Effect of Shape on Mesoporous Silica Nanoparticles for Oral Delivery of Indomethacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mesoporous Silica Nanoparticles (MSNs)
2.3. Drug Loading
2.4. Characterization
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Transmission Electron Microscopy (TEM)
2.4.3. Small-Angle X-ray Diffraction (Small-Angle XRD)
2.4.4. Nitrogen Adsorption/Desorption Measurement
2.4.5. XRD
2.5. In Vitro Release
2.6. Cell Culture and Cytotoxicity
2.7. In Vivo Pharmacokinetic Study
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of MSNs
3.1.1. Synthesis and Morphology of MSNs
3.1.2. Small-Angle XRD
3.1.3. Nitrogen Adsorption/Desorption
3.2. Drug Loading
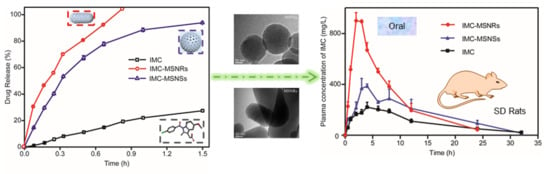
3.3. In Vitro Release
3.4. Cytotoxicity
3.5. In Vivo Pharmacokinetic Study
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Banerjee, A.; Qi, J.P.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [PubMed]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; Bijukumar, L.; do Toit, L.C.; Pillay, V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014, 32, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Herbrink, M.; Schellens, J.H.M.; Beijnen, J.H.; Nuijen, B. Improving the solubility of nilotinib through novel spray-dried solid dispersions. Int. J. Pharm. 2017, 35, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, T.M.; Shi, H.; Pietryka, J.; Hoag, S.W.; Medek, A. Investigation of Polymer/Surfactant Interactions and Their Impact on Itraconazole Solubility and Precipitation Kinetics for Developing Spray-Dried Amorphous Solid Dispersions. Mol. Pharm. 2018, 15, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Garg, T.; Das, G.U.; Gupta, P.; Rath, G.; Goyal, A.K. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif. Cells Nanomed. Biotechnol. 2014, 44, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Ding, W.; Wang, X.T.; Chen, J.P.; Li, Y.H. Dissolution and oral bioavailability enhancement of praziquantel by solid dispersions. Drug Deliv. Transl. Res. 2018, 8, 580–590. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, K.; Gawlak, K.; Paluch, M.; Jachowicz, R. Enhanced Dissolution of Solid Dispersions Containing Bicalutamide Subjected to Mechanical Stress. Int. J. Pharm. 2018, 542, 18–26. [Google Scholar] [CrossRef]
- Chaudhary, S.; Garg, T.; Rath, G.; Murthy, R.R.; Goyal, A.K. Enhancing the bioavailability of mebendazole by integrating the principles solid dispersion and nanocrystal techniques, for safe and effective management of human echinococcosis. Artif. Cells Nanomed. Biotechnol. 2015, 44, 937–942. [Google Scholar] [CrossRef]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and Evaluation of Naringenin Nanosuspensions for Bioavailability Enhancement. AAPS PharmSciTech 2017, 18, 3151–3162. [Google Scholar] [CrossRef]
- Zuo, W.; Qu, W.; Li, N.; Yu, R.; Hou, Y.H.; Liu, Y.H.; Gou, G.J.; Yang, J.H. Fabrication of multicomponent amorphous bufadienolides nanosuspension with wet milling improves dissolution and stability. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1513–1522. [Google Scholar] [CrossRef]
- Mai, K.; Al-Taani, B.; Alsmadi, M.; Zayed, A. Enhancement of the dissolution and bioavailability from freeze-dried powder of a hypocholesterolemic drug in the presence of Soluplus. Powder Technol. 2018, 329, 25–32. [Google Scholar] [CrossRef]
- Shamma, R.; Elkasabgy, N. Design of freeze-dried Soluplus/polyvinyl alcohol-based film for the oral delivery of an insoluble drug for the pediatric use. Drug Deliv. 2016, 23, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; De, P.A.; Giangregorio, N.; Miniero, D.V.; Pesce, P.; Nicolotti, O.; Altomare, C.D.; Catto, M. Mannich base approach to 5-methoxyisatin 3-(4-isopropylphenyl)hydrazone: A water-soluble prodrug for a multitarget inhibition of cholinesterases, beta-amyloid fibrillization and oligomer-induced cytotoxicity. Eur. J. Pharm. Sci. 2017, 109, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Xie, H.J.; Cao, Q.R.; Shi, L.L.; Cao, Y.; Zhu, X.Y.; Cui, J.H. Enhanced dissolution and oral bioavailability of valsartan solid dispersions prepared by a freeze-drying technique using hydrophilic polymers. Drug Deliv. 2016, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ratnatilaka, N.B.P.; El-Magboub, A.; Haworth, I.S.; Rojsitthisak, P. Enhancement of Curcumin Bioavailability Via the Prodrug Approach: Challenges and Prospects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.; Li, H.; Shang, L.; Li, S.M. Superiority of amino-modified chiral mesoporous silica nanoparticles in delivering indometacin. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1085–1094. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Leong, K.W.; Sung, H.W. Nanoparticle- and biomaterials-mediated oral delivery for drug, gene, and immunotherapy. Adv. Drug Deliv. Rev. 2013, 65, 757–758. [Google Scholar] [CrossRef]
- Niu, Z.; Conejos-Sanchez, I.; Griffin, B.T.; O’Driscoll, C.M.; Alonso, M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 337–354. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Yang, B.; Bao, Z.H.; Pan, W.S.; Li, S.M. Biomimetic synthesized chiral mesoporous silica: Structures and controlled release functions as drug carrier. Mater. Sci. Eng. C 2015, 55, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, L.; Zheng, K.; Wei, P.; Du, L.; Shen, M.; Shi, X.Y. Formation of Gold Nanostar-Coated Hollow Mesoporous Silica for Tumor Multimodality Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 5817–5827. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.; Lather, V.; Pandita, D. Mesoporous silica nanoparticles: A smart nanosystem for management of breast cancer. Drug Discov. Today 2018, 23, 315–332. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Yang, Y.; Zhu, C.; Su, Q.; Guo, S.; Sun, J.; Gan, Y.; Shi, X.; Gao, H. Rotation-Facilitated Rapid Transport of Nanorods in Mucosal Tissues. Nano Lett. 2016, 16, 7176–7182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhang, K.; Wu, Z.M.; Feng, N.P. Biotinylated-lipid bilayer coated mesoporous silica nanoparticles for improving the bioavailability and anti-leukaemia activity of Tanshinone IIA. Artif. Cells Nanomed. Biotechnol. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liang, S.; Long, M.; Xu, H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater. Sci. Eng. C 2017, 78, 12–17. [Google Scholar] [CrossRef]
- Shen, S.C.; Ng, W.K.; Chia, L.; Hu, J.; Tan, R.B.H. Physical state and dissolution of ibuprofen formulated by co-spray drying with mesoporous silica: Effect of pore and particle size. Int. J. Pharm. 2011, 410, 188–195. [Google Scholar] [CrossRef]
- Tzankov, B.; Yoncheva, K.; Popova, M.; Szegedi, A.; Momekov, G.; Mihály, J.; Lambov, N. Indometacin loading and in vitro release properties from novel carbopol coated spherical mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2013, 171, 131–138. [Google Scholar] [CrossRef]
- Pu, Y.Y.; Li, Y.; Zhuang, W.; Zhang, M.; Li, B.Z.; Yang, Y.G. Preparation and characterizations of helical mesoporous silica nanorods using CTAB and alcohols. Chin. Chem. Lett. 2012, 23, 1201–1204. [Google Scholar] [CrossRef]
- Zheng, N.; Li, J.; Xu, C.; Xu, L.S.; Li, S.M.; Xu, L. Mesoporous silica nanorods for improved oral drug absorption. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1132–1140. [Google Scholar] [CrossRef]
- Landry, C.C.; Tolbert, S.H.; Gallis, K.W.; Monnier, A.; Stucky, G.D.; Norby, F.; Hanson, J.C. Phase transformations in mesostructured silica/surfactant composites. Mechanisms for change and applications to materials synthesis. Chem. Mater. 2001, 13, 1600–1608. [Google Scholar] [CrossRef]
- O’Shea, J.P.; Nagarsekar, K.; Wieber, A.; Witt, V.; Herbert, E.; O’Driscoll, C.M.; Saal, C.; Lubda, D.; Griffin, B.T.; Dressman, J.B.; et al. Mesoporous silica-based dosage forms improve bioavailability of poorly soluble drugs in pigs: Case example fenofibrate. J. Pharm. Pharmacol. 2017, 69, 1284–1292. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Z.; Ohsuna, T.; Terasaki, O.; Inoue, Y.; Sakamoto, K.; Nakanishi, T.; Ariga, K.; Che, S. Control of Morphology and Helicity of Chiral Mesoporous Silica. Adv. Mater. 2006, 18, 593–596. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Yang, B.; Wang, H.; Bao, Z.; Pan, W.; Li, S.M. Facile synthesis of functionalized ionic surfactant templated mesoporous silica for incorporation of poorly water-soluble drug. Int. J. Pharm. 2015, 492, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhi, Z.; Zhao, Q.; Wu, C.; Zhao, P.; Jiang, H.; Jiang, T.Y.; Wang, S.L. 3D cubic mesoporous silica microsphere as a carrier for poorly soluble drug carvedilol. Microporous Mesoporous Mater. 2012, 147, 94–101. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Marmottini, F.; Giovagnoli, S.; Esposito, M.; Rossi, C. Improvement of dissolution rate of piroxicam by inclusion into MCM-41 mesoporous silicate. Eur. J. Pharm. Sci. 2007, 32, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.Y.; Lin, H.M.; Wu, X.; Li, X.F.; Qiu, S.L.; Zhu, G.S. Bio-templated synthesis of highly ordered macro-mesoporous silica material for sustained drug delivery. Solid State Sci. 2010, 12, 851–856. [Google Scholar] [CrossRef]
- Kapoor, S.; Hegde, R.; Bhattacharyya, A.J. Influence of surface chemistry of mesoporous alumina with wide pore distribution on controlled drug release. J. Control. Release 2009, 140, 34–39. [Google Scholar] [CrossRef]
- Salonen, J.; Laitinen, L.; Kaukonen, A.M.; Tuura, J.; Bjorkqvist, M.; Heikkila, T.; Vaha-Heikkila, K.; Hirvonen, J.; Lehto, V.P. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Control. Release 2005, 108, 362–374. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Q.; Xu, Y.; Wu, D.; Sun, Y.; Shen, W.; Deng, F. Controllable release of ibuprofen from size-adjustable and surface hydrophobic mesoporous silica spheres. Powder Technol. 2009, 191, 13–20. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, S.Z.; Jin, Y.G.; Cheng, L.N.; Yan, Z.F.; Lu, G.Q. Hydrophobic Functional Group Initiated Helical Mesostructured Silica for Controlled Drug Release. Adv. Funct. Mater. 2008, 18, 3834–3842. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.F.; Li, W.Z.; Yao, C.L.; Xie, S.F. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Wang, W.; Yang, W.; Wang, Y.R.; Ru, H.Q. Facile and controllable preparation of different SBA-15 platelets and their regulated drug release behaviours. Microporous Mesoporous Mater. 2017, 263, 34–41. [Google Scholar] [CrossRef]
- Maksym-Bębenek, P.; Neugebauer, D. Study on Self-Assembled Well-Defined PEG Graft Copolymers as Efficient Drug-Loaded Nanoparticles for Anti-Inflammatory Therapy. Macromol. Biosci. 2015, 15, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, N.; Wang, X.; Li, C.; Sun, M.C.; Wang, J.; Fu, Q.; He, Z.G. Interfacial interaction track of amorphous solid dispersions established by water-soluble polymer and indomethacin. Eur. J. Pharm. Sci. 2017, 106, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, X.T.; Zheng, N.; Xu, L.; Xu, J.H.; Li, S.M. Contribution of carboxyl modified chiral mesoporous silica nanoparticles in delivering doxorubicin hydrochloride in vitro pH-response controlled release, enhanced drug cellular uptake. Colloids Surf. B 2016, 141, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Von, R.C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef]
- Thanh, C.V.; Katharina, G.; Tilley, R.D.; Gooding, J.J. Rod-shaped mesoporous silica nanoparticles for nanomedicine: Recent progress and perspectives. Expert Opin. Drug Deliv. 2018, 15, 881–892. [Google Scholar] [CrossRef]







| Sample | SBET (m2/g) | Vt (cm3/g) | WBJH (nm) |
|---|---|---|---|
| MSNSs | 907.20 | 0.82 | 4.7 |
| MSNRs | 1074.22 | 0.96 | 5.8 |
| Kinetic Equations | IMC | IMC-MSNSs | IMC-MSNRs |
|---|---|---|---|
| Zero-order | F = 0.845 + 19.620t | F = 21.333 + 61.072t | F = 20.326 + 111.671t |
| F = C + kt | r2 = 0.9704 | r2 = 0.7858 | r2 = 0.8883 |
| First-order | F = 53.971 × (1 − e−0.493t) | F = 98.587 × (1 − e−2.255t) | F = 107.487 × (1 − e−3.127t) |
| F = a × (1 − e−kt) | r2 = 0.9910 | r2 = 0.9980 | r2 = 0.9859 |
| Quadratic | F = 100 × (−0.050t2 + 0.262t) | F = 100 × (−0.713 × t2 + 1.676t) | F = 100 × (−1.732 × t2 + 2.623t) |
| F = 100 × (k1 × t2 + k2 × t) | r2 = 0.9923 | r2 = 0.9824 | r2 = 0.9602 |
| Higuchi | F = 19.235 × t0.5 | F = 85.081 × t0.5 | F = 114.014 × t0.5 |
| F = k × t0.5 | r2 = 0.8765 | r2 = 0.9566 | r2 = 0.9968 |
| Hixson-Crowell | F = 100 × [1 − (1 − 0.075t)3] | F = 100 × [1 − (1 − 0.619t)3] | F = 100 × [1 − (1 − 0.980t)3] |
| F = 100 × [1 − (1 − kt)3] | r2 = 0.9823 | r2 = 0.9896 | r2 = 0.9794 |
| Weibull | F = 100 × {1 − e[−(t^0.935)/4.282]} | F = 100 × {1 − e[−(t^1.025)/0.446]} | F = 100 × {1−e[−(t^0.991)/0.277]} |
| F = 100 × {1 − e[−(t^β)/α]} | r2 = 0.9872 | r2 = 0.9979 | r2 = 0.9810 |
| Baker-Lonsdale | 1.5 × [1 − (1 − F/10)2/3] − F/100 = 0.007t | 1.5 × [1 − (1 − F/10)2/3] − F/100 = 0.198t | 1.5 × [1 − (1 − F/10)2/3] − F/100 = 0.370t |
| 1.5 × [1 − (1 − F/10)2/3] − F/100 = kt | r2 = 0.8642 | r2 = 0.9450 | r2 = 0.9627 |
| Peppas-Sahlin | F = 4.800 × t0.5 + 15.693t | F = 93.402 × t0.5 − 9.030t | F = 113.394 × t0.5 + 0.849t |
| F = k1 × t0.5 + k2t | r2 = 0.9783 | r2 = 0.9539 | r2 = 0.9963 |
| Korsmeyer-Peppas | F = 20.608 × t0.848 | F = 84.808 × t 0.488 | F = 114.483 × t0.506 |
| F = k × tn | r2 = 0.9828 | r2 = 0.9507 | r2 = 0.9963 |
| Formulation | Cmax (mg/mL) | Tmax (h) | AUC0→t (mg∙h/L) | Frel (%) |
|---|---|---|---|---|
| IMC | 234.2 ± 16.0 | 4.7 | 3240 ± 117 | - |
| IMC-MSNSs | 417.4 ± 36.3 | 3.7 | 5398 ± 746 | 167 |
| IMC-MSNRs | 928.0 ± 24.8 | 2.3 | 7275 ± 608 | 225 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zheng, N.; Chen, L.; Xie, L.; Cui, M.; Li, S.; Xu, L. Effect of Shape on Mesoporous Silica Nanoparticles for Oral Delivery of Indomethacin. Pharmaceutics 2019, 11, 4. https://doi.org/10.3390/pharmaceutics11010004
Zhang W, Zheng N, Chen L, Xie L, Cui M, Li S, Xu L. Effect of Shape on Mesoporous Silica Nanoparticles for Oral Delivery of Indomethacin. Pharmaceutics. 2019; 11(1):4. https://doi.org/10.3390/pharmaceutics11010004
Chicago/Turabian StyleZhang, Wei, Nan Zheng, Lu Chen, Luyao Xie, Mingshu Cui, Sanming Li, and Lu Xu. 2019. "Effect of Shape on Mesoporous Silica Nanoparticles for Oral Delivery of Indomethacin" Pharmaceutics 11, no. 1: 4. https://doi.org/10.3390/pharmaceutics11010004
APA StyleZhang, W., Zheng, N., Chen, L., Xie, L., Cui, M., Li, S., & Xu, L. (2019). Effect of Shape on Mesoporous Silica Nanoparticles for Oral Delivery of Indomethacin. Pharmaceutics, 11(1), 4. https://doi.org/10.3390/pharmaceutics11010004