–4802. [Google Scholar] [CrossRef]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef]
- Ko, N.R.; Nafiujjaman, M.; Lee, J.S.; Lim, H.N.; Lee, Y.K.; Kwon, I.K. Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv. 2017, 7, 11420–11427. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yan, Y.; Chen, B.; Zhang, P.; Wang, S.; Zhou, J.; Fan, H.; Wang, Y.; Huang, X. Lanthanide-doped upconversion nanoparticles complexed with nano-oxide graphene used for upconversion fluorescence imaging and photothermal therapy. Biomater. Sci. 2018, 6, 877–884. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Li, Y.; Li, X.; Zhu, J.; Fan, L.; Yang, S. Exceptionally High Payload of the IR780 Iodide on Folic Acid-Functionalized Graphene Quantum Dots for Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef]
- Lin, L.-S.; Yang, X.; Niu, G.; Song, J.; Yang, H.-H.; Chen, X. Dual-enhanced photothermal conversion properties of reduced graphene oxide-coated gold superparticles for light-triggered acoustic and thermal theranostics. Nanoscale 2016, 8, 2116–2122. [Google Scholar] [CrossRef]
- Luo, S.; Yang, Z.; Tan, X.; Wang, Y.; Zeng, Y.; Wang, Y.; Li, C.; Li, R.; Shi, C. Multifunctional Photosensitizer Grafted on Polyethylene Glycol and Polyethylenimine Dual-Functionalized Nanographene Oxide for Cancer-Targeted Near-Infrared Imaging and Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 17176–17186. [Google Scholar] [CrossRef]
- Ma, X.; Qu, Q.; Zhao, Y.; Luo, Z.; Zhao, Y.; Ng, K.W.; Zhao, Y. Graphene oxide wrapped gold nanoparticles for intracellular Raman imaging and drug delivery. J. Mater. Chem. B 2013, 1, 6495–6500. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Kim, G.; Lee, S.; Lee, H.J.; Kim, Y.B.; Byun, Y.; Oh, Y.K. Image-guided synergistic photothermal therapy using photoresponsive imaging agent-loaded graphene-based nanosheets. J. Control. Release 2015, 211, 28–36. [Google Scholar] [CrossRef]
- Nergiz, S.Z.; Gandra, N.; Tadepalli, S.; Singamaneni, S. Multifunctional Hybrid Nanopatches of Graphene Oxide and Gold Nanostars for Ultraefficient Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2014, 6, 16395–16402. [Google Scholar] [CrossRef]
- Nie, L.; Huang, P.; Li, W.; Yan, X.; Jin, A.; Wang, Z.; Tang, Y.; Wang, S.; Zhang, X.; Niu, G.; et al. Early-stage imaging of nanocarrier-enhanced chemotherapy response in living subjects by scalable photoacoustic microscopy. ACS Nano 2014, 8, 12141–12150. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, T.; Yang, S.; Xing, D. Fluorescence quenching nanoprobes dedicated to in vivo photoacoustic imaging and high-efficient tumor therapy in deep-seated tissue. Small 2015, 11, 2675–2686. [Google Scholar] [CrossRef]
- Rong, P.; Wu, J.; Liu, Z.; Ma, X.; Yu, L.; Zhou, K.; Zeng, W.; Wang, W. Fluorescence dye loaded nano-graphene for multimodal imaging guided photothermal therapy. RSC Adv. 2016, 6, 1894–1901. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Chen, Z.; Liu, W.; Pan, J.; Hou, L.; Zhang, Z. A Multi-Functional Tumor Theranostic Nanoplatform for MRI Guided Photothermal-Chemotherapy. Pharm. Res. 2016, 33, 1472–1485. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Zhang, J.; Ma, R.; Gao, J.; Liu, Y.; Zhang, C.; Zhang, Z. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials 2014, 35, 5847–5861. [Google Scholar] [CrossRef]
- Shim, G.; Kim, D.; Kim, J.; Suh, M.S.; Kim, Y.K.; Oh, Y.K. Bacteriomimetic poly-γ-glutamic acid surface coating for hemocompatibility and safety of nanomaterials. Nanotoxicology 2017, 11, 762–770. [Google Scholar] [CrossRef]
- Some, S.; Gwon, A.R.; Hwang, E.; Bahn, G.-H.; Yoon, Y.; Kim, Y.; Kim, S.-H.; Bak, S.; Yang, J.; Jo, D.-G.; et al. Cancer Therapy Using Ultrahigh Hydrophobic Drug-Loaded Graphene Derivatives. Sci. Rep. 2014, 4, 6314. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Yang, X.; Jacobson, O.; Lin, L.; Huang, P.; Niu, G.; Ma, Q.; Chen, X. Sequential Drug Release and Enhanced Photothermal and Photoacoustic Effect of Hybrid Reduced Graphene Oxide-Loaded Ultrasmall Gold Nanorod Vesicles for Cancer Therapy. ACS Nano 2015, 9, 9199–9209. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Chan, C.; Shi, J.; Tsang, M.-K.; Pan, Y.; Cheng, C.; Gerile, O.; Yang, M. A graphene quantum dot@Fe3O4@SiO2 based nanoprobe for drug delivery sensing and dual-modal fluorescence and MRI imaging in cancer cells. Biosens. Bioelectron. 2017, 92, 489–495. [Google Scholar] [CrossRef]
- Taratula, O.; Patel, M.; Schumann, C.; Naleway, M.A.; Pang, A.J.; He, H.; Taratula, O. Phthalocyanine-loaded graphene nanoplatform for imaging-guided combinatorial phototherapy. Int. J. Nanomed. 2015, 10, 2347–2362. [Google Scholar] [CrossRef]
- Thakur, M.; Mewada, A.; Pandey, S.; Bhori, M.; Singh, K.; Sharon, M.; Sharon, M. Milk-derived multi-fluorescent graphene quantum dot-based cancer theranostic system. Mater. Sci. Eng. C Mater. Boil. Appl. 2016, 67, 468–477. [Google Scholar] [CrossRef]
- Viraka Nellore, B.P.; Pramanik, A.; Chavva, S.R.; Sinha, S.S.; Robinson, C.; Fan, Z.; Kanchanapally, R.; Grennell, J.; Weaver, I.; Hamme, A.T.; et al. Aptamer-conjugated theranostic hybrid graphene oxide with highly selective biosensing and combined therapy capability. Faraday Discuss. 2014, 175, 257–271. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; He, H.; Yang, H.; Lao, J.; Song, Y.; Xia, Y.; Xu, H.; Zhang, X.; Huang, F. A two-component active targeting theranostic agent based on graphene quantum dots. J. Mater. Chem. B 2015, 3, 3583–3590. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, D.; Song, S.; Wang, X.; Zhang, H. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 2013, 34, 7715–7724. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Fu, Y.-Y.; Peng, Q.; Guo, S.-S.; Liu, G.; Li, J.; Yang, H.-H.; Chen, G.-N. Dye-enhanced graphene oxide for photothermal therapy and photoacoustic imaging. J. Mater. Chem. B 2013, 1, 5762–5767. [Google Scholar] [CrossRef]
- Wu, C.; Li, D.; Wang, L.; Guan, X.; Tian, Y.; Yang, H.; Li, S.; Liu, Y. Single wavelength light-mediated, synergistic bimodal cancer photoablation and amplified photothermal performance by graphene/gold nanostar/photosensitizer theranostics. Acta Biomater. 2017, 53, 631–642. [Google Scholar] [CrossRef]
- Yan, X.; Hu, H.; Lin, J.; Jin, A.J.; Niu, G.; Zhang, S.; Huang, P.; Shen, B.; Chen, X. Optical and photoacoustic dual-modality imaging guided synergistic photodynamic/photothermal therapies. Nanoscale 2015, 7, 2520–2526. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Tian, Z.; Liu, J.; Zhu, Y.; Hanagata, N. Mesoporous Silica Nanoparticles Capped with Graphene Quantum Dots for Potential Chemo–Photothermal Synergistic Cancer Therapy. Langmuir 2017, 33, 591–599. [Google Scholar] [CrossRef]
- Yu, J.; Lin, Y.-H.; Yang, L.; Huang, C.-C.; Chen, L.; Wang, W.-C.; Chen, G.-W.; Yan, J.; Sawettanun, S.; Lin, C.-H. Improved Anticancer Photothermal Therapy Using the Bystander Effect Enhanced by Antiarrhythmic Peptide Conjugated Dopamine-Modified Reduced Graphene Oxide Nanocomposite. Adv. Healthc. Mater. 2016, 6, 1600804. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, T.; Tao, J.; Wan, G.; Zhao, H. Co-delivery of paclitaxel and indocyanine green by PEGylated graphene oxide: A potential integrated nanoplatform for tumor theranostics. RSC Adv. 2016, 6, 15460–15468. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, Y.; Wu, H.; Zeng, B.; Zhang, Y.; Tian, Q.; Yang, S. Hydrophilic graphene oxide/bismuth selenide nanocomposites for CT imaging, photoacoustic imaging, and photothermal therapy. J. Mater. Chem. B 2017, 5, 1846–1855. [Google Scholar] [CrossRef]
- Zheng, A.; Zhang, D.; Wu, M.; Yang, H.; Liu, X.; Liu, J. Multifunctional human serum albumin-modified reduced graphene oxide for targeted photothermal therapy of hepatocellular carcinoma. RSC Adv. 2016, 6, 11167–11175. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, L.; Ge, X.; Zhou, J.; Wei, S.; Shen, J. Multicolor imaging and the anticancer effect of a bifunctional silica nanosystem based on the complex of graphene quantum dots and hypocrellin A. Chem. Commun. 2015, 51, 421–424. [Google Scholar] [CrossRef]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef]
- Soares, T.B.; Loureiro, L.; Carvalho, A.; Oliveira, M.E.C.D.R.; Dias, A.; Sarmento, B.; Lúcio, M. Lipid nanocarriers loaded with natural compounds: Potential new therapies for age related neurodegenerative diseases? Prog. Neurobiol. 2018, 168, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Cabizza, R.; Bianco, A.; Delogu, L.G. Graphene as cancer theranostic tool: Progress and future challenges. Theranostics 2015, 5, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Liang, X.-L.; Yue, X.-L.; Wang, J.-R.; Li, C.-H.; Deng, Z.-J.; Jing, L.-J.; Lin, L.; Qu, E.-Z.; Wang, S.-M.; et al. Imaging guided photothermal therapy using iron oxide loaded poly(lactic acid) microcapsules coated with graphene oxide. J. Mater. Chem. B 2014, 2, 217–223. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.-J. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ziccarelli, I.; Galvagno, S. Chapter 8—Graphene-based materials for application in pharmaceutical nanotechnology. In Fullerens, Graphenes and Nanotubes; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 297–329. [Google Scholar]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A. Graphene: Safe or Toxic? The Two Faces of the Medal. Angew. Chem. Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef]
- SCENIHR. Position Statement on Emerging and Newly Identified Health Risks to be Drawn to the Attention of the European Commission; EU Commission: Brussels, Belgium, 2014. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, F.; Li, S.; Hu, Z.; Zhao, Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362–382. [Google Scholar] [CrossRef] [PubMed]

| Graphene-Based Nanomaterial | Graphene Type | Coating | Theranostic System Size (D or D × T) | Zeta-Potential/ Colloidal Stability in Serum | Proven Graphene Therapeutic Relevance | Proven Graphene Diagnostic Relevance | Ref. |
|---|---|---|---|---|---|---|---|
| Au@PLA-(PAH/GO)n | GO | 1.5 µm | n.r. | USI (Figure 2) s.e. Au@PLA | [61] | ||
| UCNPs-NGO/ZnPc | GO | PEG | ≈ 300 × 1.5 nm (GO) + 40 nm (UCNPs) | n.r. | PTT (Figure 3 and Figure S4) | [85] | |
| GO-HA-Ce6 | GO | ≈ 440 nm | n.r. 1 d stability | PTT (Figure 3) s.e. Ce6 | [52] | ||
| ICG-GO-FA | GO | PEG-FA | ≈ 200 nm | n.r. 3 h stability | PTT (Figure S3, Figure 2 and Figure 3) s.e. ICG | [86] | |
| GO-AuNS | GO | ≈ 0.8 μm | −19 mV 10 days | PTT (Figure 6) s.e. AuNS | [70] | ||
| GQD-Cur | GO | ≈ 150 nm (GQD-Cur) 3–6 nm (GQD) | n.r. | PL (Figure 6) | [78] | ||
| GO-Abs/Cy7 | GO | 100–600 × 1.2 nm | n.r. | PAT (Figure 3 and Figure 9) s.e. Cy7 | PAI (Figure 3 and Figure 6) s.e. Cy7 | [72] | |
| GDH | GO | HA | ≈ 120 nm (GDH) ≈ 30 nm (GO) | n.r. 7 days | PTT (Figure 5a) | [62] | |
| PheoA+GO:FA-BSA-c-PheoA | GO | PEG-FA | ≈ 180 nm | ≈ −30 mV n.r. | PTT + PDT (Figure 7 and Figure 9) s.e. Pheo | [47] | |
| GO-PEG-ZnS:Mn-DOX | GO | PEG-ZnS:Mn | n.r. | n.r. | PL (Figure 4) s.e. ZnS:Mn | [53] | |
| CPGA | GO | PEG | ≈ 230 × 15 nm | ≈ −25 mV 7 d stability | PTT (Figure 3) s.e. Au and Cy5.5 | PAI (Figure 2) FI (Figure 2) s.e. Au | [56] |
| GO@Gd-PEG-FA/DOX | GO | PEG-FA | n.r | ≈ −6 mV | PTT (Figure 6) | [75] | |
| GO/AuNS-PEG/Ce6 | GO | PEG | ≈ 400 × 18 nm | ≈ −38 mV 1 d stability | PTT (Figure 2b) s.e. AuNS | [87] | |
| GO/Bi2Se3/PVP | GO | ≈ 150 nm | ≈ −18 mV (GO) n.r. | PTT (Figure 5d) s.e. Bi2Se3 | [92] | ||
| GO/UCNPs ZnFe2O4 | GO | PEG | ≈ 400 nm | ≈ −17 mV n.r. | PTT (Figure 2d) PDT+PTT (Figure 3) s.e. UCNPs | [48] | |
| GO/MnWO4/PEG | GO | PEG | ≈ 130 nm | ≈ −26 mV n.r. | PTT (Figure 3) | [5] | |
| LOGr-Pc-LHRH | LOGr | PEG | ≈ 80 nm | n.r. n.r. | PTT (Figure 3a and Figure 6b) PDT (Figure 6c) s.e. Pc | [81] | |
| GO-DOX | NGO | PEG | ≈ 30 × 6 nm | n.r. | PAI (Figure 5) s.e. Cy 5.5 | [71] | |
| ICG-FeCl3@GO | NGO | _ | ≈ 40 nm | n.r. | PTT (Figure 3d) s.e. ICG | [83] | |
| GO@Ag-DOX-NGR | NGO | DSPE-PEG-NGR | ≈ 40 nm | −29 mV | PTT (Figure 6) s.e. Ag | [76] | |
| GO-PEG-DVDMS | NGO | PEG | ≈ 20.5 × 1.5 nm | n.r. | PTT (Figure 2a, Figure 3a and Figure 3d) s.e. DVDMS | [88] | |
| IO/GO-COOH | NGO | OA | ≈ 50 × 20 nm | n.r. | PTT (Figure 5) s.e. IO | MRI (Figure 3) s.e. IO | [60] |
| GO-PEG-CysCOOH | NGO | PEG-CysCOOH | < 50 × 2 nm | n.r. 1 d stability | PTT (Figure 2 and Figure 4) s.e. CysCOOH | PAI (Figure 3) s.e. CysCOOH | [73] |
| Au@NGO | NGO | ≈ 98 nm | ≈ −28 mV n.r. | SERS (Figure 3) s.e. Au | [68] | ||
| NGO-PEG-FA | NGO | PEG-FA | ≈ 100 nm | n.r | PTT (Figure 4, Figure 5, Figure 6 and Figure 7 | FI (Figure 3) | [7] |
| NGO-IR-808 | NGO | PEG | ≈ 20–40 × 3 nm | n.r. | PTT (Figure 2, Figure 3 and Figure 7) s.e. IR-808 | FI (Figure 4 and Figure 6) IR-TI (Figure 7) s.e. IR-808 | [67] |
| NGO-PEG-ICG/PTX | NGO | PEG-ICG | < 100 × 1 nm | ≈ −30 mV 14 d stability | PTT (Figure 4, Figure S5 and Figure 6) s.e. ICG | FI (Figure 5) s.e. ICG | [91] |
| NGO-UCNPs-Ce6 | NGO | OA | ≈ 100 nm (GO)+ 48 nm (UCNPs) | n.r. | PTT (Figure S4, Figure 7 and Figure 8) PDT+PTT (Figure 3, Figure 7 and Figure 8) s.e. UCNPs | [57] | |
| UCNP@NGO | NGO | OA | ≈ 100 nm (GO)+ 55 nm (UCNPs) | n.r. | PTT (Figure 2, Figure 4 and Figure 5) s.e. UCNPs | [64] | |
| BSA/nano-rGO | rGO | BSA | ≈ 70 nm | ≈ −30 mV 30 d stability | PTT (Figure 3) | PAI (Figure 4 and Figure 5) | [74] |
| rGONM-PEG-Cy7-RGD | rGO | PEG | ≈ 61 nm | n.r. | PTT (Figure 2) | [46] | |
| rGO-Fe2O3@AuNPs | rGO | NH2-PEG | ≈ 610 nm | −21.1 mV > 5 h | PTT (Figure 3a) s.e. Fe2O3@Au NPs | [50] | |
| rGO nanosheets | rGO | HA | ≈ 115 nm | ≈ −60 mV n.r. | PTT (Figure 4a and Figure 5a) s.e. ICG | [69] | |
| 131I-RGO-PEG | rGO | PEG | ≈ 50 × 3.5 nm | n.r. 7 days | PTT (Figure 2) s.e. 131I | γ-image (Figure 3) s.e. 131I | [51] |
| rGO-AuNRVe | rGO | PEG | ≈ 74 nm | n.r. | PTT (Figure 2) s.e. AuNR | PAI (Figure 5) s.e. AuNR | [79] |
| anti-EGFR-PEG-rGO@ CPSS-Au-R6G | rGO | PEG-Anti-EGFR | < 200 nm | n.r. | PTT (Figure S5 and Figure 7b) s.e. AuNPs | [6] | |
| ICG-PDA-rGO | rGO | PDA | 1–5 μm × 1 nm | n.r. | PTT (Figure 3 and Figure 8) s.e. ICG | PAI (Figure 4 and Figure 6) IR-TI (Figure 7) s.e. ICG | [59] |
| rGO-GSPs | rGO | PEG | ≈ 100 nm | n.r. 3 d stability | PTT (Figure 2a and Figure 2b) s.e. AuNPs | PAI (Figure 2d and Figure 2e) s.e. AuNPs | [66] |
| rGO-mfHSA | rGO | mfHSA | ≈ 200 nm | −25 mV 2 d stability | PTT (Figure 3c) | [93] | |
| FA-PEG-Lip@rGO/Res | rGO | PEG-FA | ≈ 220 nm | ≈ −24 mV 7 d stability | PTT (Figure 7b) | IR-TI (Figure 7a) | [58] |
| ArGO | rGO | APGA | ≈ 115 nm | ≈ −38 mV 28 d stability | PTT (Figure 5b and Figure 6a) | IR-TI (Figure 5a) | [77] |
| AAP10-pDA/rGO | rGO | pDA | 300 nm × 3.5 nm | n.r. | PTT (Figure 2) s.e. pDA | [90] | |
| cGdots | GQDs | ≈ 5 nm | n.r. n.r. | All therapeutic outcomes came from GQDs | All diagnostic outcomes came from GQDs | [45] | |
| GQDs | GQDs | 2–6 nm | n.r. | All therapeutic outcomes came from GQDs | All diagnostic outcomes came from GQDs | [44] | |
| PLA-PEG-grafted GQDs (f-GQDs) | GQDs | PLA-PEG | ≈ 22 × 1.7 nm | ≈ −4 mV n.r. | PL (Figure 4) | [55] | |
| AS1411@GQD | GQDs | ≈ −13 mV n.r. | PTT (Figure 6a and Figure 6b) s.e. AS1411 | [84] | |||
| HA-GQD -SiO2 NPs | GQDs | ≈ 40 nm | n.r. | PDT (Figure 6) s.e. HA and SiO2 | [94] | ||
| GQDs@Cys-BHC | GQDs | ≈ 5 nm | n.r. | FI (Figure 8) | [82] | ||
| Fe3O4@SiO2@GQDs-FA/DOX | GQDs | ≈ 25 nm | ≈ −18 mV n.r. | PL (Figure 3) FI (Figure 5) | [80] | ||
| GQD-MSN--DOX | GQDs | 50-80 nm | n.r. | PTT (Figure 6 and Figure 9) | [89] | ||
| GQD-PEG-P | GQDs | PEG | 8 × 1 nm | ≈ + 14 mV n.r. | PTT (Figure 3) | FI (Figure 5) | [49] |
| DOX@GQD-P-Cy | GQDs | ≈ 15 nm | ≈ −4 mV (w/o) DOX n.r. | FI (Figure 4d–e) s.e. DOX by FRET | [54] | ||
| DL-GQD-comp | GQDs | ≈ 220 nm | n.r. | ― | PL (Figure 5) | [63] | |
| IR780/GQDs-FA | GQDs | 8.5 × 1.5 nm | n.r. | PTT (Figure 5a and Figure 5b) s.e. IR780 | FI (Figure 6) IR-TI (Figure 7a) s.e. IR780 | [65] | |
| SCNA (DOX/GQD) | GQDs | HTPGS | < 5 nm | n.r. 4 h stability | PTT (Figure 5e) | CLSMI (Figure 4) | [8] |
| Graphene-Based Nanomaterial | THERAPY | DIAGNOSTIC | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug, Bioactive, PS, or Gene | Trigger | MHT | PTT PAT | PDT | Sensing/ Targeting | Therapy Guiding | ||||||||||
| Optical | Non-Optical | |||||||||||||||
| FI, PL | 2PFI or UCLI | IR-TI | Raman, SERS | X-ray | MRI | PET, CT SPECT | PAI | USI | ||||||||
| Theranostic strategies of graphene-based nanomaterials containing GO | ||||||||||||||||
| Au@PLA-(PAH/GO)n | + | + | + | [61] | ||||||||||||
| UCNPs-NGO/ZnPc | ZnPc (PS) | + | + | + | [85] | |||||||||||
| GO-HA-Ce6 | Ce6 (PS) | HA | + | + | + | [52] | ||||||||||
| ICG-GO-FA | + | FA | + | + | [86] | |||||||||||
| GO-AuNS | + | + | [70] | |||||||||||||
| GQD-Cur | Cur (Bioactive) | + | [78] | |||||||||||||
| GO-Abs/Cy7 | +PAT | Abs | + | [72] | ||||||||||||
| GDH | DOX (Drug) | NIR, pH GDH | + | HA | + | + | [62] | |||||||||
| PheoA+GO:FA-BSA-c-PheoA | PheoA (PS) | pH | + | + | FA | + | [47] | |||||||||
| GO-PEG-ZnS:Mn DOX | DOX (Drug) | pH | + | [53] | ||||||||||||
| CPGA | Cy5.5 (PS) | + | + | MMP-14(P) | + | + | + | [56] | ||||||||
| GO@Gd-PEG-FA/DOX | DOX (Drug) | + | FA | + | [75] | |||||||||||
| GO/AuNS-PEG/Ce6 | Ce6 (PS) | + | + | + | [87] | |||||||||||
| GO/Bi2Se3/PVP | + | + | + | + | [92] | |||||||||||
| GO/UCNPs ZnFe2O4 | ZnFe2O4 (PS) | + | + | + | + | + | + | + | [48] | |||||||
| GO/MnWO4/PEG | DOX (Drug) | NIR, pH | + | + | + | [5] | ||||||||||
| LOGr-Pc-LHRH | Pc (PS) | NIR | + | + | + | [81] | ||||||||||
| Theranostic strategies of graphene-based nanomaterials containing NGO | ||||||||||||||||
| GO-DOX | DOX (Drug) | pH | + | [71] | ||||||||||||
| ICG-FeCl3@GO | ICG (PS) | + | + | AGE-Aptamer | + | [83] | ||||||||||
| GO@Ag-DOX-NGR | DOX (Drug) | NIR | + | NGR | + | [76] | ||||||||||
| GO-PEG-DVDMS | DVDMS (PS) | NIR | + | + | + | [88] | ||||||||||
| IO/GO-COOH | + | + | + | [60] | ||||||||||||
| GO-PEG-CysCOOH | + | + | + | [73] | ||||||||||||
| Au@NGO | DOX (Drug) | + | [68] | |||||||||||||
| NGO-PEG-FA | + | + | FA | + | + | [7] | ||||||||||
| NGO-IR-808 | IR-808 (PS) | NIR | + | + | BPEI | + | + | [67] | ||||||||
| NGO-PEG-ICG/PTX | PTX (Drug) | pH | + | [91] | ||||||||||||
| NGO-UCNPs-Ce6 | Ce6 (PS) | + | + | + | [57] | |||||||||||
| UCNP@NGO | + | + | + | [64] | ||||||||||||
| Theranostic strategies of graphene-based nanomaterials containing rGO | ||||||||||||||||
| BSA/nano-rGO | + | + | + | + | [74] | |||||||||||
| rGONM-PEG-Cy7 RGD | + | RGD | + | [46] | ||||||||||||
| rGO-Fe2O3 @AuNPs | DOX (drug) | NIR Magnetic | + | + | + | [50] | ||||||||||
| rGO nanosheets | + | + | [69] | |||||||||||||
| 131I-rGO-PEG | 131I (Radio- therapy) | NIR | + | + | + | [51] | ||||||||||
| rGO-AuNRVe | DOX (drug) | NIR pH | + | + | + | [79] | ||||||||||
| anti-EGFR-PEG-rGO@CPSS-Au-R6G | + | Anti-EGFR | + | + | [6] | |||||||||||
| ICG-PDA-rGO | + | + | + | [59] | ||||||||||||
| rGO-GSPs | + | + | + | [66] | ||||||||||||
| rGO-mfHSA | + | + | [93] | |||||||||||||
| FA-PEG-Lip@rGO/Res | Res (Bioactive) | + | FA | + | [58] | |||||||||||
| ArGO | + | + | [77] | |||||||||||||
| AAP10-pDA/rGO | AAP10 (Peptide) | + | + | [90] | ||||||||||||
| Theranostic strategies of graphene-based nanomaterials containing GQDs | ||||||||||||||||
| cGdots | + | + | + | [45] | ||||||||||||
| GQDs | PpIX (PS) | + | + | [44] | ||||||||||||
| PLA-PEG-grafted GQDs (f-GQDs) | IP ASODN (Gene) | + | [55] | |||||||||||||
| AS1411@GQD | AS1411 (Gene) | NIR | + | AS1411 | + | [84] | ||||||||||
| HA-GQD-SiO2 NPs | Hypocrellin (PS) | + | + | [94] | ||||||||||||
| GQDs@Cys-BHC | BHC (Bioactive) | pH | + | [82] | ||||||||||||
| Fe3O4@SiO2 @GQDs-FA/DOX | DOX (Drug) | pH | FA FRET | + | + | [80] | ||||||||||
| GQD-MSN-DOX | DOX (Drug) | + | + | [89] | ||||||||||||
| GQD-PEG-P | P (PS) | + | + | + | [49] | |||||||||||
| DOX@GQD--P-Cy | DOX (Drug) | Peptide P | FRET | + | [54] | |||||||||||
| DL-GQD-comp | DOX (Drug) | pH | HER | + | [63] | |||||||||||
| R780/GQDs-FA | + | FA | + | + | [65] | |||||||||||
| SCNA (DOX/GQD) | DOX (Drug) | NIR + HTPGS | + | + | + | [8] | ||||||||||
| Graphene-Based Nanomaterial (GBNs) | In Vitro | In Vivo | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Model | Dark Cell Viability [GBNs] (Method) | NIR Laser | Drug or Active, Dose, AL(wt%) | Therapeutic Outcomes [GBNs] (Method) | Animal Model and Dose | Toxicity (Method) | NIR Laser | Drug or Active, Dose, AL(wt%) | Therapeutic Outcomes (Method) | ||
| Therapeutic outcomes and toxicity evaluation of graphene-based nanomaterials containing GO | |||||||||||
| Au@PLA-(PAH/GO)n | HeLa HUVECs | 90% HUVECs viability with 1000 µg/mL (Trypan blue) | λ = 808 nm P = 6.67 W/cm2 t = 6 min | ― |
| Xenograft mice (HT1080 cells) 20 mg/Kg |
| λ = 808 nm P = 2.23 W/cm2 t = 10 min | ― |
| [61] |
| UCNPs-NGO/ZnPc | KB HeLa | >90% with 320 µg/mL (MTT) | - PDT: λ = 630 nm P = 50 mW/cm2 t = n.r. - PTT: λ = 808 nm P = 2 W/cm2 t = 10 min | ZnPc n.r. |
| ― | ― | ― | ― | ― | [85] |
| GO-HA-Ce6 | A549 | ≈84% with 1.8 µM Ce6 (CCK-8) | λ = 810 nm P = 4 W/cm2 t = 8 min | ― |
| ― | ― | ― | ― | ― | [52] |
| ICG-GO-FA | HeLa | ≈100% with 20 µg/mL (MTT) | λ = 808 nm P = 2 W/cm2 t = 10 min | ― |
| ― | ― | ― | ― | ― | [86] |
| GO-AuNS | SKBR-3 MCF-10a | ≈95% in SKBR-3 and MCF-10a with 40 µg/mL (MTT) | λ = 808 P = 0.75 W/cm2 t = 2 | ― |
| ― | ― | ― | ― | ― | [70] |
| GQD-Cur | HCT166 | >90% with 100 µg/mL (SRB) | ― | Cur AL ≈ 41% |
| Xenograft mice (HCT166) 10 mg/Kg |
| ― | Cur AL ≈ 41% |
| [78] |
| GO-Abs/Cy7 | U87-MG | ≈95% with 42.8 µg/mL (CCK-8) | λ = n.r. P = 0.016W/cm2 t = n.r. | ― |
| Xenograft mice (U87-MG cells) 43.4 µg/mouse |
| λ = 753 nm P = 0.02 W/cm2 t = 1 min | ― |
| [72] |
| GDH | MDCK A549 | ≈80–85% MDCK cell viability with 100 µg/mL (MTT) | λ = 670 nm P = 1 W/cm2 t = 5 min | DOX 100 μg/mL AL n.r. |
| Xenograft mice (A549 cells) 2.5 mg/Kg |
| λ = 670 nm P = 1 W/cm2 t = 30 min | DOX n.r. |
| [62] |
| PheoA+GO:FA-BSA-c-PheoA | B16F10 MCF-7 | 100% with 0.375 µg/mL (MTT) | λ = 670 nm P = 0.13 W/cm2 t = 10 min | ― |
| Xenograft mice (B16F10 cells) 2 mg/Kg |
| λ = 670 nm P = 0.13 W/cm2 t = 10 min | ― |
| [47] |
| GO-PEG-ZnS:Mn-DOX | HeLa; CHO-K1 | 100% with 1000 µg/mL (w/o DOX) (MTT) | ― | DOX 300 μg/mL AL ≈ 10% |
| ― | ― | ― | ― | ― | [53] |
| CPGA | SCC7 | ≈70% with 200 µg/mL (MTT) | λ = 808 nm P = 0.3 W/cm2 t = 10 min | ― |
| Xenograft mice (SCC7 cells) 10 mg/Kg |
| λ = 808 nm P = 0.75 W/cm2 t = 10 min | ― |
| [56] |
| GO@Gd-PEG-FA/DOX | MCF-7 | ≈85% with 10 µg/mL (w/o DOX) (SRB) | λ = 808 nm P = 2.5 W/cm2 t = 5 min | DOX 10 µg/mL AL≈94% |
| Xenograft mice (S180 cells) 10 mg/Kg |
| λ = 808 nm P = 2 W/cm2 t = 5 min | DOX 5 mg/kg AL ≈ 94% |
| [75] |
| GO/AuNS- -PEG/Ce6 | EMT6 | >95% with 100 µg/mL (CCK-8) | λ = 660 nm P = 2 W/cm2 t = 8 min | ― |
| Xenograft mice (EMT6 cells) 10 mg/kg |
| λ = 660 nm P = 3 W/cm2 t = 10 min | ― |
| [87] |
| GO/Bi2Se3/ PVP | HeLa | >90% with 75 µg/mL (MTT) | λ = 808 nm P = 0.3 W/cm2 t = 10 min | ― |
| Xenograft mice (HeLa) 0.2 mg/mouse |
| λ = 808 nm P = 0.4 W/cm2 t = 5 min | ― |
| [92] |
| GO/UCNPs ZnFe2O4 | HeLa; L929 | 80% with 500 µg/mL (MTT) | λ = 980 nm P = 0.8 W/cm2 t = 15 min | ― |
| Xenograft mice (U14 cells) n.r. |
| λ = 980 nm P = 0.8 W/cm2 t = 15 min | ― |
| [48] |
| GO/MnWO4/PEG | 4T1 HUVEC | 90% and 80% (4T1 and HUVEC cell) with 100 µg/mL (MTT) | λ = 808 nm P = 0.6 W/cm2 t = 10 min | DOX 5 µg/mL AL ≈ 55% |
| Xenograft mice (U14 cells) 0.6 mg/mouse |
| λ = 808 nm P = 0.6 W/cm2 t = 10 min | DOX 0.2 mg/mouse AL ≈ 55% |
| [5] |
| LOGr-Pc-LHRH | A2780/AD RBC |
| λ = 690 nm P = 0.95W/cm2 t = 15 min | Pc 4 µg/mL |
| Xenograft mice (A2780/AD cells) 1 mg/Kg | ― | ― | ― |
| [81] |
| Therapeutic outcomes and toxicity evaluation of graphene-based nanomaterials containing NGO | |||||||||||
| GO-DOX | ― | ― | ― | ― | ― | Xenograft mice (H1975 cells) n.r. |
| ― | DOX 8 mg/kg AL = 133% |
| [71] |
| ICG-FeCl3@GO | G361 | ― | λ = 785 nm P = 1 W/cm2 t = 20 min | ICG n.r. |
| ― | ― | ― | ― | ― | [83] |
| GO@Ag-DOX-NGR | MCF-7 | ≈90% with 10 µg/mL (SRB) | λ = 808 nm P = 2 W/cm2 t = 3 min | DOX 4 µg/mL AL ≈ 82% |
| Xenograft mice (S180) 6.1 mg/Kg |
| λ = 808 nm P = 2 W/cm2 t = 3 min | DOX 5 mg/kg AL ≈ 82% |
| [76] |
| GO-PEG-DVDMS | PC9 | ≈70% with 3 µg/mL (MTT) | - PDT: λ = 630 nm P = 3 J/well t = n.r. - PTT: λ = 808 nm P = 1 W/cm2 t = 3 min | DVDMS n.r |
| Xenograft mice (PC9 cells) 1 mg/Kg |
| - PDT: λ = 630 nm 50 J t = n.r. - PTT: λ = 808 nm P = 1 W/cm2 t = 10 min | DVDMS 2 mg/kg |
| [88] |
| IO/GO-COOH | HeLa | 95% with [Fe] = 200 µg/mL (MTT) | λ = 808 nm P = 2 W/cm2 t = 5 min | ― |
| Xenograft mice (S180 cells) 37.5 µg /mouse |
| λ = 808 nm P = 1 W/cm2 t = 5 min | ― |
| [60] |
| GO-PEG-CysCOOH | 4T1 | ≈100% with 250 µg/mL (MTT) | λ = 808 nm P = 0.5 W/cm2 t = 3 min | ― |
| Xenograft mice (4T1) 450 μg/mouse |
| λ = 808 nm P = 0.5 W/cm2 t = 5 min | ― |
| [73] |
| Au@NGO | HeLa cells | ≈80% with 200 µg/mL (MTT) | ― | DOX 25 µg/mL AL ≈ 2% |
| ― | ― | ― | ― | ― | [68] |
| NGO-PEG-FA | B16F0 | ≈90% with 75 µg/mL (MTT) |
λ = 808 nm P = 0.32 W/cm2 t = 15 min
λ = 980 nm P = 0.32 W/cm2 t = 18 min | ― |
| Xenograft mice (B16F10 cells) 8 mg/Kg | ― |
λ = 808 n P = 0.25 W/cm t = 8 mi
λ = 980 nm P = 0.25 W/cm2 t = 10 min | ― |
| [7] |
| NGO-IR-808 | A549; Lewis lung | Negligible toxicity with 10 µM (CCK-8) | λ = 808 nm P = 2 W/cm2 t = 5 min | ― |
| Xenograft mice (A549; Lewis lung cells) 10 mg/Kg |
| λ = 808 nm P = 1 W/cm2 t = 5 min | ― |
| [67] |
| NGO-PEG-ICG/PTX | MG-63 | ≈100% with 200 µg/mL (w/o PTX) (CCK-8) | ― | PTX 100 µg/mL AL ≈ 90% |
| Xenograft mice (MG-63) 10 mg/kg |
| ― | PTX 9 mg/kg AL ≈ 90% |
| [91] |
| NGO-UCNPs-Ce6 | HeLa; L929 | >95% with 800 µg/mL (MTT) | λ = 808 nm P = 0.72 W/cm2 t = 10 min | ― |
| Xenograft mice (U14 cells) n.r. |
| λ = 808 nm P = 0.72 W/cm2 t = 10 min | ― |
| [57] |
| UCNP@ NGO | 4T1 | >90% with 400 µg/mL (MTT) | λ = 808 nm P = 2 W/cm2 t = 10 min | ― |
| Xenograft mice (4T1 cells) n.r. |
| λ = 808 nm P = 1 W/cm2 t = 5 min | ― |
| [64] |
| Therapeutic outcomes and toxicity evaluation of graphene-based nanomaterials containing rGO | |||||||||||
| BSA/nano-rGO | MCF-7 cells | 100% with 0.04 µg/mL (MTT) | λ = 808 nm P = 6 W/cm2 t = 5 min | ― |
| Xenograft mice (MCF-7) 20 mg/kg |
| λ = 808 nm P = 0.6 W/cm2 t = 5 min | ― |
| [74] |
| rGONM-PEG-Cy7-RGD | ― | ― | ― | ― | ― | Xenograft mice (U87MG cells) 0.2 mg/mouse |
| λ = 808 nm P = 0.1 W/cm2 t = 7 min | ― |
| [46] |
| rGO- Fe2O3@Au NPs | HeLa | ≈90% with 50 µg/mL (MTT) | λ = 808 nm P = 2 W/cm2 t = 5 min | DOX n.r. AL≈100% |
| ― | ― | ― | ― | ― | [50] |
| rGO nanosheets | KB | ≈95% with 20 µg/mL (CCK-8) | λ = 808 nm P = 1.2 W/cm2 t = 3 min | ― |
| Xenograft and orthotopic mice (KB cells) 5 mg/kg |
| λ = 808 nm P = 1.2 W/cm2 t = 3 min | ― |
| [69] |
| 131I-RGO-PEG | 4T1 | ≈90% with 200 µg/mL (CCK-8) | λ = 808 nm P = 0.5 W/cm2 t = 10 min | 131I 100 µCi |
| Xenograft and orthotopic mice (4T1cells) 10 mg/kg |
| λ = 808 nm P = 0.2 W/cm2 T = 20 min | 131I 200 µCi/ mouse |
| [51] |
| rGO-AuNRVe | U87MG | 100% with 2.4 nM (CCK-8) | λ = 808 nm P = 0.25 W/cm2 t = 5 min | DOX 6.4 µg/mL AL ≈ 65% |
| Xenograft and orthotopic mice (U87MG) 10 mg/kg |
| λ = 808 nm P = 0.25 W/cm2 t = 5 min | DOX AL ≈ 65% |
| [79] |
| anti-EGFR-PEG-rGO@ CPSS-Au-R6G | A549 | 100% with 100 µg/mL (MTT) | λ = 808 nm P = 0.5 W/cm2 t = 5 min | ― |
| ― | ― | ― | ― | ― | [6] |
| ICG-PDA-rGO | 4T1 | ≈90% with 20 µg/mL (MTT) | λ = 808 nm P = 0.6 W/cm2 t = 5 min | ― |
| Xenograft and orthotopic mice (4T1 cells) 200 µg/mouse |
| λ = 808 nm P = 0.6 W/cm2 t = 5 min | ― |
| [59] |
| rGO-GSPs | U87MG | ≈100% with 100 µg/mL (MTT) | λ = 808 nm P = 0.8 W/cm2 t = 5 min | ― |
| Xenograft mice (U87MG cells) 200 μg/mouse |
| λ = 808 nm P = 0.8 W/cm2 t = 5 min | ― |
| [66] |
| rGO-mfHSA | HepG2 | ≈100% with 20 μg/mL (CCK-8) | λ = 808 nm P = 2 W/cm2 t = 5 min | ― |
| Xenograft mice (HepG2) 200 μg/mouse |
| λ = 808 nm P = 1 W/cm2 t = 10 min | ― |
| [93] |
| FA-PEG-Lip@rGO/ Res | A549 MCF-7 | >90% with 80 µg/mL (w/o Res) (MTT) | λ = 780 nm P = 0.6 W/cm2 t = 10 min | Res 56 µg/mL AL ≈ 70% |
| Xenograft mice (MCF-7 cells) 2.2 mg/kg |
| λ = 780 nm P = 0.6 W/cm2 t = 5 min | Res AL ≈ 70% |
| [58] |
| ArGO | ― | ― | ― | ― | ― | Xenograft mice (SCC7 cells) 5 mg/kg |
| λ = 808 nm P = 1.5 W/cm2 t = 3 min | ― |
| [77] |
| AAP10-pDA/rGO | MCF-7 | ≈100% with 160 µg/mL (MTT) | λ = 808 nm P = 1.5 W/cm2 t = 5 min | AAP10 50 nM AL ≈ 0.024% |
| Xenograft mice (4T1 cells) 0.3 mg/mouse |
| λ = 808 nm P = 1.5 W/cm2 t = 5 min | AAP10 AL ≈ 0.024% |
| [90] |
| Therapeutic outcomes and toxicity evaluation of graphene-based nanomaterials containing GQDs | |||||||||||
| cGdots | MDA-MB231 | >70% with 500 µg/mL (MTT) | λ = 670 nm P = 0.3 W/cm2 t = 30 min | ― |
| Xenograft mice (MDA-MB231 cells) 75 µg/mouse |
| λ = 670 nm P = 0.3 W/cm2 t = 30 min irr. every other day | ― |
| [45] |
| GQDs | HeLa | >90% with 1.8 µM (MTT) | λ = 670 nm P = 6.5 mW/cm2 t = 10 min | PpIX n.r. |
| Xenograft mice (MDA-MB231 cells) 80 µg/mouse |
| λ = 400–800 nm P = 80 mW/cm2 t = 10 min irr. day 1 and 7 | PpIX n.r. |
| [44] |
| PLA-PEG-grafted GQDs (f-GQDs) | HeLa | ≈90% cell viability with 140 µg/mL (MTT) | ― | IP and ASODN 50 nM |
(Cytometry) | ― | ― | ― | ― | ― | [55] |
| AS1411@ GQD | A549 | 100% cell viability with 5 µM (MTT) | λ = 808 nm P = 2 W/cm2 t = 10 min | AS1411n.r. |
| ― | ― | ― | ― | ― | [84] |
| HA-GQD -SiO2 NPs | HeLa | 100% with 4 µM Hypo-crellin (MTT) | λ = 470 nm | Hypocre-llin 4 µM |
| ― | ― | ― | ― | ― | [94] |
| GQDs@Cys-BHC | L929 HeLa MDA-MB-231 | Low toxicity with 200 µg/mL (w/o BHC) (Trypan blue, MTT) | ― | BHC ≈0.4 mM AL ≈ 88% |
| ― | ― | ― | ― | ― | [82] |
| Fe3O4@SiO2 @GQDs-FA/DOX | HeLa | >90% with 50 µg/mL (MTT) | λ = 808 nm P = 0.3 W/cm2 t = 10 min | ― |
| ― | ― | ― | ― | ― | [80] |
| GQD-MSN- -DOX | 4T1 | ≈95% with 100 μg/mL (CCK-8) | λ = 808 nm P = 2.5 W/cm2 t = 3 min | DOX 4.5 μg/mL AL ≈ 4.8% |
| ― | ― | ― | ― | ― | [89] |
| GQD-PEG-P | A549 MCF-7 | 100% with 100 µg/mL (MTT) | λ = 980 + 636 nm P = 0.72 W/cm2 t = 10 min | ― |
| ― | ― | ― | ― | ― | [49] |
| DOX@GQD- -P-Cy | 4T1 | ≈95% with 4 µg/mL (w/o DOX) (MTT) | ― | DOX 3.3 µg/mL AL ≈ 82.5% |
| Xenograft mice (4T1 cells) 1 µg /mouse |
| ― | DOX 0.8 µg /mouse AL ≈ 82.5% |
| [54] |
| DL-GQD-comp | BT-474 | ≈90% with 100 µg/mL (w/o DOX) (CCK-8) | ― | DOX 8.8 µM AL ≈ 5.3% |
| ― | ― | ― | ― | ― | [63] |
| IR780/GQD-FA | HeLa | ≈90% with 30 µg/mL (CCK-8) | λ = 808 nm P = 1 W/cm2 t = 5 min | ― |
| Xenograft mice (HeLa cells) 2 mg/kg |
| λ = 808 nm P = 1 W/cm2 t = 5 min | ― |
| [65] |
| SCNA (DOX/GQD) | RG2 | ≈100% with 10 µg/mL (w/o DOX) (alamar blue) | λ = 808 nm P = 2 W/cm2 t = 5 min | DOX 2 µg/mL AL n.r. |
| Xenograft mice (RG2 cells) 0.2 mg/mouse |
| λ = 808 nm P = 2 W/cm2 t = 10 min | DOX 2 µg/mL AL n.r |
| [8] |
| Ligands | Function | Ref. |
|---|---|---|
| Abs | Targets integrin (αvβ3) receptor overexpressed in cancer cells | [72] |
| AGE-aptamer | Targets melanoma inhibitor of apoptosis protein (ML-IAP) overexpressed in melanoma cells | [83] |
| Anti-EGFR | Targets the epidermal growth factor receptor (EGFR) of lung cancer cells | [6] |
| AS1411 | Aptamer specific to malignant melanoma | [84] |
| BPEI | Targets the organic anion transporting polypeptides (OATPs) overexpressed in cancer cells | [46] |
| FA | Targets folic acid receptors overexpressed in cancer cells | [7,47,58,65,75,80,86] |
| HA | Targets CD44 receptors, a cell surface adhesion receptor that is highly expressed in many cancers and regulates metastasis | [62] |
| HER | Targets HER2+ receptors in breast cancer cells | [63] |
| HSA-LA | Generates galactose residues that targets asialoglycoprotein receptor (ASGP-R), highly expressed on the surface of hepatocellular carcinoma cells (HCC) | [93] |
| MMP-14(P) | Targets the overexpressed endoperoxidase in tumor cell membrane | [56] |
| NGR | Targets CD13 isoform selectively overexpressed in tumor vasculature and certain tumor cells | [76] |
| RGD | Targets integrin αvβ3 mAb overexpressed in cancer cells | [46] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viseu, T.; Lopes, C.M.; Fernandes, E.; Real Oliveira, M.E.C.D.; Lúcio, M. A Systematic Review and Critical Analysis of the Role of Graphene-Based Nanomaterials in Cancer Theranostics. Pharmaceutics 2018, 10, 282. https://doi.org/10.3390/pharmaceutics10040282
Viseu T, Lopes CM, Fernandes E, Real Oliveira MECD, Lúcio M. A Systematic Review and Critical Analysis of the Role of Graphene-Based Nanomaterials in Cancer Theranostics. Pharmaceutics. 2018; 10(4):282. https://doi.org/10.3390/pharmaceutics10040282
Chicago/Turabian StyleViseu, Teresa, Carla M. Lopes, Eduarda Fernandes, Maria Elisabete C.D. Real Oliveira, and Marlene Lúcio. 2018. "A Systematic Review and Critical Analysis of the Role of Graphene-Based Nanomaterials in Cancer Theranostics" Pharmaceutics 10, no. 4: 282. https://doi.org/10.3390/pharmaceutics10040282
APA StyleViseu, T., Lopes, C. M., Fernandes, E., Real Oliveira, M. E. C. D., & Lúcio, M. (2018). A Systematic Review and Critical Analysis of the Role of Graphene-Based Nanomaterials in Cancer Theranostics. Pharmaceutics, 10(4), 282. https://doi.org/10.3390/pharmaceutics10040282









