Development of Folate-Functionalized PEGylated Zein Nanoparticles for Ligand-Directed Delivery of Paclitaxel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Folate-PEG
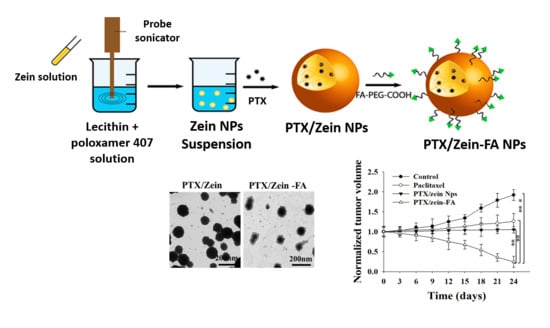
2.3. Preparation and Characterization of PTX/Zein-FA
2.4. Characterization of PTX/Zein-FA
2.4.1. Particle Size and Zeta Potential
2.4.2. Morphological Characterization
2.4.3. FTIR Analysis
2.4.4. X-ray Diffraction Analysis
2.4.5. Colloidal Stability
2.4.6. Encapsulation Efficiency and Loading Capacity
2.5. In Vitro Release Study
2.6. In Vitro Cytotoxicity
2.7. In Vitro Cellular Study
2.7.1. Intracellular Uptake Efficacy
2.7.2. Live Cell Analysis
2.7.3. Cell Cycle Analysis
2.7.4. Cell Migration Studies
2.7.5. Western Blot Analysis
2.8. In Vivo Imaging and Biodistribution Analysis
2.9. In Vivo Antitumor Efficacy
2.10. Histopathological Characterization
2.11. Statistical Analyses
3. Results and Discussion
3.1. Preparation and Characterization of PTX/Zein-FAs
3.2. In Vitro Cellular Assays for PTX/Zein-FA
3.3. In Vivo Imaging and Biodistribution Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| ABS | Acetate-buffered saline |
| AO | Acridine orange |
| DL | Drug loading |
| DLS | Dynamic light scattering |
| DMSO | dimethyl sulfoxide |
| DCC | N’,N’-dicyclohexyl carbodiimide |
| EE | Encapsulation efficiency |
| EDC | 1-ethyl-3-(3-dimethyl amino propyl) carbodiimide hydrochloride |
| FACS | Fluorescence-activated cell sorting |
| FDA | Food and Drug Administration |
| FTIR | Fourier-transform infrared spectroscopy |
| HPLC | High performance liquid chromatography |
| H&E | Hematoxylin and eosin staining |
| 1H NMR | proton nuclear magnetic resonance |
| LC | loading capacity |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NHS | N-hydroxy succinimide |
| PTX | Paclitaxel |
| PTX/Zein NPs | Paclitaxel loaded zein nanoparticles |
| PTX/Zein-FA | Paclitaxel loaded zein nanoparticles conjugated with folate |
| PBS | Phosphate-buffered saline |
| PEG | Polyethylene Glycol |
| PI | Propidium iodide |
| XRD | X-ray diffraction |
References
- Choi, Y.; Han, H. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Al-azzawi, S.; Masheta, D. Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. J. Pharm. Investig. 2019, 49, s40005–s40018. [Google Scholar] [CrossRef]
- Xue, X.; Wang, F.; Liu, X. Emerging functional nanomaterials for therapeutics. J. Mater. Chem. 2011, 21, 13107–13127. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Nurra, S.; Pala, N.; Marceddu, S.; Pathania, D.; Neamati, N.; Sechi, M. Targeted nanoparticles for the delivery of novel bioactive molecules to pancreatic cancer cells. J. Med. Chem. 2016, 59, 5209–5220. [Google Scholar] [CrossRef]
- Mittal, V.; Sheth, S.; Patel, S. Development and characterization of folate targeted nanoparticle drug delivery system. Int. J. Pharma. Bio. Sci. 2010. [Google Scholar] [CrossRef]
- Gu, L.; Shi, T.; Sun, Y.; You, C.; Wang, S.; Wen, G.; Chen, L.; Zhang, X.; Zhu, J.; Sun, B. Folate-modified, indocyanine green-loaded lipid-polymer hybrid nanoparticles for targeted delivery of cisplatin. J. Biomater. Sci. Polym. Ed. 2017, 28, 690–702. [Google Scholar] [CrossRef]
- Bi, D.; Zhao, L.; Yu, R.; Li, H.; Guo, Y.; Wang, X.; Han, M. Surface modification of doxorubicin-loaded nanoparticles based on polydopamine with pH-sensitive property for tumor targeting therapy. Drug Deliv. 2018, 25, 564–575. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Gong, P.; Zheng, C.; Zhao, P.; Luo, Z.; Ma, Y.; Cai, L. Lipid-Polymer nanoparticles for folate-receptor targeting delivery of doxorubicin. J. Nanosci. Nanotechnol. 2015, 15, 4792–4798. [Google Scholar] [CrossRef]
- Montagner, I.M.; Banzato, A.; Zuccolotto, G.; Renier, D.; Campisi, M.; Bassi, P.; Zanovello, P.; Rosato, A. Paclitaxel-hyaluronan hydrosoluble bioconjugate: Mechanism of action in human bladder cancer cell lines. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1261–1269. [Google Scholar] [CrossRef]
- Weaver, B.A.; Bement, W. How taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A. nab-Paclitaxel mechanisms of action and delivery. J. Controll. Release 2013, 170, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Pliarchopoulou, K.; Laschos, K.; Pectasides, D. Current chemotherapeutic options for the treatment of advanced bladder cancer: A review. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Huang, Q.; Zhou, B.; He, L.; Lin, L.; An, Y.; Li, Y.; Liu, S.; Chen, Y.; Li, B. Self-assembled zein–sodium carboxymethyl cellulose nanoparticles as an effective drug carrier and transporter. J. Mater. Chem. B 2015, 3, 3242–3253. [Google Scholar] [CrossRef]
- Song, F.; Li, X.; Wang, Q.; Liao, L.; Zhang, C. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J. Biomed. Nanotech. 2015, 11, 40–52. [Google Scholar] [CrossRef]
- Tran, P.; Lee, S.E.; Kim, D.H.; Pyo, Y.C.; Park, J.S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2019. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res. Int. 2014, 12. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Q.; Reddy, N.; Yang, Y. Hollow nanoparticles from zein for potential medical applications. J. Mater. Chem. 2011, 21, 18227–18235. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticle. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Seok, H.Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Controll. Release 2018, 280, 20–30. [Google Scholar] [CrossRef]
- Xu, H.; Shen, L.; Xu, L.; Yang, Y. Controlled delivery of hollow corn protein nanoparticles via non-toxic crosslinking: In vivo and drug loading study. Biomed. Microdevices 2015, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Jeong, H.J.; Kim, E.M.; Lee, C.M.; Kwon, T.H.; Sohn, M.H. Folate receptor targeted imaging using poly(ethylene glycol)-folate: In vitro and in vivo studies. J. Korean Med. Sci. 2007, 22, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, M.; Zhang, N. Folate-targeted docetaxel-lipid-based-nanosuspensions for active-targeted cancer therapy. Int. J. Nanomed. 2012, 7, 281–283. [Google Scholar] [CrossRef]
- Thapa, R.K.; Nguyen, H.T.; Jeong, J.H.; Shin, B.S.; Ku, S.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Synergistic anticancer activity of combined histone deacetylase and proteasomal inhibitor-loaded zein nanoparticles in metastatic prostate cancers. Nanomed. Nanotechnol. Biology Med. 2017, 13, 885–896. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin–PEG–folate conjugate. J. Controll. Release 2004, 100, 247–256. [Google Scholar] [CrossRef]
- Hong, S.S.; Thapa, R.K.; Kim, J.H.; Kim, S.Y.; Kim, J.O.; Kim, J.K.; Choi, H.G.; Lim, S.J. Role of zein incorporation on hydrophobic drug-loading capacity and colloidal stability of phospholipid nanoparticles. Colloids Surf. B 2018, 171, 514–521. [Google Scholar] [CrossRef]
- Poudel, B.K.; Soe, Z.C.; Ruttala, H.B.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Gautam, M.; Ou, W.; Nguyen, H.T.; Jeong, J.H.; et al. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int. J. Pharm. 2018, 548, 92–103. [Google Scholar] [CrossRef]
- Poudel, B.K.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Youn, Y.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of polymeric irinotecan nanoparticles using a novel lactone preservation strategy. Int. J. Pharm. 2016, 512, 75–86. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Layer-by-layer assembly of hierarchical nanoarchitectures to enhance the systemic performance of nanoparticle albumin-bound paclitaxel. Int. J. Pharm. 2017, 519, 11–21. [Google Scholar] [CrossRef]
- Erdoğar, N.; Esendağlı, G.; Nielsen, T.T.; Esendağlı-Yılmaz, G.; Yöyen-Ermis, D.; Erdoğdu, B.; Sargon, M.F.; Eroğlu, H.; Bilensoy, E. Therapeutic efficacy of folate receptor-targeted amphiphilic cyclodextrin nanoparticles as a novel vehicle for paclitaxel delivery in breast cancer. J. Drug Target. 2018, 26, 66–74. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Preparation and characterization of a novel conformed bipolymer paclitaxel-nanoparticle using tea polysaccharides and zein. Carbohydr. Polym. 2016, 146, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.H.; Jeong, J.H.; Ku, S.K.; Choi, H.G.; Yong, C.S.; et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Controll. Release 2018, 281, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.; Ko, Y.T. Enhanced photodyamic therapy via photosensitizer-loaded nanoparticles for cancer treatment. J. Pharm. Investig. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Poudel, B.K.; Kim, J.O.; Byeon, J.H. Photoinduced rapid transformation from Au nanoagglomerates to drug-conjugated Au nanovesicles. Adv. Sci. 2018, 5, 1700563. [Google Scholar] [CrossRef]
- Ou, W.; Jiang, L.; Thapa, R.K.; Chi, S.Z.; Poudel, B.K.; Chang, J.H.; Kwang, K.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Combination of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles for effective cancer photoimmunotherapy. Theranostics 2018, 8, 4574–4590. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ahir, M.; Patra, P.; Mukherjee, S.; Ghosh, S.; Mazumdar, M.; Chattopadhyay, S.; Das, T.; Chattopadhyay, D.; Adhikary, A. PEGylated-thymoquinone-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR-34a. Biomaterials 2015, 51, 91–107. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S.; Cheng, S.; Jiang, S.; Liu, Y.; Li, D.; Huang, H.; Zhang, K.; Zheng, X. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour. peel extract. Nanoscale Res. Lett. 2016, 11, 300. [Google Scholar] [CrossRef]
- Ou, W.; Byeon, J.H.; Thapa, R.K.; Ku, S.K.; Yong, C.S.; Kim, J.O. Plug-and-Play nanorization of coarse black phosphorus for targeted chemo-photoimmunotherapy of colorectal cancer. ACS Nano 2018, 12, 10061–10074. [Google Scholar] [CrossRef]
- Gupta, D.; Kumar, M.; Tyagi, P.; Kapoor, S.; Tyagi, A.; Barman, T.K.; Kharbanda, S.; Kufe, D.; Singh, H. Concomitant delivery of paclitaxel and NuBCP-9 peptide for synergistic enhancement of cancer therapy. Nanomed. Nanotechnol. Biology Med. 2018, 14, 1301–1313. [Google Scholar] [CrossRef]
- Chan, W.T.; Liu, C.C.; Chiang Chiau, J.S.; Tsai, S.T.; Liang, C.K.; Cheng, M.L.; Lee, H.C.; Yeung, C.Y.; Hou, S.Y. In vivo toxicologic study of larger silica nanoparticles in mice. Int. J. Nanomed. 2017, 12, 3421–3432. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Luo, L.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Yang, J.; Zhu, C.; Du, Y.; et al. External magnetic field-enhanced chemo-photothermal combination tumor therapy via iron oxide nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 16581–16593. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; He, L.; Zhou, B.; Li, B.; Li, J. Folate-functionalized assembly of low density lipoprotein/sodium carboxymethyl cellulose nanoparticles for targeted delivery. Colloids Surf. B 2017, 156, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Na, Y.G.; Lee, H.S.; Son, G.H.; Jeon, S.H.; Bang, K.H.; Kim, S.J.; Lee, H.J.; Cho, C.W. Improvement of cellular uptake of hydrophilic molecule, calcein, formulated by liposome. J. Pharm. Investig. 2018, 48, 595–601. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Liu, C.; Zhu, J.; Fan, M.; Sun, X.; Wang, T.; Xu, Y.; Cao, Y. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2019, 87, 342–351. [Google Scholar] [CrossRef]
- Pang, J.; Li, Z.; Li, S.; Lin, S.; Wang, H.; Xie, Q.; Jiang, Y. Folate-conjugated zein/Fe3O4 nanocomplexes for the enhancement of cellular uptake and cytotoxicity of gefitinib. J. Mater. Sci. 2018, 53, 14907–14921. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Zein nanoparticles as delivery systems for covalently linked and physically entrapped folic acid. J. Nanoparticle Res. 2017, 19, 81. [Google Scholar] [CrossRef]
- Pokharkar, V.; Patil-Gadhe, A.; Kaur, G. Physicochemical and pharmacokinetic evaluation of rosuvastatin loaded nanostructured lipid carriers: Influence of long- and medium-chain fatty acid mixture. J. Pharm. Investig. 2018, 48, 465–476. [Google Scholar] [CrossRef]
- Phung, D.C.; Nguyen, H.T.; Phuong Tran, T.T.; Jin, S.G.; Truong, D.H.; Tran, T.H.; Yong, C.S.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Investig. 2019, 49, 519–526. [Google Scholar] [CrossRef]
- Sobh, R.A.; Nasr, H.E.; Moustafa, A.B.; Mohamed, W.S. Tailoring of anticancer drugs loaded in MWCNT/Poly(MMA-co-HEMA) nanosphere composite by using in situ microemulsion polymerization. J. Pharm. Investig. 2019, 49, 45–55. [Google Scholar] [CrossRef]
- Wang, M.; Huang, M.; Wang, J.; Ye, M.; Deng, Y.; Li, H.; Qian, W.; Zhu, B.; Zhang, Y.; Gong, R. Facile one-pot synthesis of self-assembled folate-biotin-pullulan nanoparticles for targeted intracellular anticancer drug delivery. J. Nanomater. 2016, 5752921, 10. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-modified lipid–polymer hybrid nanoparticles for targeted paclitaxel delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef]
- Rathinaraj, P.; Lee, K.; Park, S.Y.; Kang, I.K. Targeted images of KB cells using folate-conjugated gold nanoparticles. Nanoscale Res. Lett. 2015, 10, 5. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soe, Z.C.; Ou, W.; Gautam, M.; Poudel, K.; Kim, B.K.; Pham, L.M.; Phung, C.D.; Jeong, J.-H.; Jin, S.G.; Choi, H.-G.; et al. Development of Folate-Functionalized PEGylated Zein Nanoparticles for Ligand-Directed Delivery of Paclitaxel. Pharmaceutics 2019, 11, 562. https://doi.org/10.3390/pharmaceutics11110562
Soe ZC, Ou W, Gautam M, Poudel K, Kim BK, Pham LM, Phung CD, Jeong J-H, Jin SG, Choi H-G, et al. Development of Folate-Functionalized PEGylated Zein Nanoparticles for Ligand-Directed Delivery of Paclitaxel. Pharmaceutics. 2019; 11(11):562. https://doi.org/10.3390/pharmaceutics11110562
Chicago/Turabian StyleSoe, Zar Chi, Wenquan Ou, Milan Gautam, Kishwor Poudel, Bo Kyun Kim, Le Minh Pham, Cao Dai Phung, Jee-Heon Jeong, Sung Giu Jin, Han-Gon Choi, and et al. 2019. "Development of Folate-Functionalized PEGylated Zein Nanoparticles for Ligand-Directed Delivery of Paclitaxel" Pharmaceutics 11, no. 11: 562. https://doi.org/10.3390/pharmaceutics11110562