Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy
Abstract
:1. Introduction
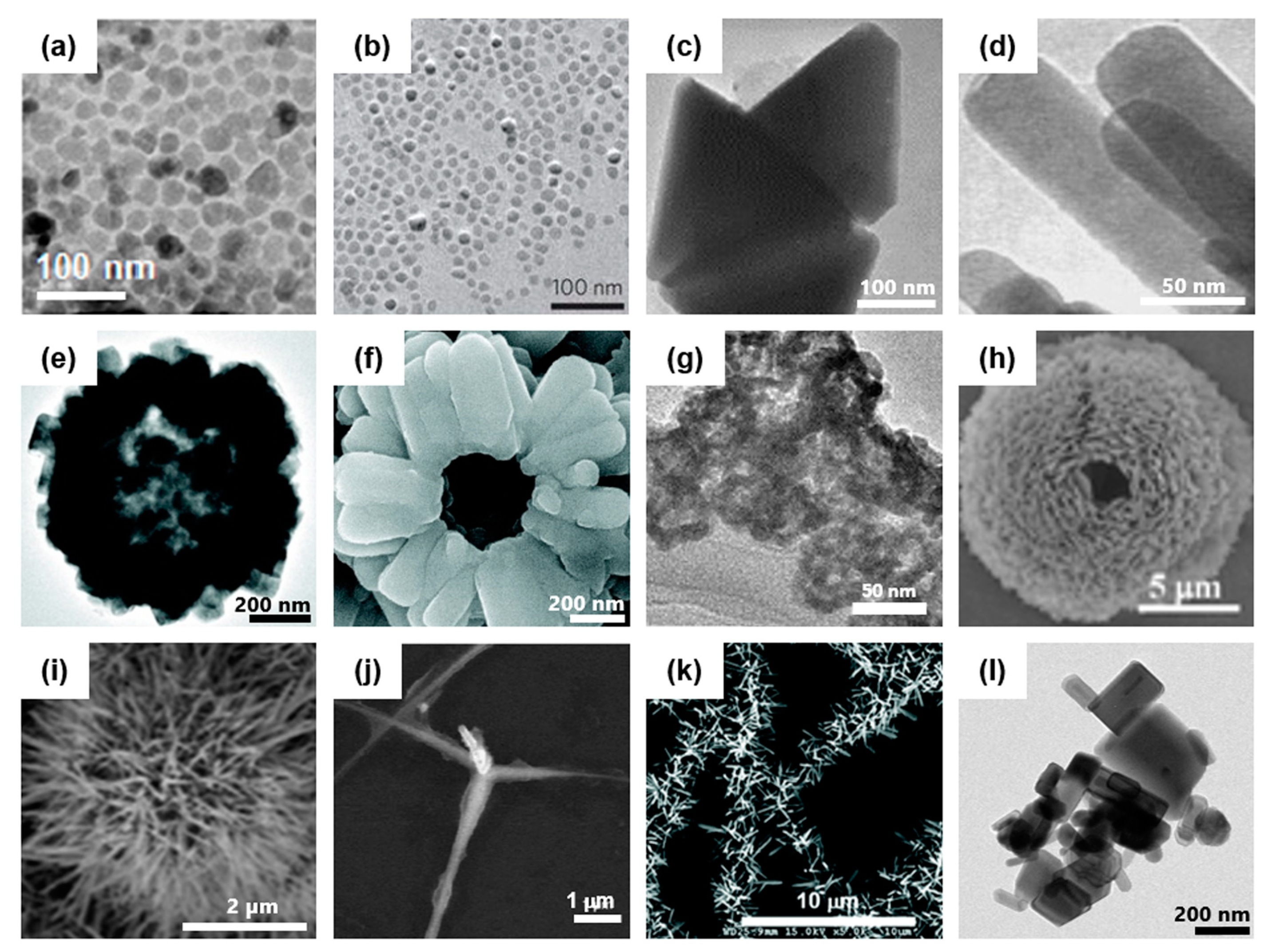
1.1. Synthesis of ZnO Nanostructure
1.2. History of ZnO Application in Biomedical Fields
2. Interactions of ZnO Particles with Immune Cells
2.1. ZnO Uptake by Immune Cells
2.2. Stimulation of Toll-Like Receptors (TLRs)
2.3. Zn2+ Dissolution and ROS Generation
2.4. Induction of Proinflammatory Cytokines and Costimulatory Molecules in Antigen-Presenting Cells
3. Toxicity of ZnO Nanocomposites In Vivo
3.1. In Vivo Toxicity by Systemically Administered ZnO Nanocomposites
3.2. In Vivo Toxicity by Locally Administered ZnO Nanocomposites
3.3. Selective Toxicity to Cancer Cells by ZnO Nanocomposites
4. Application of ZnO Nanocomposites for Vaccines and Immunotherapy
4.1. ZnO Nanocomposites as an Antigen Delivery System
4.2. Induction of Antigen-Specific Adaptive Immunity by ZnO Nanocomposites
4.3. Generation of Protective Immunity against Infections and Cancers by ZnO Nanocomposites
4.4. Remaining Challenges
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhao, X.; Hu, H.; Qiao, M.; Deng, Y.; Chen, D. Nanoparticles for tumor immunotherapy. Eur. J. Pharm. Biopharm. 2017, 115, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R. Immunotherapy of autoimmunity and cancer: The penalty for success. Nat. Rev. Immunol. 2008, 8, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Touchette, N.A.; Folkers, G.K. Emerging infectious diseases: A 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerg. Infect. Dis. 2005, 11, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wu, T.; Bao, Y.; Zhang, Z. Nanotechnology based therapeutic modality to boost anti-tumor immunity and collapse tumor defense. J. Control. Release 2017, 256, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.Y.; Shin, H.M.; Sharma, P.; Cho, H.A.; Min, C.K.; Kim, H.I.; Yen, N.T.; Kang, J.S.; Kim, I.S.; Choi, M.S.; et al. Generation of protective immunity against Orientia tsutsugamushi infection by immunization with a zinc oxide nanoparticle combined with ScaA antigen. J. Nanobiotechnol. 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Cheong, T.C.; Min, J.H.; Wu, J.H.; Lee, S.J.; Kim, D.; Yang, J.S.; Kim, S.; Kim, Y.K.; Seong, S.Y. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat. Nanotechnol. 2011, 6, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Agelidis, A.; Koujah, L.; Suryawanshi, R.; Yadavalli, T.; Adelung, R.; Mishra, Y.K. An Intra-Vaginal Zinc Oxide Tetrapod Nanoparticles (ZOTEN) and Genital Herpesvirus Cocktail Can Provide a Novel Platform for Live Virus Vaccine. Front. Immunol. 2019, 10, 500. [Google Scholar] [Green Version]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced Nanobiomaterials: Vaccines, Diagnosis and Treatment of Infectious Diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.M.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development. Front. Immunol. 2017, 8, 239. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Mandal, G.; Dass, R.; Isore, D.; Garg, A.; Ram, G. Effect of zinc supplementation from two sources on growth, nutrient utilization and immune response in male crossbred cattle (Bos indicus× Bos taurus) bulls. Anim. Feed. Sci. Technol. 2007, 138, 1–12. [Google Scholar] [CrossRef]
- Chang, H.; Ho, C.-C.; Yang, C.S.; Chang, W.-H.; Tsai, M.-H.; Tsai, H.-T.; Lin, P. Involvement of MyD88 in zinc oxide nanoparticle-induced lung inflammation. Exp. Toxicol. Pathol. 2013, 65, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Shen, C.C.; Cheng, Y.W.; Huang, S.H.; Wu, C.C.; Kao, C.C.; Liao, J.W.; Kang, J.J. Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxide nanoparticles in mice. Nanotoxicology 2012, 6, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Yoon, J.H.; Kang, B.C.; Cho, N.H.; Seok, S.H.; Min, S.K.; Min, J.H.; Che, J.H.; Kim, Y.K. The toxicity and distribution of iron oxide–zinc oxide core-shell nanoparticles in C57BL/6 mice after repeated subcutaneous administration. J. Appl. Toxicol. 2015, 35, 593–602. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Kim, P.; Jo, E.; Kim, H.M.; Lee, M.Y.; Jin, S.M.; Park, K. Toxicity of zinc oxide nanoparticles in rats treated by two different routes: Single intravenous injection and single oral administration. J. Toxicol. Environ. Health A 2015, 78, 226–243. [Google Scholar] [CrossRef]
- James, S.A.; Feltis, B.N.; de Jonge, M.D.; Sridhar, M.; Kimpton, J.A.; Altissimo, M.; Mayo, S.; Zheng, C.; Hastings, A.; Howard, D.L. Quantification of ZnO nanoparticle uptake, distribution, and dissolution within individual human macrophages. ACS Nano 2013, 7, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Kumar, D.; Sharma, A.; Gupta, P.; Chaudhari, B.P.; Tripathi, A.; Das, M.; Dwivedi, P.D. ZnO nanoparticles induced adjuvant effect via toll-like receptors and Src signaling in Balb/c mice. Toxicol. Lett. 2014, 230, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Kumar, S.; Verma, A.K.; Sharma, A.; Chaudhari, B.P.; Tripathi, A.; Das, M.; Dwivedi, P.D. Zinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a Th2 response in Balb/c mice. Int. Immunol. 2013, 26, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Tsuji, N.M. Hollow ZnO Nanospheres Enhance Anticancer Immunity by Promoting CD4(+) and CD8(+) T Cell Populations In Vivo. Small 2017, 13, 1701816. [Google Scholar] [CrossRef] [PubMed]
- Afroz, S.; Medhi, H.; Maity, S.; Minhas, G.; Battu, S.; Giddaluru, J.; Kumar, K.; Paik, P.; Khan, N. Mesoporous ZnO nanocapsules for the induction of enhanced antigen-specific immunological responses. Nanoscale 2017, 9, 14641–14653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Shin, J.B.; Park, B.C.; Lee, J.W.; Byun, S.W.; Jang, N.Y.; Kim, Y.J.; Kim, Y.; Kim, Y.K.; Cho, N.H. Application of radially grown ZnO nanowires on poly-l-lactide microfibers complexed with a tumor antigen for cancer immunotherapy. Nanoscale 2019, 11, 4591–4600. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Synthesis and Growth of ZnO Nanoparticles. J. Phys. Chem. B 1998, 5566–5572. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor Clusters in the Sol-Gel Process: Quantized Aggregation, Gelation, and Crystal Growth in Concentrated ZnO Colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Joo, J.; Chow, B.Y.; Prakash, M.; Boyden, E.S.; Jacobson, J.M. Face-selective electrostatic control of hydrothermal zinc oxide nanowire synthesis. Nat. Mater. 2011, 10, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Dong, W.; Keeter-Brewer, M.; Konar, S.; Njabon, R.N.; Tian, Z.R. Site-specific Nucleation and Growth Kinetics in Hierarchial Nanosyntheses of Branched ZnO Crystallites. J. Am. Chem. Soc. 2006, 128, 10960–10968. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Melosh, N.A. Fabrication of sub-cell size “spiky” nanoparticles and their interfaces with biological cells. J. Mater. Chem. B 2015, 3, 5155–5160. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Nutting, J. 2000 years of zinc and brass. Int. Mater. Rev. 1991, 36, 81–84. [Google Scholar] [CrossRef]
- Oleson, J.P. The Oxford Handbook of Engineering and Technology in the Classical World; Oxford University Press: Oxford, UK, 2010; p. 112. [Google Scholar]
- Craddock, P.T. The origins and inspirations of zinc smelting. J. Mater. Sci. 2009, 44, 2181–2191. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- Craddock, P.T.; Freestone, I.; Gurjar, L.; Middleton, A.; Wilkes, L. Zinc in India; British Museum Publications Ltd.: London, UK, 1998; pp. 29–72. [Google Scholar]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Xiong, H.M.; Xu, Y.; Ren, Q.G.; Xia, Y.Y. Stable aqueous ZnO@polymer core-shell nanoparticles with tunable photoluminescence and their application in cell imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef] [PubMed]
- Yearian, H. Intensity of diffraction of electrons by ZnO. Phys. Rev. 1935, 48, 631. [Google Scholar] [CrossRef]
- Hong, H.; Shi, J.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Wang, X.; Cai, W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano. Lett. 2011, 11, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Gao, L.; Yan, X.; Wang, T. Functionalized tetrapod-like ZnO nanostructures for plasmid DNA purification, polymerase chain reaction and delivery. Nanotechnology 2006, 18, 015101. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xu, Y.D.; Ma, Y.Y.; Qiu, L.L.; Wang, Y.; Kong, J.L.; Xiong, H.M. Biodegradable ZnO@ polymer core–shell nanocarriers: pH-triggered release of doxorubicin in vitro. Angew. Chem. Int. Ed. 2013, 52, 4127–4131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Cho, H.A.; Lee, J.W.; Ham, W.S.; Park, B.C.; Cho, N.H.; Kim, Y.K. Efficient intracellular delivery of biomacromolecules employing clusters of zinc oxide nanowires. Nanoscale 2017, 9, 15371–15378. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Gao, L.; Feng, P.; Zhang, J.; Fu, X.; Liu, Y.; Yan, X.; Wang, T. Three-Dimensional Functionalized Tetrapod-like ZnO Nanostructures for Plasmid DNA Delivery. Small 2006, 2, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Antoine, T.E.; Hadigal, S.R.; Yakoub, A.M.; Mishra, Y.K.; Bhattacharya, P.; Haddad, C.; Valyi-Nagy, T.; Adelung, R.; Prabhakar, B.S.; Shukla, D. Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents against Genital Herpes. J. Immunol. 2016, 196, 4566–4575. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, T.; Shukla, D. Could zinc oxide tetrapod nanoparticles be used as an effective immunotherapy against HSV-2? Future Med. 2016, 11, 2239–2242. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.W.; Wu, H.P.; Ge, M.Y.; Yang, L.; Zeng, Y.W.; Wang, Y.W.; Jiang, J.Z. Triangle-shape ZnO prepared by thermal decomposition. Mater. Lett. 2007, 61, 3416–3420. [Google Scholar] [CrossRef]
- Shabannia, R. Vertically aligned ZnO nanorods on porous silicon substrates: Effect of growth time. Prog. Nat. Sci. Mater. Int. 2015, 25, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gao, L. A new route toward ZnO hollow spheres by a base-erosion mechanism. Cryst. Growth Des. 2008, 8, 460–464. [Google Scholar] [CrossRef]
- Pan, J.H.; Zhang, X.; Du, A.J.; Bai, H.; Ng, J.; Sun, D. A hierarchically assembled mesoporous ZnO hemisphere array and hollow microspheres for photocatalytic membrane water filtration. Phys. Chem. Chem. Phys. 2012, 14, 7481–7489. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Chang, L.W.; Lin, P. Metal-based nanoparticles and the immune system: Activation, inflammation, and potential applications. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Parashar, V.; Chauhan, L.K.; Shanker, R.; Das, M.; Tripathi, A.; Dwivedi, P.D. Mechanism of uptake of ZnO nanoparticles and inflammatory responses in macrophages require PI3K mediated MAPKs signaling. Toxicol. In Vitro 2014, 28, 457–467. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.; Vijayaraghavan, R. Size-dependent effect of zinc oxide on toxicity and inflammatory potential of human monocytes. J. Toxicol. Environ. Health 2014, 77, 177–191. [Google Scholar] [CrossRef]
- Roy, R.; Das, M.; Dwivedi, P.D. Toxicological mode of action of ZnO nanoparticles: Impact on immune cells. Mol. Immunol. 2015, 63, 184–192. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Huang, K.L.; Lee, Y.H.; Chen, H.I.; Liao, H.S.; Chiang, B.L.; Cheng, T.J. Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. J. Hazard. Mater. 2015, 297, 304–312. [Google Scholar] [CrossRef]
- Mohamud, R.; Xiang, S.D.; Selomulya, C.; Rolland, J.M.; O’Hehir, R.E.; Hardy, C.L.; Plebanski, M. The effects of engineered nanoparticles on pulmonary immune homeostasis. Drug Metab. Rev. 2014, 46, 176–190. [Google Scholar] [CrossRef]
- Simon-Vazquez, R.; Lozano-Fernandez, T.; Davila-Grana, A.; Gonzalez-Fernandez, A. Analysis of the activation routes induced by different metal oxide nanoparticles on human lung epithelial cells. Future Sci. OA 2016, 2, FSO118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Roy, R.; Singh, S.K.; Das, M.; Tripathi, A.; Dwivedi, P.D. Toll-like receptor 6 mediated inflammatory and functional responses of zinc oxide nanoparticles primed macrophages. Immunology 2014, 142, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Choy, J.H. Biokinetics of zinc oxide nanoparticles: Toxicokinetics, biological fates, and protein interaction. Int. J. Nanomed. 2014, 9, 261–269. [Google Scholar] [CrossRef]
- Simon-Vazquez, R.; Lozano-Fernandez, T.; Davila-Grana, A.; Gonzalez-Fernandez, A. Metal oxide nanoparticles interact with immune cells and activate different cellular responses. Int. J. Nanomed. 2016, 11, 4657–4668. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Zhang, F.; Chen, D.; Ding, W. ZnO nanoparticle-induced oxidative stress triggers apoptosis by activating JNK signaling pathway in cultured primary astrocytes. Nanoscale Res. Lett. 2014, 9, 117. [Google Scholar] [CrossRef]
- Scherzad, A.; Meyer, T.; Kleinsasser, N.; Hackenberg, S. Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity Short Running Title: Genotoxicity of ZnO NPs. Materials 2017, 10, 1427. [Google Scholar] [CrossRef]
- Shen, C.; James, S.A.; de Jonge, M.D.; Turney, T.W.; Wright, P.F.; Feltis, B.N. Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle-exposed human immune cells. Toxicol. Sci. 2013, 136, 120–130. [Google Scholar] [CrossRef]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef]
- Matsumura, M.; Nagata, M.; Nakamura, K.; Kawai, M.; Baba, T.; Yamaki, K.; Yoshino, S. Adjuvant effect of zinc oxide on Th2 but not Th1 immune responses in mice. Immunopharmacol. Immunotoxicol. 2010, 32, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Palomäki, J.; Karisola, P.; Pylkkänen, L.; Savolainen, K.; Alenius, H. Engineered nanomaterials cause cytotoxicity and activation on mouse antigen presenting cells. Toxicology 2010, 267, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Fernandez, T.; Ballester-Antxordoki, L.; Perez-Temprano, N.; Rojas, E.; Sanz, D.; Iglesias-Gaspar, M.; Moya, S.; Gonzalez-Fernandez, A.; Rey, M. Potential impact of metal oxide nanoparticles on the immune system: The role of integrins, L-selectin and the chemokine receptor CXCR4. Nanomedicine 2014, 10, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhao, X.; Tan, E.C.; Khamis, N.; Assodani, A.; Xiong, S.; Ruedl, C.; Ng, K.W.; Loo, J.S. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch. Toxicol. 2011, 85, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, M.; Moosavi, M.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 2699–2711. [Google Scholar] [CrossRef]
- Müller, K.H.; Kulkarni, J.; Motskin, M.; Goode, A.; Winship, P.; Skepper, J.N.; Ryan, M.P.; Porter, A.E. PH-dependent toxicity of high aspect ratio ZnO nanowires in macrophages due to intracellular dissolution. ACS Nano 2010, 4, 6767–6779. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. In Vitro 2011, 25, 657–663. [Google Scholar] [CrossRef]
- Pandurangan, M.; Kim, D.H. In vitro toxicity of zinc oxide nanoparticles: A review. J. Nanopart. Res. 2015, 17, 158. [Google Scholar] [CrossRef]
- Elshama, S.S.; Abdallah, M.E.; Abdel-Karim, R.I. Zinc Oxide Nanoparticles: Therapeutic Benefits and Toxicological Hazards. Open Nanomed. J. 2018, 5. [Google Scholar] [CrossRef]
- Hong, T.K.; Tripathy, N.; Son, H.J.; Ha, K.T.; Jeong, H.S.; Hahn, Y.B. A comprehensive in vitro and in vivo study of ZnO nanoparticles toxicity. J. Mater. Chem. B 2013, 1, 2985–2992. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hevia, L.; Valiente, R.; Martin-Rodriguez, R.; Renero-Lecuna, C.; Gonzalez, J.; Rodriguez-Fernandez, L.; Aguado, F.; Villegas, J.C.; Fanarraga, M.L. Nano-ZnO leads to tubulin macrotube assembly and actin bundling, triggering cytoskeletal catastrophe and cell necrosis. Nanoscale 2016, 8, 10963–10973. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.Y.; Chen, Y.C.; Cheng, T.J.; Chiung, Y.M.; Liu, P.S. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol. Sci. 2012, 125, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Fraietta, J.A.; Gracias, D.T.; Hope, J.L.; Stairiker, C.J.; Patel, P.R.; Mueller, Y.M.; McHugh, M.D.; Jablonowski, L.J.; Wheatley, M.A.; et al. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology 2015, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Biological reactivity of zinc oxide nanoparticles with mammalian test systems: An overview. Nanomedicine 2015, 10, 2075–2092. [Google Scholar] [CrossRef] [PubMed]
- Toduka, Y.; Toyooka, T.; Ibuki, Y. Flow Cytometric Evaluation of Nanoparticles Using Side-Scattered Light and Reactive Oxygen Species-Mediated Fluorescence-Correlation with Genotoxicity. Environ. Sci. Technol. 2012, 46, 7629–7636. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Gaiser, B.K.; Hutchison, G.R.; Stone, V. An in vitro liver model--assessing oxidative stress and genotoxicity following exposure of hepatocytes to a panel of engineered nanomaterials. Part. Fibre Toxicol. 2012, 9, 28. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson-Willman, B.; Gehrmann, U.; Cansu, Z.; Buerki-Thurnherr, T.; Krug, H.F.; Gabrielsson, S.; Scheynius, A. Effects of subtoxic concentrations of TiO2 and ZnO nanoparticles on human lymphocytes, dendritic cells and exosome production. Toxicol. Appl. Pharmacol. 2012, 264, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Aula, S.; Lakkireddy, S.; Kapley, A.; Adimadhyam, V.N.; Sharma, R.K.; Uppin, S.G.; Jamil, K. Route of administration induced in vivo effects and toxicity responses of Zinc Oxide nanorods at molecular and genetic levels. Int. J. Nano Dimens. 2018, 9, 158–169. [Google Scholar]
- Karmakar, A.; Zhang, Q.; Zhang, Y. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014, 22, 147–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Tian, Y.; Zhang, T.; Ren, G.; Yang, Z. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int. J. Nanomed. 2011, 6, 1453–1461. [Google Scholar] [CrossRef]
- Tian, L.; Lin, B.; Wu, L.; Li, K.; Liu, H.; Yan, J.; Liu, X.; Xi, Z. Neurotoxicity induced by zinc oxide nanoparticles: Age-related differences and interaction. Sci Rep. 2015, 5, 16117. [Google Scholar] [CrossRef] [PubMed]
- Ashajyothi, C.; Handral, H.K.; Kelmani, R.C. A Comparative In Vivo Scrutiny of Biosynthesized Copper and Zinc Oxide Nanoparticles by Intraperitoneal and Intravenous Administration Routes in Rats. Nanoscale Res. Lett. 2018, 13, 93. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation. Int. J. Mol. Sci. 2016, 17, 1241. [Google Scholar] [CrossRef]
- Beckett, W.S.; Chalupa, D.F.; Pauly-Brown, A.; Speers, D.M.; Stewart, J.C.; Frampton, M.W.; Utell, M.J.; Huang, L.S.; Cox, C.; Zareba, W.; et al. Comparing inhaled ultrafine versus fine zinc oxide particles in healthy adults: A human inhalation study. Am. J. Respir. Crit. Care Med. 2005, 171, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.R.; Stoeger, T.; van den Brule, S.; Saber, A.T.; Beyerle, A.; Vietti, G.; Mortensen, A.; Szarek, J.; Budtz, H.C.; Kermanizadeh, A.; et al. Acute and subacute pulmonary toxicity and mortality in mice after intratracheal instillation of ZnO nanoparticles in three laboratories. Food Chem. Toxicol. 2015, 85, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Bisht, G.; Rayamajhi, S. ZnO nanoparticles: A promising anticancer agent. Nanobiomedicine 2016, 3, 3–9. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845. [Google Scholar]
- Hu, Y.; Zhang, H.R.; Dong, L.; Xu, M.R.; Zhang, L.; Ding, W.P.; Zhang, J.Q.; Lin, J.; Zhang, Y.J.; Qiu, B.S.; et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 2019, 11, 11789–11807. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Smith, J.D.; Morton, L.D.; Ulery, B.D. Nanoparticles as synthetic vaccines. Curr. Opin. Biotechnol. 2015, 34, 217–224. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Gerner, M.Y.; Casey, K.A.; Kastenmuller, W.; Germain, R.N. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J. Exp. Med. 2017, 214, 3105–3122. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.N.; Yoon, T.J.; Minai-Tehrani, A.; Kim, J.E.; Park, S.J.; Jeong, M.S.; Ha, S.W.; Lee, J.K.; Kim, J.S.; Cho, M.H. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol. Vitro 2013, 27, 1187–1195. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+) T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Tsuji, N.M.; Ohno, T. Biodegradable Metal Ion-Doped Mesoporous Silica Nanospheres Stimulate Anticancer Th1 Immune Response in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 43538–43544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Ramani, M.; Mudge, M.C.; Morris, R.T.; Zhang, Y.; Warcholek, S.A.; Hurst, M.N.; Riviere, J.E.; DeLong, R.K. Zinc oxide nanoparticle–poly I: C RNA complexes: Implication as therapeutics against experimental melanoma. Mol. Pharm. 2017, 14, 614–625. [Google Scholar] [CrossRef] [PubMed]


| ZnO Nanocomposites | Disease/Antigen * | Host Mouse | Vaccination Route | Biological Responses ** | Reference |
|---|---|---|---|---|---|
| ZnO NP | N.A./OVA | DBA/1J | Intraperitoneal | Increase in IL-4, IL-5, and IL-17; increase in IgG1 and IgE | [73] |
| Fe3O4-ZnO coreshell NP | Cancer/CEA | C57BL/6 | Subcutaneous (DCs) | Enhanced IFN-γ+ CD4 and CD8 T cells; delayed tumor growth | [8] |
| ZnO NP | N.A./OVA | BALB/c | Intraperitoneal | Increased inflammation in intestine | [22] |
| ZnO tetrapod (ZOTEN) | HSV2/HSV2 | BALB/c | Intravaginal | Enhance T cell and Ab responses; decreased mortality | [50] |
| ZnO NP | Scrub typhus/ScaA | C57BL/6 | Subcutaneous | Enhanced IFN-γ+ CD4 and CD8 T cells; protective immunity | [7] |
| ZnO NP/poly(I:C) | Cancer/N.A. | BALB/c | Intratumoral | Suppress tumor growth | [116] |
| Hollow ZnO NP | Cancer/aTA | C57BL/6J | Subcutaneous | Enhanced CD4 and CD8 T cells; delayed tumor growth | [114] |
| Mesophorous ZnO NP | N.A./OVA | BALB/c | Subcutaneous | Enhanced IFN-γ+ CD4 and CD8 T cells, elevated IgG2 | [25] |
| ZnO NWs on PLLA fiber | Cancer/CEA | C57BL/6 | Subcutaneous | Enhanced IFN-γ+ CD4 and CD8 T cells, delayed tumor growth | [26] |
| ZnO tetrapod (ZOTEN) | HSV2/HSV2 | BALB/c | Intravaginal | Blocks viral shedding and reduced inflammation | [9] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Jang, N.-Y.; Lee, J.-W.; Park, B.C.; Kim, Y.K.; Cho, N.-H. Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy. Pharmaceutics 2019, 11, 493. https://doi.org/10.3390/pharmaceutics11100493
Sharma P, Jang N-Y, Lee J-W, Park BC, Kim YK, Cho N-H. Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy. Pharmaceutics. 2019; 11(10):493. https://doi.org/10.3390/pharmaceutics11100493
Chicago/Turabian StyleSharma, Prashant, Na-Yoon Jang, Jae-Won Lee, Bum Chul Park, Young Keun Kim, and Nam-Hyuk Cho. 2019. "Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy" Pharmaceutics 11, no. 10: 493. https://doi.org/10.3390/pharmaceutics11100493
APA StyleSharma, P., Jang, N.-Y., Lee, J.-W., Park, B. C., Kim, Y. K., & Cho, N.-H. (2019). Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy. Pharmaceutics, 11(10), 493. https://doi.org/10.3390/pharmaceutics11100493





