Influence of Short Cationic Lipopeptides with Fatty Acids of Different Chain Lengths on Bacterial Biofilms Formed on Polystyrene and Hydrogel Surfaces
Abstract
:1. Introduction
2. Materials and Methods
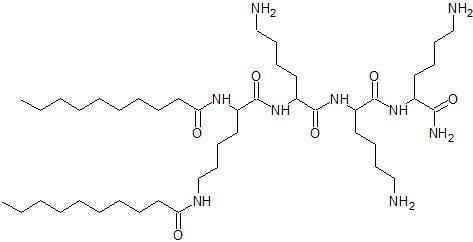
2.1. Peptide Synthesis
2.2. Bacterial Strains and Media
2.3. Antimicrobial Activities of Lipopeptides and Conventional Antimicrobials
2.3.1. Minimum Inhibitory Concentration (MIC) Assay
2.3.2. Minimum Biofilm Formation Inhibitory Concentration (MBFIC) Assay
2.3.3. Minimum Biofilm Eradication Concentration (MBEC) Assay
3. Results
3.1. MIC of Lipopeptides
3.2. MBFIC of Lipopeptides
3.3. MBEC of Lipopeptides
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AMPs | Antimicrobial peptides |
| Boc | tert-butyloxycarbonyl |
| ATCC | American Type Culture Collection |
| C | Cysteine residue |
| CL | Contact lenses |
| CLSI | Clinical and Laboratory Standards Institute |
| DCM | Dichloromethane |
| DIC | N,N′-diisopropylcarbodiimide |
| DMF | N,N-dimethylformamide |
| EC | Escherichia coli |
| EF | Enterococcus faecalis |
| ESI-MS | Electrospray-ionization mass spectrometry |
| Fmoc | 9-Fluorenylmethoxycarbonyl group |
| G | Glycine residue |
| K | Lysine residue |
| KP | Klebsiella pneumoniae |
| MBEC | Minimum biofilm eradication concentration |
| MBFIC | Minimum biofilm formation inhibitory concentration |
| MIC | Minimum inhibitory concentration |
| PA | Pseudomonas aeruginosa |
| PBS | Phosphate-buffer saline |
| PM | Proteus mirabilis |
| PS | Polystyrene plates |
| R | Arginine residue |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
| SA | Staphylococcus aureus |
| SE | Staphylococcus epidermidis |
| SPPS | Solid-phase peptide synthesis |
| SV | Streptococcus viridans |
| TFA | Trifluoroacetic acid |
| TIS | Triisopropylsilane |
References
- Hasan, A.; Waibhaw, G.; Tiwari, S.; Dharmalingam, K.; Shukla, I.; Pandey, L.M. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J. Biomed. Mater. Res. Part A 2017, 105, 2391–2404.2. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Boil. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.H.A.; Sileika, T.S.; Park, S.H.; Sousa, A.M.; Burch, P.; Szleifer, I.; Messersmith, P.B. Molecular Design of Antifouling Polymer Brushes Using Sequence-Specific Peptoids. Adv. Mater. Interfaces 2015, 2, 1400225. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.H.A.; Ren, C.; Park, S.H.; Szleifer, I.; Messersmith, P.B. An Experimental–Theoretical Analysis of Protein Adsorption on Peptidomimetic Polymer Brushes. Langmuir 2012, 28, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Pattanayek, S.K.; Pandey, L.M. Effect of Functional Groups of Self-Assembled Monolayers on Protein Adsorption and Initial Cell Adhesion. ACS Biomater. Sci. Eng. 2018, 4, 3224–3233. [Google Scholar] [CrossRef]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Riool, M.; de Breij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Antimicrobial Peptides in Biomedical Device Manufacturing. Front Chem. 2017, 5, 63. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- O’Gara, J.P.; Humphreys, H. Staphylococcus epidermidis biofilms: importance and implications. J. Med. Microbiol. 2001, 50, 582–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, W. Infection and musculoskeletal conditions: Prosthetic-joint-associated infections. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1045–1063. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Debeer, D.; Caldwell, D.; Korber, D.; James, G. Biofilms, the customized microniche. J. Bacteriol. 1994, 176, 2137–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, M. Staphylococcal biofilms. Curr. Top Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [PubMed]
- Otto, M. Staphylococcus epidermidis-the “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Lund, B.; Agvald-Öhman, C.; Hultberg, A.; Edlund, C. Frequent Transmission of Enterococcal Strains between Mechanically Ventilated Patients Treated at an Intensive Care Unit. J. Clin. Microbiol. 2002, 40, 2084–2088. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Baldassarri, L.; Campoccia, D.; Creti, R.; Pirini, V.; Huebner, J.; Montanaro, L. Strong biofilm production, antibiotic multi-resistance and high gelE expression in epidemic clones of Enterococcus faecalis from orthopaedic implant infections. Biomaterials 2008, 29, 580–586. [Google Scholar] [CrossRef]
- Chatterjee, S.; Maiti, P.; Kundu, A.; Dey, R. Biofilms on Indwelling Urologic Devices: Microbes and Antimicrobial Management Prospect. Ann. Med. Heal. Sci. Res. 2014, 4, 100–104. [Google Scholar]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Dawgul, M.; Maciejewska, M.; Jaskiewicz, M.; Karafova, A.; Kamysz, W. Antimicrobial peptides as potential tool to fight bacterial biofilm. Acta Pol. Pharm. 2014, 71, 39–47. [Google Scholar]
- Pletzer, D.; Wolfmeier, H.; Bains, M.; Hancock, R.E.W. Synthetic Peptides to Target Stringent Response-Controlled Virulence in a Pseudomonas aeruginosa Murine Cutaneous Infection Model. Front. Microbiol. 2017, 8, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, A.M. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009, 41, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Hell, E.; Giske, C.G.; Nelson, A.; Römling, U.; Marchini, G. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett. Appl. Microbiol. 2010, 50, 211–215. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Nunez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef]
- De la Fuente-Nunez, C.; Reffuveille, F.; Mansour, S.C.; Reckseidler-Zenteno, S.L.; Hernández, D.; Brackman, G.; Coenye, T.; Hancock, R.E. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 2015, 22, 196–205. [Google Scholar] [PubMed]
- Avrahami, D.; Shai, Y. A New Group of Antifungal and Antibacterial Lipopeptides Derived from Non-membrane Active Peptides Conjugated to Palmitic Acid. J. Boil. Chem. 2004, 279, 12277–12285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avrahami, D.; Shai, Y. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to D,L-amino acid-containing antimicrobial peptides: A plausible mode of action. Biochemistry 2003, 42, 14946–14956. [Google Scholar] [CrossRef]
- Makovitzki, A.; Baram, J.; Shai, Y. Antimicrobial lipopolypeptides composed of palmitoyl Di- and tricationic peptides: In vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 2008, 47, 10630–10636. [Google Scholar] [CrossRef]
- Hancock, R.E.; Straus, S.K. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: Comparison with cationic antimicrobial peptides and lipopeptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1215–1223. [Google Scholar] [Green Version]
- Mangoni, M.L.; Shai, Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. The Potential of Antimicrobial Peptides as Biocides. Int. J. Mol. Sci. 2011, 12, 6566–6596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaśkiewicz, M.; Neubauer, D.; Kamysz, W. Comparative study on antistaphylococcal activity of lipopeptides in various culture medi. Antibiotics 2017, 6, 1–11. [Google Scholar]
- Kamysz, W.; Silvestri, C.; Cirioni, O.; Giacometti, A.; Licci, A.; Della Vittoria, A.; Okroj, M.; Scalise, G. In vitro activities of the lipopeptides palmitoyl (Pal)-Lys-Lys-NH(2) and Pal-Lys-Lys alone and in combination with antimicrobial agents against multiresistant gram-positive cocci. Antimicrob. Agents Chemother. 2007, 51, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.N.; Zhanel, G.G.; Schweizer, F. Antibacterial activity of ultrashort cationic lipo-beta-peptides. Antimicrob. Agents Chemother. 2009, 53, 2215–2217. [Google Scholar] [CrossRef]
- Laverty, G.; McLaughlin, M.; Shaw, C.; Gorman, S.P.; Gilmore, B.F. Antimicrobial Activity of Short, Synthetic Cationic Lipopeptides. Chem. Boil. Drug Des. 2010, 75, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Dawgul, M.; Baranska-Rybak, W.; Kamysz, E.; Karafova, A.; Nowicki, R.; Kamysz, W. Activity of short lipopeptides and conventional antimicrobials against planktonic cells and biofilms formed by clinical strains ofStaphylococcus aureus. Futur. Med. Chem. 2012, 4, 1541–1551. [Google Scholar] [CrossRef]
- Greber, K.E.; Dawgul, M.; Kamysz, W.; Sawicki, W. Cationic Net Charge and Counter Ion Type as Antimicrobial Activity Determinant Factors of Short Lipopeptides. Front. Microbiol. 2017, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M.; Kamysz, W.; Sawicki, W.; Łukasiak, J. Biological and surface-active properties of double-chain cationic amino acid-based surfactants. Amino Acids 2014, 46, 1893–1898. [Google Scholar] [CrossRef]
- Wikler, M.A.; Hindler, J.F.; Cockerill, F.R.; Patel, J.B.; Bush, K.; Powell, M.; Dudley, M.N.; Turnidge, J.D.; Elopoulos, G.M.; Weinstein, M.P.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Paduszynska, M.A.; Maciejewska, M.; Greber, K.E.; Sawicki, W.; Kamysz, W. Antibacterial Activities of Lipopeptide (C10)2-KKKK-NH2 Applied Alone and in Combination with Lens Liquids to Fight Biofilms Formed on Polystyrene Surfaces and Contact Lenses. Int. J. Mol. Sci. 2019, 20, 393. [Google Scholar] [CrossRef]
- Bionda, N.; Fleeman, R.M.; De La Fuente-Núñez, C.; Rodriguez, M.C.; Reffuveille, F.; Shaw, L.N.; Pastar, I.; Davis, S.C.; Hancock, R.E.W.; Cudic, P. Identification of novel cyclic lipopeptides from a positional scanning combinatorial library with enhanced antibacterial and antibiofilm activities. Eur. J. Med. Chem. 2016, 108, 354–363. [Google Scholar] [CrossRef]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, P.; Grzywacz, D.; Kamysz, W.; Lourenço, A.; Pereira, M.O. Searching for new strategies against biofilm infections: Colistin-AMP combinations against Pseudomonas aeruginosa and Staphylococcus aureus single- and double-species biofilms. PLoS ONE 2017, 12, 0174654. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination Strategies to Enhance the Efficacy of Antimicrobial Peptides against Bacterial Biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Maccari, G.; Nifosì, R. Treatment of microbial biofilms in the post-antibiotic era: prophylactic and therapeutic use of antimicrobial peptides and their design by bioinformatics tools. Pathog. Dis. 2014, 70, 257–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdiero, E.; Lombardi, L.; Falanga, A.; Libralato, G.; Guida, M.; Carotenuto, R. Biofilms: Novel Strategies Based on Antimicrobial Peptides. Pharmaceutics 2019, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Park, Y.; Hahm, K.-S. The Role of Antimicrobial Peptides in Preventing Multidrug-Resistant Bacterial Infections and Biofilm Formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [Green Version]
- Pletzer, D.; Hancock, R.E.W. Antibiofilm Peptides: Potential as Broad-Spectrum Agents. J. Bacteriol. 2016, 198, 2572–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente-Nunez, C.; Cardoso, M.H.; de Souza Cândido, E.; Franco, O.L.; Hancock, R.E. Synthetic antibiofilm peptides. Biochim. Biophys. Acta. 2016, 1858, 1061–1069. [Google Scholar] [CrossRef]
- Lohan, S.; Cameotra, S.S.; Bisht, G.S. Systematic Study of Non-Natural Short Cationic Lipopeptides as Novel Broad-Spectrum Antimicrobial Agents. Chem. Boil. Drug Des. 2013, 82, 557–566. [Google Scholar] [CrossRef]
- Jacobsen, S.M.; Shirtliff, M.E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2011, 2, 460–465. [Google Scholar] [CrossRef]
- Kwiecinska-Pirog, J.; Skowron, K.; Bartczak, W.; Gospodarek-Komkowska, E. The Ciprofloxacin Impact on Biofilm Formation by Proteus Mirabilis and P. Vulgaris Strains. Jundishapur J. Microbiol. 2016, 9, 32656. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Boil. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining conditions for biofilm inhibition and eradication assays for Gram-positive clinical reference strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, A.E.; Sridhar, J.; Flynn, H.W.; Smiddy, W.E.; Albini, T.A.; Berrocal, A.M.; Forster, R.K.; Belin, P.J.; Miller, D. Endophthalmitis Caused by Enterococcus faecalis: Clinical Features, Antibiotic Sensitivities, and Outcomes. Am. J. Ophthalmol. 2014, 158, 1018–1023.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranska-Rybak, W.; Cirioni, O.; Dawgul, M.; Sokolowska-Wojdylo, M.; Naumiuk, L.; Szczerkowska-Dobosz, A.; Nowicki, R.; Roszkiewicz, J.; Kamysz, W. Activity of Antimicrobial Peptides and Conventional Antibiotics against Superantigen Positive Staphylococcus aureus Isolated from the Patients with Neoplastic and Inflammatory Erythrodermia. Chemother. Res. Pr. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Pollett, S.; Sakoulas, G.A. Current Perspective on Daptomycin for the Clinical Microbiologist. Clin. Microbiol. Rev. 2013, 26, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Dawgul, M.; Baranska-Rybak, W.; Piechowicz, L.; Bauer, M.; Neubauer, D.; Nowicki, R.; Kamysz, W. The Antistaphylococcal Activity of Citropin 1.1 and Temporin A against Planktonic Cells and Biofilms Formed by Isolates from Patients with Atopic Dermatitis: An Assessment of Their Potential to Induce Microbial Resistance Compared to Conventional Antimicrobials. Pharmaceuticals 2016, 9. [Google Scholar]
- Barańska-Rybak, W.; Pikula, M.; Dawgul, M.; Kamysz, W.; Trzonkowski, P.; Roszkiewicz, J. Safety profile of antimicrobial peptides: camel, citropin, protegrin, temporin a and lipopeptide on HaCaT keratinocytes. Acta Pol. Pharm. 2013, 70, 795–801. [Google Scholar]
- Malhotra, R.; Dhawan, B.; Garg, B.; Shankar, V.; Nag, T.C. A Comparison of Bacterial Adhesion and Biofilm Formation on Commonly Used Orthopaedic Metal Implant Materials: An In vitro Study. Indian J. Orthop. 2019, 53, 148–153. [Google Scholar]
- Schmidlin, P.R.; Müller, P.; Attin, T.; Wieland, M.; Hofer, D.; Guggenheim, B. Polyspecies biofilm formation on implant surfaces with different surface characteristics. J. Appl. Oral Sci. 2013, 21, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Song, C.W.; Jung, J.H.; Ahn, S.J.; Ferracane, J.L. The effects of surface roughness of composite resin on biofilm formation of Streptococcus mutans in the presence of saliva. Oper. Dent. 2012, 37, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Gkana, E.N.; Doulgeraki, A.I.; Chorianopoulos, N.G.; Nychas, G.-J.E. Anti-adhesion and Anti-biofilm Potential of Organosilane Nanoparticles against Foodborne Pathogens. Front. Microbiol. 2017, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Bonsaglia, E.C.R.; Silva, N.C.C.; Fernades, A., Jr.; Araújo, J.P., Jr.; Tsunemi, M.H.; Rall, V.L.M. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control 2014, 35, 386–391. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Breij, A.; Riool, M.; Kwakman, P.H.; de Boer, L.; Cordfunke, R.A.; Drijfhout, J.W.; Cohen, O.; Emanuel, N.; Zaat, S.A.; Nibbering, P.H.; et al. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J. Control Release 2016, 222, 1–8. [Google Scholar] [CrossRef]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riool, M.; de Breij, A.; de Boer, L.; Kwakman, P.H.S.; Cordfunke, R.A.; Cohen, O.; Malanovic, N.; Emanuel, N.; Lohner, K.; Drijfhout, J.W.; et al. Controlled release of LL-37-derived Synthetic Antimicrobial and Anti-Biofilm Peptides SAAP-145 and SAAP-276 prevents experimental biomaterial-associated Staphylococcus aureus infection. Adv. Funct. Mater. 2017, 27, 1606623. [Google Scholar]
- Arslan, S.Y.; Leung, K.P.; Wu, C.D. The effect of lactoferrin on oral bacterial attachment. Oral Microbiol. Immunol. 2009, 24, 411–416. [Google Scholar] [CrossRef]
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Shiba, K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling 2010, 26, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Willcox, M.; Hume, E.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Dutta, D.; Ozkan, J.; Willcox, M.D.P. Biocompatibility of antimicrobial melimine lenses: rabbit and human studies. Optom. Vis. Sci. 2014, 91, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Kamphuis, B.; Ozcelik, B.; Thissen, H.; Pinarbasi, R.; Kumar, N.; Willcox, M.D.P. Development of Silicone Hydrogel Antimicrobial Contact Lenses with Mel4 Peptide Coating. Optom. Vis. Sci. 2018, 95, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Shahrour, H.; Ferrer-Espada, R.; Dandache, I.; Bárcena-Varela, S.; Sánchez-Gómez, S.; Chokr, A.; Martínez-De-Tejada, G. AMPs as Anti-biofilm Agents for Human Therapy and Prophylaxis. Adv. Exp. Med. Biol. 2019, 1117, 257–279. [Google Scholar] [PubMed]
- Alves, D.; Magalhães, A.; Grzywacz, D.; Neubauer, D.; Kamysz, W.; Pereira, M.O. Co-immobilization of Palm and DNase I for the development of an effective anti-infective coating for catheter surfaces. Acta Biomater. 2016, 44, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Lipopeptide | Sequence | Average Mass (Da) | Net Charge | MS Analysis | HPLC Analysis | |||
|---|---|---|---|---|---|---|---|---|
| za | m/zb | m/zc | tR’ (min) | % ACN | ||||
| 1 | C12-KKC-NH2* | 1115.64 | +4 | 1 2 3 | 1115.78 558.39 372.60 | 1115.96 558.91 373.18 | 3.10 | 38.6 |
| 2 | C12-KR-NH2 | 483.70 | +2 | 1 2 | 484.40 242.70 | 484.68 243.03 | 2.32 | 33.9 |
| 3 | C14-KKC-NH2 * | 1171.75 | +4 | 1 2 3 | 1171.84 586.42 391.72 | 1172.18 586.79 391.77 | 3.88 | 43.3 |
| 4 | C14-KR-NH2 | 511.75 | +2 | 1 2 | 512.43 256.72 | 512.68 257.08 | 3.29 | 39.7 |
| 5 | C16-KGK-NH2 | 568.84 | +2 | 1 2 | 569.48 285.24 | 569.69 285.71 | 3.95 | 43.7 |
| 6 | C16-KKC-NH2 * | 1227.86 | +4 | 1 2 3 | 1227.90 614.46 409.97 | 1228.21 614.95 410.91 | 4.63 | 47.8 |
| 7 | C16-KKK-NH2 | 639.97 | +3 | 1 2 | 640.55 320.78 | 640.90 321.09 | 2.98 | 37.9 |
| 8 | C16-KR-NH2 | 539.81 | +2 | 1 2 | 540.46 270.73 | 540.68 271.00 | 4.06 | 44.3 |
| 9 | (C10)2-KKKK-NH2 | 838.23 | +3 | 1 2 3 | 838.69 419.85 280.23 | 838.94 420.28 280.68 | 3.27 | 39.6 |
| Bacterial Group | Species | Number |
|---|---|---|
| Gram-positive | Staphylococcus aureus | ATCC 25923 |
| Staphylococcus epidermidis | ATCC 14990 | |
| Enterococcus faecalis | ATCC 29212 | |
| Gram-negative | Escherichia coli | ATCC 25922 |
| Pseudomonas aeruginosa | ATCC 9029 | |
| Proteus mirabilis | PCM 543 |
| Ordinal Number | Lipopeptide | S. aureus | S. epidermidis | E. faecalis | E. coli | P. aeruginosa | P. mirabilis |
|---|---|---|---|---|---|---|---|
| 1 | C12-KKC-NH2 | 8 | 2 | 4 | 16 | 32 | >512 |
| 2 | C12-KR-NH2 | 64 | 32 | 64 | 512 | 512 | >512 |
| 3 | C14-KKC-NH2 | 32 | 4 | 16 | 64 | 128 | 512 |
| 4 | C14-KR-NH2 | 32 | 4 | 32 | 32 | 128 | 512 |
| 5 | C16-KGK-NH2 | 64 | 32 | 32 | 16 | 256 | 512 |
| 6 | C16-KKC-NH2 | 256 | 128 | 256 | 256 | 256 | >512 |
| 7 | C16-KKK-NH2 | 64 | 16 | 16 | 32 | 64 | 256 |
| 8 | C16-KR-NH2 | 32 | 8 | 64 | 64 | 128 | 512 |
| 9 | (C10)2-KKKK-NH2 | 8 | 2 | 16 | 16 | 16 | 512 |
| Ordinal Number | Lipopeptide | S. aureus | S. epidermidis | E. faecalis | E. coli | P. aeruginosa | P. mirabilis |
|---|---|---|---|---|---|---|---|
| PS/CL | PS/CL | PS/CL | PS/CL | PS/CL | PS/CL | ||
| 1 | C12-KKC-NH2 | 32/32 | 8/16 | 16/64 | 128/128 | 512/128 | >512/>512 |
| 2 | C12-KR-NH2 | 64/128 | 16/32 | 512/512 | 512/128 | 512/512 | >512/512 |
| 3 | C14-KKC-NH2 | 64/32 | 64/32 | 256/64 | 256/64 | 512/512 | 512/512 |
| 4 | C14-KR-NH2 | 16/2 | 32/32 | 128/64 | 128/128 | 256/128 | 512/>512 |
| 5 | C16-KGK-NH2 | 32/32 | 32/64 | 64/64 | 64/128 | 512/256 | >512/512 |
| 6 | C16-KKC-NH2 | 256/64 | 256/64 | 256/256 | 512/128 | 512/256 | >512/512 |
| 7 | C16-KKK-NH2 | 32/32 | 8/32 | 64/64 | 64/32 | 512/512 | 512/256 |
| 8 | C16-KR-NH2 | 8/16 | 16/32 | 64/64 | 64/64 | 512/128 | 512/512 |
| 9 | (C10)2-KKKK-NH2 | 8/16 | 8/16 | 64/64 | 128/64 | 64/64 | 512/512 |
| Ordinal Number | Lipopeptide | S. aureus | S. epidermidis | E. faecalis | E. coli | P. aeruginosa | P. mirabilis |
|---|---|---|---|---|---|---|---|
| PS/CL | PS/CL | PS/CL | PS/CL | PS/CL | PS/CL | ||
| 1 | C12-KKC-NH2 | 32/256 | 16/128 | 32/128 | 256/256 | >512/256 | >512/>512 |
| 2 | C12-KR-NH2 | 64/>512 | 16/256 | 256/128 | 256/512 | >512/>512 | >512/>512 |
| 3 | C14-KKC-NH2 | 128/128 | 128/512 | 256/256 | 512/512 | 256/>512 | 512/>512 |
| 4 | C14-KR-NH2 | 16/512 | 4/32 | 64/256 | 128/256 | >512/>512 | >512/>512 |
| 5 | C16-KGK-NH2 | 32/512 | 8/128 | 64/32 | 128/64 | >512/256 | >512/>512 |
| 6 | C16-KKC-NH2 | 256/512 | 256/256 | 512/512 | 512/512 | >512/>512 | >512/>512 |
| 7 | C16-KKK-NH2 | 32/256 | 8/64 | 64/16 | 64/128 | 512/>512 | 512/>512 |
| 8 | C16-KR-NH2 | 32/128 | 16/32 | 64/64 | 256/128 | >512/32 | 256/>512 |
| 9 | (C10)2-KKKK-NH2 | 64/128 | 16/64 | 32/128 | 64/128 | 512/256 | >512/>512 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paduszynska, M.A.; Maciejewska, M.; Neubauer, D.; Golacki, K.; Szymukowicz, M.; Bauer, M.; Kamysz, W. Influence of Short Cationic Lipopeptides with Fatty Acids of Different Chain Lengths on Bacterial Biofilms Formed on Polystyrene and Hydrogel Surfaces. Pharmaceutics 2019, 11, 506. https://doi.org/10.3390/pharmaceutics11100506
Paduszynska MA, Maciejewska M, Neubauer D, Golacki K, Szymukowicz M, Bauer M, Kamysz W. Influence of Short Cationic Lipopeptides with Fatty Acids of Different Chain Lengths on Bacterial Biofilms Formed on Polystyrene and Hydrogel Surfaces. Pharmaceutics. 2019; 11(10):506. https://doi.org/10.3390/pharmaceutics11100506
Chicago/Turabian StylePaduszynska, Malgorzata Anna, Magdalena Maciejewska, Damian Neubauer, Krzysztof Golacki, Magdalena Szymukowicz, Marta Bauer, and Wojciech Kamysz. 2019. "Influence of Short Cationic Lipopeptides with Fatty Acids of Different Chain Lengths on Bacterial Biofilms Formed on Polystyrene and Hydrogel Surfaces" Pharmaceutics 11, no. 10: 506. https://doi.org/10.3390/pharmaceutics11100506
APA StylePaduszynska, M. A., Maciejewska, M., Neubauer, D., Golacki, K., Szymukowicz, M., Bauer, M., & Kamysz, W. (2019). Influence of Short Cationic Lipopeptides with Fatty Acids of Different Chain Lengths on Bacterial Biofilms Formed on Polystyrene and Hydrogel Surfaces. Pharmaceutics, 11(10), 506. https://doi.org/10.3390/pharmaceutics11100506