The Role of Nanovaccine in Cross-Presentation of Antigen-Presenting Cells for the Activation of CD8+ T Cell Responses
Abstract
:1. Introduction
2. Nanoparticles Incorporated with Antigens and Adjuvants for CD8+ T Cell Response
2.1. Nanoparticle Incorporated with Antigens
2.2. Nanoparticles Incorporated with TLR Ligands for Enhanced Adjuvanticity
3. Targeting Strategies with Nanovaccines for CD8+ T Cell Responses
3.1. Nanovaccines Targeting Lymph Nodes
3.2. Nanovaccines Targeting APCs
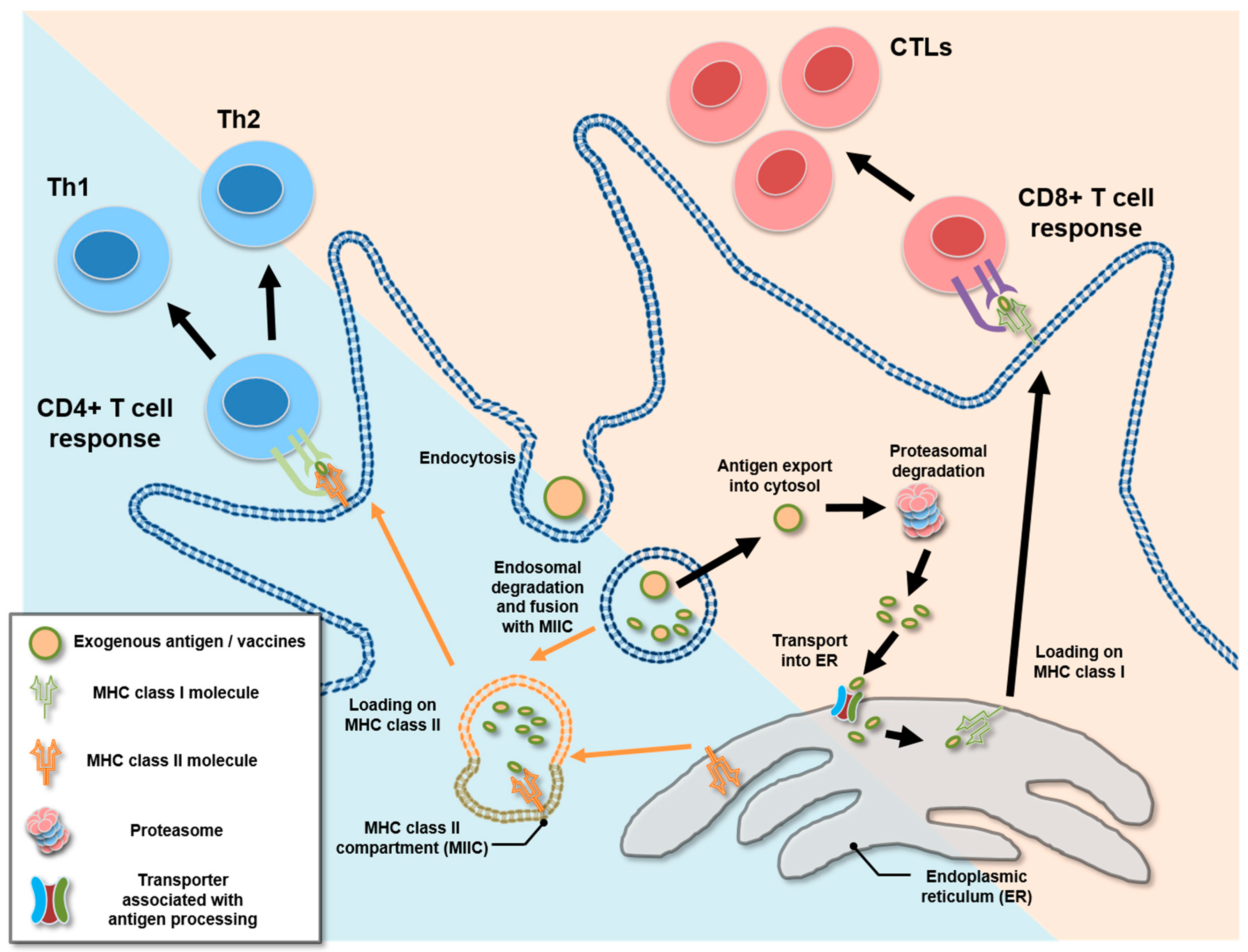
4. Cross-Presentation and Cytosolic Exportation of Nanovaccines
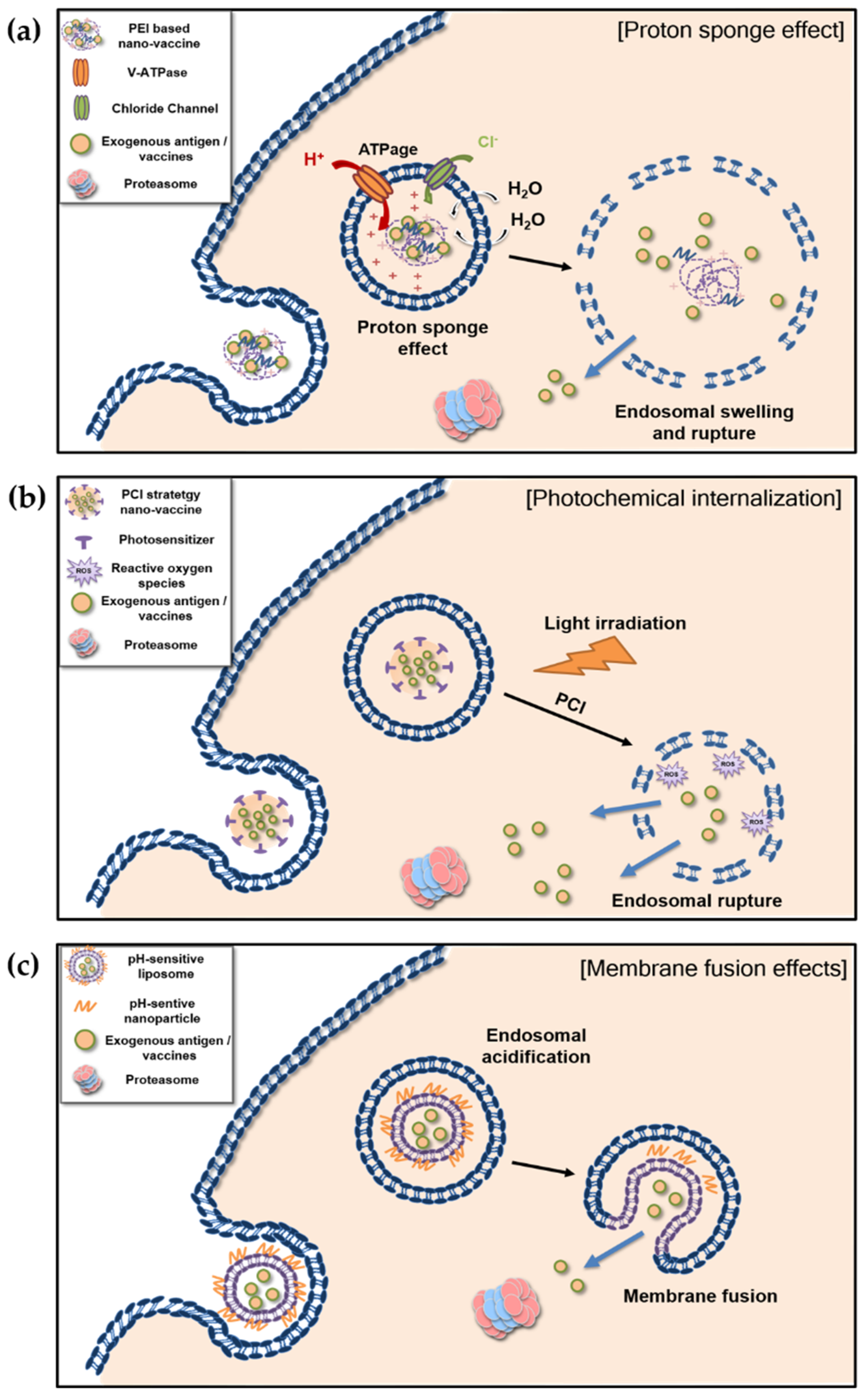
4.1. Proton Sponge Effect and Photochemical Internalization of Polymer-Based Nanovaccines
4.1.1. Proton Sponge Effect
4.1.2. Photochemical Internalization
4.2. Membrane Fusion of Liposome-Based pH-Sensitive Nanovaccines
5. Conclusions
Funding
Conflicts of Interest
References
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Roopngam, P.E. Liposome and polymer-based nanomaterials for vaccine applications. Nanomed. J. 2019, 6, 1–10. [Google Scholar]
- Nakamura, T.; Harashima, H. Integration of nano drug-delivery system with cancer immunotherapy. Ther. Deliv. 2017, 8, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Alloatti, A.; Kotsias, F.; Magalhaes, J.G.; Amigorena, S. Dendritic cell maturation and cross-presentation: Timing matters! Immunol. Rev. 2016, 272, 97–108. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current concepts of antigen cross-presentation. Front. Immunol. 2018, 9, 10. [Google Scholar] [CrossRef]
- Gros, M.; Amigorena, S. Regulation of antigen export to the cytosol during cross-presentation. Front. Immunol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Mumper, R.J.; Cui, Z.; Oyewumi, M.O. Nanotemplate engineering of cell specific nanoparticles. J. Dispers. Sci. Technol. 2003, 24, 569–588. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Kim, M.-G.; Park, J.Y.; Shon, Y.; Kim, G.; Shim, G.; Oh, Y.-K. Nanotechnology and vaccine development. Asian J. Pharm. Sci. 2014, 9, 227–235. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed]
- Wendorf, J.; Singh, M.; Chesko, J.; Kazzaz, J.; Soewanan, E.; Ugozzoli, M.; O’Hagan, D. A practical approach to the use of nanoparticles for vaccine delivery. J. Pharm. Sci. 2006, 95, 2738–2750. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.; Cocucci, E.; Podini, P.; Kirchhausen, T.; Clementi, E.; Meldolesi, J. Macropinocytosis: Regulated coordination of endocytic and exocytic membrane traffic events. J. Cell Sci. 2006, 119, 4758–4769. [Google Scholar] [CrossRef]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef]
- Jia, J.B.; Zhang, Y.; Xin, Y.; Jiang, C.J.; Yan, B.; Zhai, S.M. Interactions between nanoparticles and dendritic cells: From the perspective of cancer immunotherapy. Front. Oncol. 2018, 8, 11. [Google Scholar] [CrossRef]
- Gao, S.; Yang, D.J.; Fang, Y.; Lin, X.J.; Jin, X.C.; Wang, Q.; Wang, X.Y.; Ke, L.Y.; Shi, K. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics 2019, 9, 126–151. [Google Scholar] [CrossRef]
- Silva, J.M.; Vandermeulen, G.; Oliveira, V.G.; Pinto, S.N.; Rodrigues, C.; Salgado, A.; Afonso, C.A.; Viana, A.S.; Jerome, C.; Silva, L.C.; et al. Development of functionalized nanoparticles for vaccine delivery to dendritic cells: A mechanistic approach. Nanomedicine 2014, 9, 2639–2656. [Google Scholar] [CrossRef]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef]
- Di Marco, M.; Shamsuddin, S.; Razak, K.A.; Aziz, A.A.; Devaux, C.; Borghi, E.; Levy, L.; Sadun, C. Overview of the main methods used to combine proteins with nanosystems: Absorption, bioconjugation, and encapsulation. Int. J. Nanomed. 2010, 5, 37–49. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrom, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J. Control. Release 2010, 145, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef] [PubMed]
- Slutter, B.; Soema, P.C.; Ding, Z.; Verheul, R.; Hennink, W.; Jiskoot, W. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. J. Control. Release 2010, 143, 207–214. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mitchell, A.R.; Johnson, S.L.; Wagner-Bartak, C.; Morcol, T.; Bell, S.J.D. Calcium phosphate nanoparticle adjuvant. Clin. Diagn. Lab. Immunol. 2000, 7, 899–903. [Google Scholar] [CrossRef]
- Koppolu, B.; Zaharoff, D.A. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials 2013, 34, 2359–2369. [Google Scholar] [CrossRef]
- Koppolu, B.P.; Smith, S.G.; Ravindranathan, S.; Jayanthi, S.; Suresh Kumar, T.K.; Zaharoff, D.A. Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials 2014, 35, 4382–4389. [Google Scholar] [CrossRef]
- Moon, H.; Lee, J.; Min, J.; Kang, S. Developing genetically engineered encapsulin protein cage nanoparticles as a targeted delivery nanoplatform. Biomacromolecules 2014, 15, 3794–3801. [Google Scholar] [CrossRef]
- Choi, B.; Moon, H.; Hong, S.J.; Shin, C.; Do, Y.; Ryu, S.; Kang, S. Effective delivery of antigen-encapsulin nanoparticle fusions to dendritic cells leads to antigen-specific cytotoxic T cell activation and tumor rejection. ACS Nano 2016, 10, 7339–7350. [Google Scholar] [CrossRef]
- Gonzalez, F.E.; Gleisner, A.; Falcon-Beas, F.; Osorio, F.; Lopez, M.N.; Salazar-Onfray, F. Tumor cell lysates as immunogenic sources for cancer vaccine design. Hum. Vaccin Immunother. 2014, 10, 3261–3269. [Google Scholar] [CrossRef]
- de Gruijl, T.D.; van den Eertwegh, A.J.; Pinedo, H.M.; Scheper, R.J. Whole-cell cancer vaccination: From autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol. Immunother. 2008, 57, 1569–1577. [Google Scholar] [CrossRef]
- Ochyl, L.J.; Bazzill, J.D.; Park, C.; Xu, Y.; Kuai, R.; Moon, J.J. Pegylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials 2018, 182, 157–166. [Google Scholar] [CrossRef]
- Silva, A.L.; Rosalia, R.A.; Varypataki, E.; Sibuea, S.; Ossendorp, F.; Jiskoot, W. Poly-(lactic-co-glycolic-acid)-based particulate vaccines: Particle uptake by dendritic cells is a key parameter for immune activation. Vaccine 2015, 33, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, E.; Curato, C.; Paisana, M.; Rodrigues, C.; Porat, Z.; Viana, A.S.; Afonso, C.A.M.; Pinto, J.; Gaspar, R.; Moreira, J.N.; et al. Rational design of nanoparticles towards targeting antigen-presenting cells and improved T cell priming. J. Control. Release 2017, 258, 182–195. [Google Scholar] [CrossRef] [PubMed]
- San Roman, B.; Irache, J.M.; Gomez, S.; Tsapis, N.; Gamazo, C.; Espuelas, M.S. Co-encapsulation of an antigen and CpG oligonucleotides into PLGA microparticles by TROMS technology. Eur. J. Pharm. Biopharm. 2008, 70, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdy, S.; Molavi, O.; Ma, Z.; Haddadi, A.; Alshamsan, A.; Gobti, Z.; Elhasi, S.; Samuel, J.; Lavasanifar, A. Co-delivery of cancer-associated antigen and toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 2008, 26, 5046–5057. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Lee, Y.H.; Im, S.A.; Yang, I.H.; Ahn, G.W.; Kim, K.; Lee, C.K. Biodegradable nanoparticles containing TLR3 or TLR9 agonists together with antigen enhance MHC-restricted presentation of the antigen. Arch. Pharm. Res. 2010, 33, 1859–1866. [Google Scholar] [CrossRef]
- Barker, C.I.S.; Snape, M.D. Pandemic influenza A H1N1 vaccines and narcolepsy: Vaccine safety surveillance in action. Lancet Infect. Dis. 2014, 14, 227–238. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.-A.; Pollard, A.J. AS03- and MF59-adjuvanted influenza vaccines in children. Front. Immunol. 2017, 8, 943. [Google Scholar] [CrossRef] [Green Version]
- Palm, N.W.; Medzhitov, R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 2009, 227, 221–233. [Google Scholar] [CrossRef]
- Jones, L.H. Recent advances in the molecular design of synthetic vaccines. Nat. Chem. 2015, 7, 952–960. [Google Scholar] [CrossRef]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological principles guiding the rational design of particles for vaccine delivery. ACS Nano 2017, 11, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Q.; Li, L.; Zeng, Q.; Li, H.; Gong, T.; Zhang, Z.; Sun, X. Turning the old adjuvant from gel to nanoparticles to amplify CD8+ T cell responses. Adv. Sci. 2018, 5, 1700426. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Sun, B.; Tran, K.K.; Shen, H. Effects of particle size on toll-like receptor 9-mediated cytokine profiles. Biomaterials 2011, 32, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Spadaro, F.; Lapenta, C.; Donati, S.; Abalsamo, L.; Barnaba, V.; Belardelli, F.; Santini, S.M.; Ferrantini, M. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 2012, 119, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Kolumam, G.A.; Thomas, S.; Thompson, L.J.; Sprent, J.; Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005, 202, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, J.; Krieg, A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204. [Google Scholar] [CrossRef]
- Puig, M.; Grajkowski, A.; Boczkowska, M.; Ausin, C.; Beaucage, S.L.; Verthelyi, D. Use of thermolytic protective groups to prevent G-tetrad formation in CpG ODN type D: Structural studies and immunomodulatory activity in primates. Nucleic Acids Res. 2006, 34, 6488–6495. [Google Scholar] [CrossRef]
- McHutchison, J.G.; Bacon, B.R.; Gordon, S.C.; Lawitz, E.; Shiffman, M.; Afdhal, N.H.; Jacobson, I.M.; Muir, A.; Al-Adhami, M.; Morris, M.L.; et al. Phase 1B, randomized, double-blind, dose-escalation trial of CpG 10101 in patients with chronic hepatitis C virus. Hepatology 2007, 46, 1341–1349. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Kobiyama, K.; Aoshi, T.; Narita, H.; Kuroda, E.; Hayashi, M.; Tetsutani, K.; Koyama, S.; Mochizuki, S.; Sakurai, K.; Katakai, Y.; et al. Nonagonistic dectin-1 ligand transforms CpG into a multitask nanoparticulate TLR9 agonist. Proc. Natl. Acad. Sci. USA 2014, 111, 3086–3091. [Google Scholar] [CrossRef] [Green Version]
- Kitahata, Y.; Kanuma, T.; Hayashi, M.; Kobayashi, N.; Ozasa, K.; Kusakabe, T.; Temizoz, B.; Kuroda, E.; Yamaue, H.; Coban, C.; et al. Circulating nano-particulate TLR9 agonist scouts out tumor microenvironment to release immunogenic dead tumor cells. Oncotarget 2016, 7, 48860–48869. [Google Scholar] [CrossRef] [Green Version]
- Homhuan, A. Maturation of dendritic cells induced by nano-liposomes containing imiquimod. Asian Biomed. 2008, 2, 233–239. [Google Scholar]
- Jimenez-Sanchez, G.; Pavot, V.; Chane-Haong, C.; Handke, N.; Terrat, C.; Gigmes, D.; Trimaille, T.; Verrier, B. Preparation and in vitro evaluation of imiquimod loaded polylactide-based micelles as potential vaccine adjuvants. Pharm. Res. 2015, 32, 311–320. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Goldinger, S.M.; Dummer, R.; Baumgaertner, P.; Mihic-Probst, D.; Schwarz, K.; Hammann-Haenni, A.; Willers, J.; Geldhof, C.; Prior, J.O.; Kundig, T.M.; et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur. J. Immunol. 2012, 42, 3049–3061. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Son, S.; Moon, J.J. Adjuvant-loaded spiky gold nanoparticles for activation of innate immune cells. Cell Mol. Bioeng 2017, 10, 341–355. [Google Scholar] [CrossRef]
- Bocanegra Gondan, A.I.; Ruiz-de-Angulo, A.; Zabaleta, A.; Gomez Blanco, N.; Cobaleda-Siles, B.M.; Garcia-Granda, M.J.; Padro, D.; Llop, J.; Arnaiz, B.; Gato, M.; et al. Effective cancer immunotherapy in mice by polyic-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials 2018, 170, 95–115. [Google Scholar] [CrossRef] [Green Version]
- Giannini, S.L.; Hanon, E.; Moris, P.; Van Mechelen, M.; Morel, S.; Dessy, F.; Fourneau, M.A.; Colau, B.; Suzich, J.; Losonksy, G.; et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006, 24, 5937–5949. [Google Scholar] [CrossRef]
- Duthie, M.S.; Raman, V.S.; Piazza, F.M.; Reed, S.G. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine 2012, 30, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Wu, T.; Zhao, Y.; Hu, X.; Bao, Y.; Guo, Y.; Song, Q.; Li, G.; Tan, S.; Zhang, Z. Lipid-enveloped zinc phosphate hybrid nanoparticles for codelivery of H-2Kb and H-2Db-restricted antigenic peptides and monophosphoryl lipid a to induce antitumor immunity against melanoma. J. Control. Release 2016, 228, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; Lopez, S.C.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jong, S.; Chikh, G.; Sekirov, L.; Raney, S.; Semple, S.; Klimuk, S.; Yuan, N.; Hope, M.; Cullis, P.; Tam, Y. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered cpg odn. Cancer Immunol. Immunother. 2007, 56, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, C.; Anz, D.; Zwiorek, K.; Lanz, A.L.; Fuchs, S.; Weigel, S.; Wurzenberger, C.; von der Borch, P.; Golic, M.; Moder, S.; et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J. Immunol. 2008, 181, 2990–2998. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Sun, X. Lymph node targeting strategies to improve vaccination efficacy. J. Control. Release 2017, 267, 47–56. [Google Scholar] [CrossRef]
- van der Vlies, A.J.; O’Neil, C.P.; Hasegawa, U.; Hammond, N.; Hubbell, J.A. Synthesis of pyridyl disulfide-functionalized nanoparticles for conjugating thiol-containing small molecules, peptides, and proteins. Bioconjugate Chem. 2010, 21, 653–662. [Google Scholar] [CrossRef]
- Kourtis, I.C.; Hirosue, S.; de Titta, A.; Kontos, S.; Stegmann, T.; Hubbell, J.A.; Swartz, M.A. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS ONE 2013, 8, e61646. [Google Scholar] [CrossRef]
- Hirosue, S.; Kourtis, I.C.; van der Vlies, A.J.; Hubbell, J.A.; Swartz, M.A. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and t cell activation. Vaccine 2010, 28, 7897–7906. [Google Scholar] [CrossRef]
- Nembrini, C.; Stano, A.; Dane, K.Y.; Ballester, M.; van der Vlies, A.J.; Marsland, B.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc. Natl. Acad. Sci. USA 2011, 108, E989–E997. [Google Scholar] [CrossRef] [Green Version]
- de Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; van der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 2013, 110, 19902–19907. [Google Scholar] [CrossRef] [Green Version]
- Jeanbart, L.; Ballester, M.; de Titta, A.; Corthesy, P.; Romero, P.; Hubbell, J.A.; Swartz, M.A. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2014, 2, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Meng, C.; Zhi, X.; Li, C.; Li, C.; Chen, Z.; Qiu, X.; Ding, C.; Ma, L.; Lu, H.; Chen, D.; et al. Graphene oxides decorated with carnosine as an adjuvant to modulate innate immune and improve adaptive immunity in vivo. ACS Nano 2016, 10, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.; Copland, A.; Diogo, G.R.; Harris, S.; Spallek, R.; Oehlmann, W.; Singh, M.; Basile, J.; Rottenberg, M.; Paul, M.J.; et al. Nanoparticle-fusion protein complexes protect against mycobacterium tuberculosis infection. Mol. Ther. 2018, 26, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadière, C.; Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Benechet, A.P.; Lefrancois, L.; Khanna, K.M. CD8 T cells enter the splenic T cell zones independently of CCR7, but the subsequent expansion and trafficking patterns of effector T cells after infection are dysregulated in the absence of CCR7 migratory cues. J. Immunol. 2015, 195, 5227–5236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, M.D.; Tangemann, K.; Tam, C.; Cyster, J.G.; Rosen, S.D.; Williams, L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Sallusto, F.; Lanzavecchia, A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 2000, 177, 134–140. [Google Scholar] [CrossRef]
- Castellino, F.; Huang, A.Y.; Altan-Bonnet, G.; Stoll, S.; Scheinecker, C.; Germain, R.N. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell–dendritic cell interaction. Nature 2006, 440, 890–895. [Google Scholar] [CrossRef]
- Zaric, M.; Lyubomska, O.; Poux, C.; Hanna, M.L.; McCrudden, M.T.; Malissen, B.; Ingram, R.J.; Power, U.F.; Scott, C.J.; Donnelly, R.F.; et al. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and th1 immune responses by murine langerhans cells. J. Investig. Derm. 2015, 135, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Mencia, A.; Bi, L.; Taylor, A.; Yao, Y.; HogenEsch, H. Dendrimer-like alpha-d-glucan nanoparticles activate dendritic cells and are effective vaccine adjuvants. J. Control. Release 2015, 204, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Mosley, Y.C.; Rodriguez Rosales, R.J.; Carmichael, B.E.; Elesela, S.; Yao, Y.; HogenEsch, H. Alpha-d-glucan nanoparticulate adjuvant induces a transient inflammatory response at the injection site and targets antigen to migratory dendritic cells. NPJ Vaccines 2017, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin. Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, J.; Shi, Y.; Peng, S.; Cai, Y.; Zhan, X.; Song, N.; Liu, Y.; Wang, Z.; Yu, Y.; et al. A new cancer immunotherapy via simultaneous DC-mobilization and DC-targeted IDO gene silencing using an immune-stimulatory nanosystem. Int. J. Cancer 2018, 143, 2039–2052. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Higuchi, T.; Tubbe, I.; Voltz, N.; Krummen, M.; Pektor, S.; Montermann, E.; Rausch, K.; Schmidt, M.; Schild, H.; et al. A trifunctional dextran-based nanovaccine targets and activates murine dendritic cells, and induces potent cellular and humoral immune responses in vivo. PLoS ONE 2013, 8, e80904. [Google Scholar] [CrossRef] [Green Version]
- Gulla, S.K.; Rao, B.R.; Moku, G.; Jinka, S.; Nimmu, N.V.; Khalid, S.; Patra, C.R.; Chaudhuri, A. In vivo targeting of DNA vaccines to dendritic cells using functionalized gold nanoparticles. Biomater. Sci. 2019, 7, 773–788. [Google Scholar] [CrossRef]
- Arosio, D.; Chiodo, F.; Reina, J.J.; Marelli, M.; Penades, S.; van Kooyk, Y.; Garcia-Vallejo, J.J.; Bernardi, A. Effective targeting of DC-sign by α-fucosylamide functionalized gold nanoparticles. Bioconjugate Chem. 2014, 25, 2244–2251. [Google Scholar] [CrossRef] [Green Version]
- Fehres, C.M.; Duinkerken, S.; Bruijns, S.C.M.; Kalay, H.; van Vliet, S.J.; Ambrosini, M.; de Gruijl, T.D.; Unger, W.W.J.; Garcia-Vallejo, J.J.; van Kooyk, Y. Langerin-mediated internalization of a modified peptide routes antigens to early endosomes and enhances cross-presentation by human langerhans cells. Cell. Mol. Immunol. 2017, 14, 360–370. [Google Scholar] [CrossRef]
- Wamhoff, E.C.; Schulze, J.; Bellmann, L.; Rentzsch, M.; Bachem, G.; Fuchsberger, F.F.; Rademacher, J.; Hermann, M.; Del Frari, B.; van Dalen, R.; et al. A specific, glycomimetic langerin ligand for human langerhans cell targeting. ACS Cent. Sci. 2019, 5, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Oishi, M.; Kataoka, K.; Nagasaki, Y. pH-responsive three-layered PEGylated polyplex micelle based on a lactosylated ABC triblock copolymer as a targetable and endosome-disruptive nonviral gene vector. Bioconjug Chem. 2006, 17, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G.; Zhao, X.; Qiao, M.; Cheng, L.; Cheng, L.; et al. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan—Pamam-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132. [Google Scholar] [CrossRef] [PubMed]
- Tambe, P.; Kumar, P.; Karpe, Y.A.; Paknikar, K.M.; Gajbhiye, V. Triptorelin tethered multifunctional PAMAM-histidine-PEG nanoconstructs enable specific targeting and efficient gene silencing in LHRH overexpressing cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 35562–35573. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.; Huang, H.; Yang, Y.; Ding, Q.; Mai, J.; Guo, W.; Xu, Y. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int. J. Nanomed. 2011, 6, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Luo, Z.; Liu, P.; Gao, N.; Zhang, Y.; Pan, H.; Liu, L.; Wang, C.; Cai, L.; Ma, Y. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J. Control. Release 2013, 168, 271–279. [Google Scholar] [CrossRef]
- Dong, H.; Wen, Z.F.; Chen, L.; Zhou, N.; Liu, H.; Dong, S.; Hu, H.M.; Mou, Y. Polyethyleneimine modification of aluminum hydroxide nanoparticle enhances antigen transportation and cross-presentation of dendritic cells. Int. J. Nanomed. 2018, 13, 3353–3365. [Google Scholar] [CrossRef] [Green Version]
- Firdous, J.; Islam, M.A.; Park, S.M.; Cheon, I.S.; Shim, B.S.; Yoon, H.S.; Song, M.; Chang, J.; Choi, Y.J.; Park, Y.M.; et al. Induction of long-term immunity against respiratory syncytial virus glycoprotein by an osmotic polymeric nanocarrier. Acta Biomater. 2014, 10, 4606–4617. [Google Scholar] [CrossRef]
- Kye, Y.C.; Park, S.M.; Shim, B.S.; Firdous, J.; Kim, G.; Kim, H.W.; Ju, Y.J.; Kim, C.G.; Cho, C.S.; Kim, D.W.; et al. Intranasal immunization with pneumococcal surface protein a in the presence of nanoparticle forming polysorbitol transporter adjuvant induces protective immunity against the streptococcus pneumoniae infection. Acta Biomater. 2019, 90, 362–372. [Google Scholar] [CrossRef]
- Jiang, D.; Mu, W.; Pang, X.; Liu, Y.; Zhang, N.; Song, Y.; Garg, S. Cascade cytosol delivery of dual-sensitive micelle-tailored vaccine for enhancing cancer immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 37797–37811. [Google Scholar] [CrossRef]
- Hakerud, M.; Waeckerle-Men, Y.; Selbo, P.K.; Kundig, T.M.; Hogset, A.; Johansen, P. Intradermal photosensitisation facilitates stimulation of mhc class-I restricted CD8 T-cell responses of co-administered antigen. J. Control. Release 2014, 174, 143–150. [Google Scholar] [CrossRef]
- Hakerud, M.; Selbo, P.K.; Waeckerle-Men, Y.; Contassot, E.; Dziunycz, P.; Kundig, T.M.; Hogset, A.; Johansen, P. Photosensitisation facilitates cross-priming of adjuvant-free protein vaccines and stimulation of tumour-suppressing CD8 T cells. J. Control. Release 2015, 198, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Waeckerle-Men, Y.; Hakerud, M.; Kundig, T.M.; Gander, B.; Johansen, P. Photosensitizer and light pave the way for cytosolic targeting and generation of cytosolic CD8 T cells using PLGA vaccine particles. J. Immunol. 2015, 195, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Hjalmsdottir, A.; Buhler, C.; Vonwil, V.; Roveri, M.; Hakerud, M.; Wackerle-Men, Y.; Gander, B.; Johansen, P. Cytosolic delivery of liposomal vaccines by means of the concomitant photosensitization of phagosomes. Mol. Pharm. 2016, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Shi, G.; Song, H.; Shi, S.; Zhang, X.; Huang, P.; Wang, Z.; Wang, W.; Wang, C.; et al. A light responsive nanoparticle-based delivery system using pheophorbide a graft polyethylenimine for dendritic cell-based cancer immunotherapy. Mol. Pharm. 2017, 14, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, N.; Kojima, C.; Harada, A.; Kono, K. Preparation of pH-sensitive poly(glycidol) derivatives with varying hydrophobicities: Their ability to sensitize stable liposomes to pH. Bioconjug Chem. 2008, 19, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Kono, K. Carboxylated hyperbranched poly(glycidol)s for preparation of pH-sensitive liposomes. J. Control. Release 2011, 149, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Watarai, S.; Kono, K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 2013, 34, 3042–3052. [Google Scholar] [CrossRef]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, Y.; Yuba, E.; Sakaguchi, N.; Koiwai, K.; Harada, A.; Kono, K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials 2014, 35, 8186–8196. [Google Scholar] [CrossRef]
- Behr, J.P. The proton sponge: A trick to enter cells the viruses did not exploit. Chimia 1997, 51, 34–36. [Google Scholar]
- De Smedt, S.C.; Demeester, J.; Hennink, W.E. Cationic polymer based gene delivery systems. Pharm. Res. 2000, 17, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.; Futaki, S.; Harashima, H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS J. 2009, 11, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.Q.; Xu, X.; Bertrand, N.; Pridgen, E.; Swami, A.; Farokhzad, O.C. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv. Drug Deliv Rev. 2012, 64, 1363–1384. [Google Scholar] [CrossRef] [PubMed]
- Norum, O.J.; Selbo, P.K.; Weyergang, A.; Giercksky, K.E.; Berg, K. Photochemical internalization (PCI) in cancer therapy: From bench towards bedside medicine. J. Photochem. Photobiol. B 2009, 96, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Selbo, P.K.; Bostad, M.; Olsen, C.E.; Edwards, V.T.; Hogset, A.; Weyergang, A.; Berg, K. Photochemical internalisation, a minimally invasive strategy for light-controlled endosomal escape of cancer stem cell-targeting therapeutics. Photochem. Photobiol. Sci. 2015, 14, 1433–1450. [Google Scholar] [CrossRef]
- Adigbli, D.K.; MacRobert, A.J. Photochemical internalisation: The journey from basic scientific concept to the threshold of clinical application. Curr. Opin. Pharm. 2012, 12, 434–438. [Google Scholar] [CrossRef]
- Martinez de Pinillos Bayona, A.; Moore, C.M.; Loizidou, M.; MacRobert, A.J.; Woodhams, J.H. Enhancing the efficacy of cytotoxic agents for cancer therapy using photochemical internalisation. Int. J. Cancer 2016, 138, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Waeckerle-Men, Y.; Mauracher, A.; Hakerud, M.; Mohanan, D.; Kundig, T.M.; Hogset, A.; Johansen, P. Photochemical targeting of antigens to the cytosol for stimulation of MHC class-I-restricted T-cell responses. Eur. J. Pharm. Biopharm. 2013, 85, 34–41. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, J.; Chu, C.-C. Enhanced MHC-I antigen presentation from the delivery of ovalbumin by light-facilitated biodegradable poly(ester amide)s nanoparticles. J. Mater. Chem. B 2018, 6, 1930–1942. [Google Scholar] [CrossRef]
- Allison, A.C.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Bungener, L.; Serre, K.; Bijl, L.; Leserman, L.; Wilschut, J.; Daemen, T.; Machy, P. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine 2002, 20, 2287–2295. [Google Scholar] [CrossRef]
- Jahn, R.; Lang, T.; Sudhof, T.C. Membrane fusion. Cell 2003, 112, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Bullough, P.A.; Hughson, F.M.; Skehel, J.J.; Wiley, D.C. Structure of influenza hemagglutinin at the pH of membrane-fusion. Nature 1994, 371, 37–43. [Google Scholar] [CrossRef]
- Kunisawa, J.; Nakanishi, T.; Takahashi, I.; Okudaira, A.; Tsutsumi, Y.; Katayama, K.; Nakagawa, S.; Kiyono, H.; Mayumi, T. Sendai virus fusion protein mediates simultaneous induction of MHC class I/II-dependent mucosal and systemic immune responses via the nasopharyngeal-associated lymphoreticular tissue immune system. J. Immunol. 2001, 167, 1406–1412. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Okada, N.; Tsujino, M.; Gao, J.Q.; Hayashi, A.; Tsutsumi, Y.; Mayumi, T.; Yamamoto, A.; Nakagawa, S. Vaccine efficacy of fusogenic liposomes containing tumor cell-lysate against murine B16BL6 melanoma. Biol. Pharm. Bull. 2006, 29, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.; Zhou, F.; Huang, L.; Carbone, F.; Bevan, M.; Rouse, B.T. pH sensitive liposomes provide an efficient means of sensitizing target-cells to class-I restricted ctl recognition of a soluble-protein. J. Immunol. Methods 1991, 141, 157–163. [Google Scholar] [CrossRef]
- Seki, K.; Tirrell, D.A. pH-dependent complexation of poly(acrylic acid) derivatives with phospholipid vesicle membranes. Macromolecules 1984, 17, 1692–1698. [Google Scholar] [CrossRef]
- Kono, K.; Igawa, T.; Takagishi, T. Cytoplasmic delivery of calcein mediated by liposomes modified with a pH-sensitive poly(ethylene glycol) derivative. Biochim. Et Biophys. Acta-Biomembr. 1997, 1325, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Murthy, N.; Robichaud, J.R.; Tirrell, D.A.; Stayton, P.S.; Hoffman, A.S. The design and synthesis of polymers for eukaryotic membrane disruption. J. Control. Release 1999, 61, 137–143. [Google Scholar] [CrossRef]
- Nakamura, T.; Moriguchi, R.; Kogure, K.; Shastri, N.; Harashima, H. Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes. Mol. Ther. 2008, 16, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, R.; Harashima, H.; Shono, M.; Azumano, M.; Niwa, M.; Futaki, S.; Kiwada, H. Intracellular regulation of macromolecules using pH-sensitive liposomes and nuclear localization signal: Qualitative and quantitative evaluation of intracellular trafficking. Biochem. Biophys. Res. Commun. 1998, 251, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Vieira, O.V.; Botelho, R.J.; Grinstein, S. Phagosome maturation: Aging gracefully. Biochem. J. 2002, 366, 689–704. [Google Scholar] [CrossRef]
- Samie, M.; Cresswell, P. The transcription factor TFEB acts as a molecular switch that regulates exogenous antigen-presentation pathways. Nat. Immunol. 2015, 16, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.S.; Villadangos, J.A. Regulation of antigen presentation and cross-presentation in the dendritic cell network: Facts, hypothesis, and immunological implications. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 86, pp. 241–305. [Google Scholar]
- Ackerman, A.L.; Kyritsis, C.; Tampe, R.; Cresswell, P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc. Natl. Acad. Sci. USA 2003, 100, 12889–12894. [Google Scholar] [CrossRef] [Green Version]
- Delamarre, L.; Mellman, O.I.; Trombetta, S. Differential lysosomal proteolysis in antigen presenting cells determines antigen fate and immunogenicity. Cell Struct. Funct. 2005, 30, 74. [Google Scholar]
| Applied Nanomaterial | Targeted Antigen | Host Species | Type of Evaluation Model | Delivery Method | Ref. | |
|---|---|---|---|---|---|---|
| Proton sponge effect | PEI | Ovalbumin (OVA) | Mouse | In vitro | [94] | |
| Cationic alginate PEI nanogel with 3,3′-dithiobis (AP-SS) | OVA | Mouse | In vitro/In vivo | i.p. | [95] | |
| PEI-modified aluminum hydroxide | Tumor derived autophagosomes (DRibbles) | Mouse | In vitro/In vivo | s.c. (DC-based vaccine) | [96] | |
| PEI-based polysorbitor transpoter (PST) | OVA | Mouse | In vitro/In vivo | i.n. | [97] | |
| PEI-based polysorbitor transpoter (PST) | Pneumococcal surface protein A (PspA) | Mouse | In vitro/In vivo | i.n. | [98] | |
| Amphiphilic poly(l-histidine)−poly(ethylene glycol) | OVA | Mouse | In vitro/In vivo | i.p./s.c. | [99] | |
| Photochemical internalization | TPCS2a | OVA | Mouse | In vitro/In vivo | i.d. | [100] |
| TPCS2a-PLGA | OVA | Mouse | In vitro/In vivo | i.v. | [101] | |
| TPCS2-Liposome | OVA | Mouse | In vitro/In vivo | i.d. | [102] | |
| PheoA-PEI | OVA | Mouse | In vitro/In vivo | i.v. (DC-based vaccine) | [103] | |
| AlPcS2 | OVA | Mouse | In vitro/In vivo | i.d. | [103] | |
| Membrane fusion | MGlu-PG | OVA | Mouse | In vitro/In vivo | i.n./s.c. | [104] |
| MGlu-Dex | OVA | Mouse | In vitro/In vivo | s.c. | [105] | |
| Cationic lipid-incorporated MGlu | OVA | Mouse | In vitro/In vivo | s.c. | [106] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.G.; Kye, Y.-C.; Yun, C.-H. The Role of Nanovaccine in Cross-Presentation of Antigen-Presenting Cells for the Activation of CD8+ T Cell Responses. Pharmaceutics 2019, 11, 612. https://doi.org/10.3390/pharmaceutics11110612
Kim CG, Kye Y-C, Yun C-H. The Role of Nanovaccine in Cross-Presentation of Antigen-Presenting Cells for the Activation of CD8+ T Cell Responses. Pharmaceutics. 2019; 11(11):612. https://doi.org/10.3390/pharmaceutics11110612
Chicago/Turabian StyleKim, Cheol Gyun, Yoon-Chul Kye, and Cheol-Heui Yun. 2019. "The Role of Nanovaccine in Cross-Presentation of Antigen-Presenting Cells for the Activation of CD8+ T Cell Responses" Pharmaceutics 11, no. 11: 612. https://doi.org/10.3390/pharmaceutics11110612
APA StyleKim, C. G., Kye, Y.-C., & Yun, C.-H. (2019). The Role of Nanovaccine in Cross-Presentation of Antigen-Presenting Cells for the Activation of CD8+ T Cell Responses. Pharmaceutics, 11(11), 612. https://doi.org/10.3390/pharmaceutics11110612





