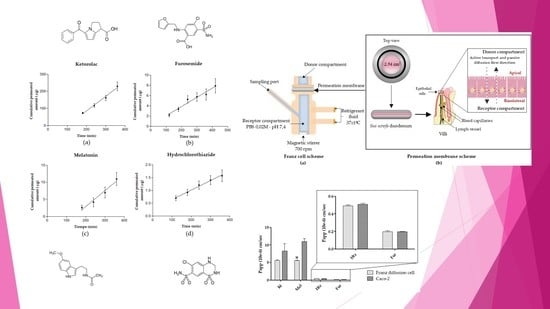
Validation of an Ex Vivo Permeation Method for the Intestinal Permeability of Different BCS Drugs and Its Correlation with Caco-2 In Vitro Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Instrumentation
HPLC-UV Procedure
2.3. Validation and Verification of Analytical Methods
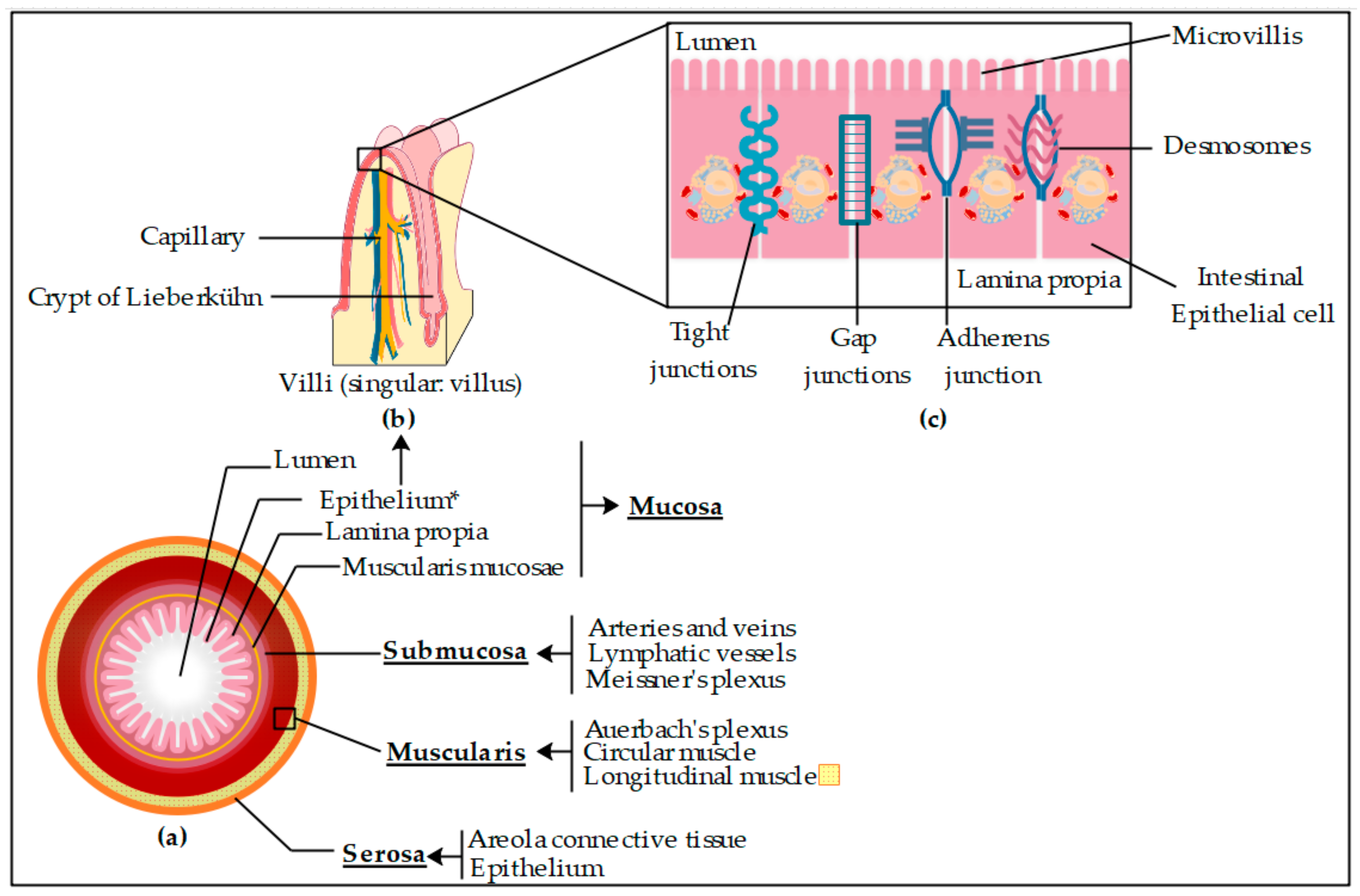
2.4. Ex Vivo Permeation Studies through Pig Intestine
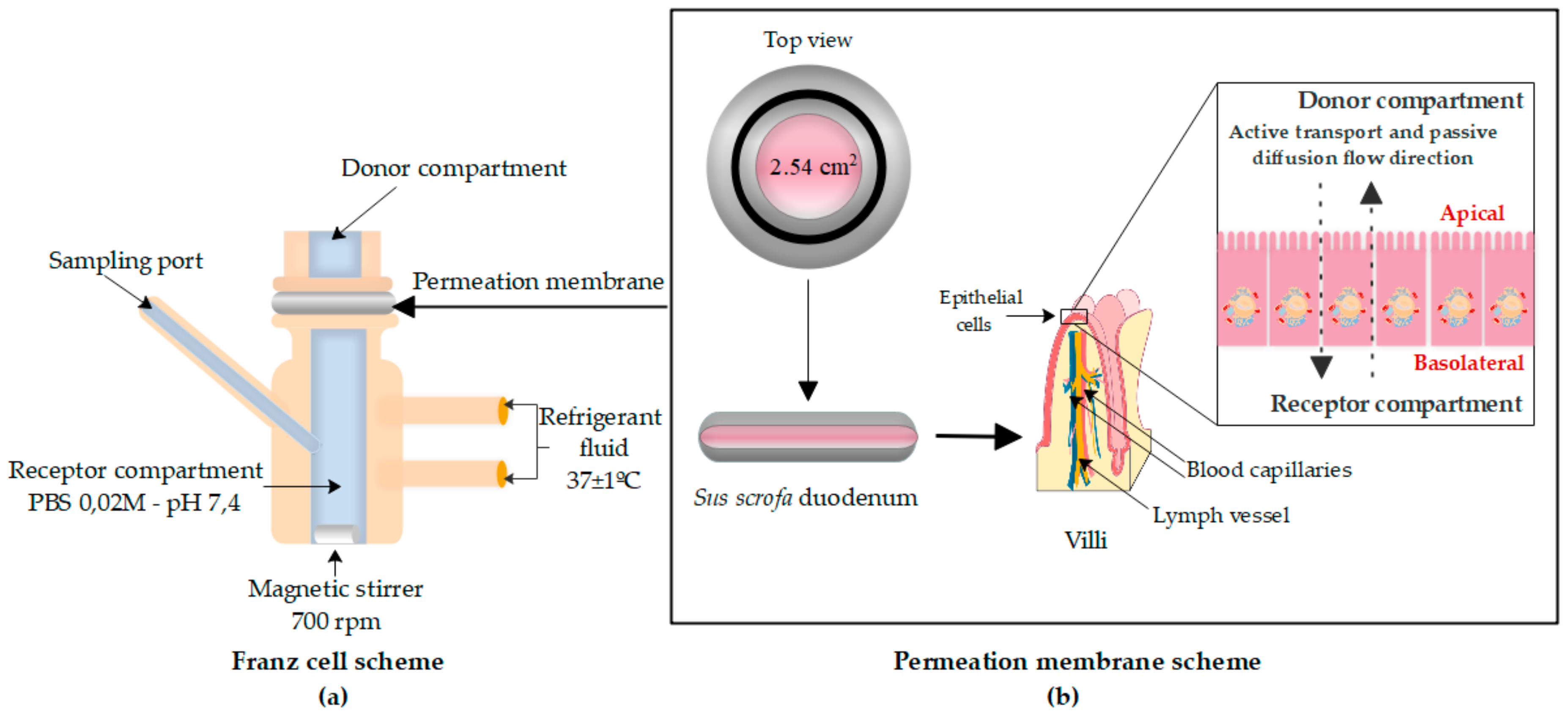
2.4.1. Franz Cell System and Intestinal Membrane
2.4.2. Donor Solution Preparation and Sampling Method
2.4.3. Sample Analysis
2.4.4. Data Treatment and Statistical Analysis
3. Results and Discussion
3.1. Obtained Kinetics Parameters and Papp Calculation
3.2. Specificity
3.3. Correlation between Papp in Caco-2 versus Franz Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugano, K. Biopharmaceutics Modeling and Simulations: Theory, Practice, Methods, and Applications, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 66–102. [Google Scholar]
- Palumbo, P.; Picchini, U.; Beck, B.; Gelder, J.; Van Delbar, N. A general approach to the apparent permeability index. J. Pharm. Pharm. 2008, 35, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H.; Palm, K.; Fagerholm, U.; Artursson, P. Comparison between active and passive drug transport in human intestinal epithelial (Caco-2) cells in vitro and human jejunum in vivo. Int. J. Pharm. 1996, 127, 103–107. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, C.; Jiao, R.; Bai, W.; Zheng, J.; Ou, S. Adducts formed during protein digestion decreased the toxicity of five carbonyl compounds against Caco-2 cells. J. Hazard. Mater. 2019, 363, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Z.; Xie, Y.; Liu, L.; Cao, Y. Cyanidin chloride modestly protects Caco-2 cells from ZnO nanoparticle exposure probably through the induction of autophagy. Food Chem. Toxicol. 2019, 127, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Juan-García, A.; Montesano, D.; Mañes, J.; Juan, C. Cytoprotective effects of carotenoids-rich extract from Lycium barbarum L. on the beauvericin-induced cytotoxicity on Caco-2 cells. Food Chem. Toxicol. 2019, 133, 110798. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A. In-vitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, X.; Guo, N.; Hu, L.; E, M.P.; Wu, Y.; Wang, K. Protective effects of Bee pollen extract on the Caco-2 intestinal barrier dysfunctions induced by dextran sulfate sodium. Biomed. Pharmacother. 2019, 117, 109200. [Google Scholar] [CrossRef]
- Ciappellano, S.G.; Tedesco, E.; Marco Venturini, M.; Benetti, F. In vitro toxicity assessment of oral nanocarriers. Adv. Drug Deliv. Rev. 2016, 106, 381–401. [Google Scholar] [CrossRef]
- Dou, T.; Wang, J.; Han, C.; Shao, X.; Zhang, J.; Lu, W. Cellular uptake and transport characteristics of chitosan modified nanoparticles in Caco-2 cell monolayers. Int. J. Biol. Macromol. 2019, 138, 791–799. [Google Scholar] [CrossRef]
- Yan, X.; Cao, S.; Li, Y.; Xiao, P.; Huang, Z.; Li, H.; Ma, Y. Internalization and subcellular transport mechanisms of different curcumin loaded nanocarriers across Caco-2 cell model. J. Drug Deliv. Sci. Technol. 2019, 52, 660–669. [Google Scholar] [CrossRef]
- Lu, C.; Fu, K.; Cao, K.; Wei, J.; Zhou, J.; Zhao, D.; Zhang, Y. Permeability and transport mechanism of trihexyphenidyl hydrochloride in Caco-2 cell monolayer model with a validated UPLC-MS/MS method. J. Pharm. Biomed. Anal. 2019, 112924. [Google Scholar] [CrossRef] [PubMed]
- Xue-Qing, C.; Stefanski, K.; Shen, H.; Huang, C.; Caporuscio, C.; Yang, W.; Lam, P.; Su, C.; Gudmundsson, O.; Hageman, M. Oral delivery of highly lipophilic poorly water-soluble drugs: spray-dried dispersions to improve oral absorption and enable high-dose toxicology studies of a P2Y1 antagonist. J. Pharm. Sci. 2014, 12, 3924–3931. [Google Scholar]
- Bu, P.; Ji, Y.; Narayanan, S.; Dalrymple, D.; Cheng, X.; Serajuddin, A.T. Assessment of cell viability and permeation enhancement in presence of lipid-based self-emulsifying drug delivery systems using Caco-2 cell model: Polysorbate 80 as the surfactant. Eur. J. Pharm. Sci. 2017, 99, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Xu, Y.; Chen, S.; Cheng, B.; Hu, F.; You, J.; Du, Y.Z.; Yuan, H. Transport mechanisms of solid lipid nanoparticles across Caco-2 cell monolayers and their related cytotoxicology. ACS Appl. Mater. Interfaces 2016, 8, 5929–5940. [Google Scholar] [CrossRef] [PubMed]
- Markowska, M.; Oberle, R.; Juzwin, S.; Hsu, C.P.; Gryszkiewicz, M.; Streeter, A.J. Optimizing Caco-2 cell monolayers to increase throughput in drug intestinal absorption analysis. J. Pharm. Toxicol. Methods 2001, 46, 51–55. [Google Scholar] [CrossRef]
- Yamashita, S.; Furubayashi, T.; Kataoka, M.; Sakane, T.; Sezaki, H.; Tokuda, H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 2000, 10, 195–204. [Google Scholar] [CrossRef]
- Committee for Human Medicinal Products. ICH guideline M9 on biopharmaceutics classification system based biowaivers; European Medicines Agency: Amsterdam, The Netherlands, 2018.
- Welcome, M.O. Gastrointestinal Physiology, 1st ed.; Springer: Basel, Switzerland, 2018; pp. 53–219. [Google Scholar]
- Balimane, P.V.; Chong, S. Evaluation of permeability and P-glycoprotein interactions: Industry outlook. In Biopharmaceutics Applications in Drug Development, 1st ed.; Krishna, R., Yu, L., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 2008; pp. 101–105. [Google Scholar]
- Nejdfors, P.; Ekelund, M.; Jeppsson, B. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: Species- and region-related differences. Scand. J. Gastroenterol. 2009, 35, 501–507. [Google Scholar]
- United States Pharmacopeial Convention. USP DI, 21th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2006; Volume 1, Parts 1–2; Volumes 2–3; pp. 39–40. [Google Scholar]
- Römsing, S. Development and Validation of Bioanalytical Methods, Application to Melatonin and Selected Anti-Infective Drugs. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 16 November 2010. [Google Scholar]
- O’Neil, M.J.; Smith, A.; Henckelman, P.E.; Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Kenilworth, NJ, USA, 2001; p. 854. [Google Scholar]
- Khan, S.J.; Ongerth, J.E. Modelling of pharmaceutical residues in Australian sewage by quantities of use and fugacity calculations. Chemosphere 2004, 54, 355–367. [Google Scholar] [CrossRef]
- Jung, S.J.; Choi, S.O.; Um, S.Y.; Kim, J.I.; Choo, H.Y.; Choi, S.Y.; Chung, S.Y. Prediction of the permeability of drugs through study on quantitative structure-permeability relationship. J. Pharm. Biomed. Anal. 2016, 41, 469–475. [Google Scholar] [CrossRef]
- Yeleswaram, K.; Lg, M.; Jo, K.; Schabdach, D. Pharmacokinetics and oral bioavailability of exogenous melatonin in preclinical animal models and clinical implications. J. Pineal Res. 1997, 22, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Glynn, S.L.; Wright, J.L.; Hawi, A. Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm. Res. 1998, 15, 1490. [Google Scholar] [CrossRef] [PubMed]
- Bock, U.; Flototto, T.; Haltner, E. Validation of cell culture models for the intestine and the blood-brain barrier and comparison of drug permeation. ALTEX 2004, 21 (Suppl. 3), 57–64. [Google Scholar]
- Lee, P.P.N.; Shiu, S.Y.W.; Chow, P.H.; Pang, S.F. regional and diurnal studies of melatonin and melatonin binding sites in the duck gastro-intestinal tract. Neurosignals 1995, 4, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Söderquist, F.; Hellström, P.M.; Cunningham, J.L. Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2. PLoS ONE 2015, 10, e0120195. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Aguado, A.; Cernuda-Cernuda, R.; Álvarez, A.; Cepas, V.; González, I.Q.; Rosa, M.S. Melatonin uptake by cells: An answer to its relationship with glucose? Molecules 2018, 23, 1999. [Google Scholar] [CrossRef] [PubMed]
- Res, P. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor – dependent pathway in rats in vivo. J. Pineal Res. 2013, 54, 282–291. [Google Scholar]
- Tran, H.T.; Tran, P.H.; Lee, B. New findings on melatonin absorption and alterations by pharmaceutical excipients using the Ussing chamber technique with mounted rat gastrointestinal segments. Int. J. Pharm. 2009, 378, 9–16. [Google Scholar] [CrossRef]
- Batista, A.P.; Da Silva, T.G.; Teixeira, A.A.; De Medeiros, P.L.; Teixeira, V.W.; Alves, L.C.; Dos Santos, F.A.; Silva, E.C. Ultrastructural aspects of melatonin cytotoxicity on Caco-2 cells in vitro. Micron 2014, 59, 17–23. [Google Scholar] [CrossRef]




| Molecule | Column | UV-ʎ (nm) | Mobil Phase | Flow Rate (mL/min) | IV2 (µL) |
|---|---|---|---|---|---|
| Kt | C8, 150 × 2.1 mm, 5 µm | 313 | NH4H2PO:THF (70:30) | 0.25 | 100 |
| Mel | C18, 100 × 4.6 mm, 3.5 µm | 223 | H2O:MeOH (55:45) | 0.60 | 100 |
| Fur | C18, 150 × 3.9 mm, 4 µm | 230 | KH2PO4:CAN (80:20) | 0.50 | 100 |
| Htz | 100 ODS2, 100 × 4.6, 3 µm | 224 | NaH2PO4:MeOH:THF1 | 0.70 | 100 |
| Molecule | Structure | BCS | pKa | Dissolution Media | Concentration |
|---|---|---|---|---|---|
| Ketorolac tromethamine | 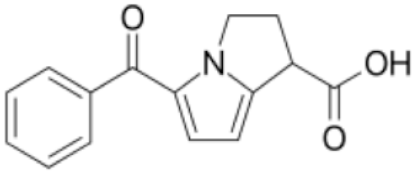 | Class 1 | 3.5 [22] | PBS pH 7.4 | 1 mg/mL |
| Melatonin | 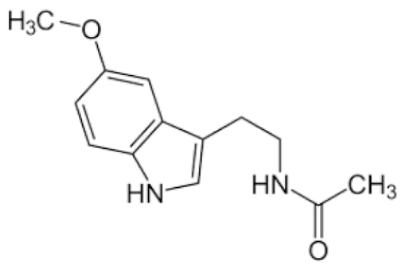 | Class 2 | 16.5 [23] | PBS pH 7.4 | 0.8 mg/mL |
| Hydrochlorothiazide | 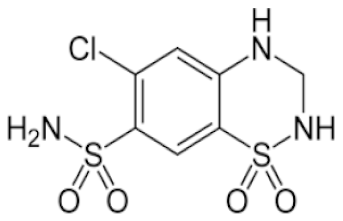 | Class 3 | 7.9 [24] | PBS pH 7.4 | 0.05 mg/mL |
| Furosemide |  | Class 4 | 3.9 [25] | PBS pH 7.4 | 0.6 mg/mL |
| Permeation Parameters | Kt | Mel | Htz | Fur |
|---|---|---|---|---|
| Flux (µg/min) | 0.855 ± 0.069 | 0.683 ± 0.016 | 0.004 ± 0.0002 | 0.0180 ± 0.0018 |
| Flux/sup (µg/(cm/min)) | 0.336 ± 0.027 | 0.268 ± 0.006 | 0.0015 ± 0.0001 | 0.0071 ± 0.0001 |
| Co (µg/mL) | 1000 | 800 | 50 | 600 |
| Papp (x10−6) (cm/s) | 5.609 ± 0.452 | 5.598 ± 0.130 | 0.487 ± 0.026 | 0.196 ± 0.020 |
| Drug | Papp (×10−6) (cm/s) (AP → BL)) Caco-2 Cells |
|---|---|
| Kt | 8.30 ± 5.20 (n = 6) (HBBS1 pH 7.4) [26] |
| Mel | 12.50 ± 0.01 (n = 3) (HEPES2 pH 7.4) [27] |
| Htz | 0.51 ± 0.02 (n = 3) (HBBS1 pH 7.4) [28] |
| Fur | 0.19 ± 0.01 (n = 3) (KBR3 pH 7.4) [29] |
| Drug | Papp (×10−6) (cm/s) (AP → BL) | ||
|---|---|---|---|
| Franz Diffusion Cells (PBS pH 7.4) | Caco-2 (pH 7.4) 1 | Unpaired t-Test (p) | |
| Kt | 5.61 ± 0.45 (n = 5) | 8.3 ± 5.2 (n = 6) [26] | 0.28 (p > 0.05) |
| Mel | 5.60 ± 0.13 (n = 5) | 12.50 ± 0.01 (n = 3) [27] | 0.0001 (p < 0.05) * |
| Htz | 0.49 ± 0.03 (n = 5) | 0.42 ± 0.33 (n = 3) [28] | 0.30 (p > 0.05) |
| Fur | 0.20 ± 0.020 (n = 5) | 0.19 ± 0.01 (n = 3) [29] | 0.87 (p > 0.05) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
B. Sánchez, A.; C. Calpena, A.; Mallandrich, M.; Clares, B. Validation of an Ex Vivo Permeation Method for the Intestinal Permeability of Different BCS Drugs and Its Correlation with Caco-2 In Vitro Experiments. Pharmaceutics 2019, 11, 638. https://doi.org/10.3390/pharmaceutics11120638
B. Sánchez A, C. Calpena A, Mallandrich M, Clares B. Validation of an Ex Vivo Permeation Method for the Intestinal Permeability of Different BCS Drugs and Its Correlation with Caco-2 In Vitro Experiments. Pharmaceutics. 2019; 11(12):638. https://doi.org/10.3390/pharmaceutics11120638
Chicago/Turabian StyleB. Sánchez, Aroha, Ana C. Calpena, Mireia Mallandrich, and Beatriz Clares. 2019. "Validation of an Ex Vivo Permeation Method for the Intestinal Permeability of Different BCS Drugs and Its Correlation with Caco-2 In Vitro Experiments" Pharmaceutics 11, no. 12: 638. https://doi.org/10.3390/pharmaceutics11120638
APA StyleB. Sánchez, A., C. Calpena, A., Mallandrich, M., & Clares, B. (2019). Validation of an Ex Vivo Permeation Method for the Intestinal Permeability of Different BCS Drugs and Its Correlation with Caco-2 In Vitro Experiments. Pharmaceutics, 11(12), 638. https://doi.org/10.3390/pharmaceutics11120638