Investigation of Silicone-Containing Semisolid in Situ Film-Forming Systems Using QbD Tools
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quality by Design Methodology
2.2.1. Definition of TPP and QTPP
2.2.2. Definition of CQA, CMA, CPP
2.2.3. Risk Assessment: Quality Tools
2.3. Preparation of FFSs
2.4. Investigation of the Mechanical Parameters of the Films
2.4.1. Measurement of Skin Adhesion
2.4.2. Measurement of Film Flexibility
2.4.3. Measurement of Film Burst Strength
2.5. Investigation of Film Integrity and Appeariance
2.6. Investigation of the Drying Time of FFSs
2.7. Statistical Analysis
3. Results & Discussion
3.1. Determination of QTPP and CQAs for In Situ FFSs
3.2. Initial Risk Assessment
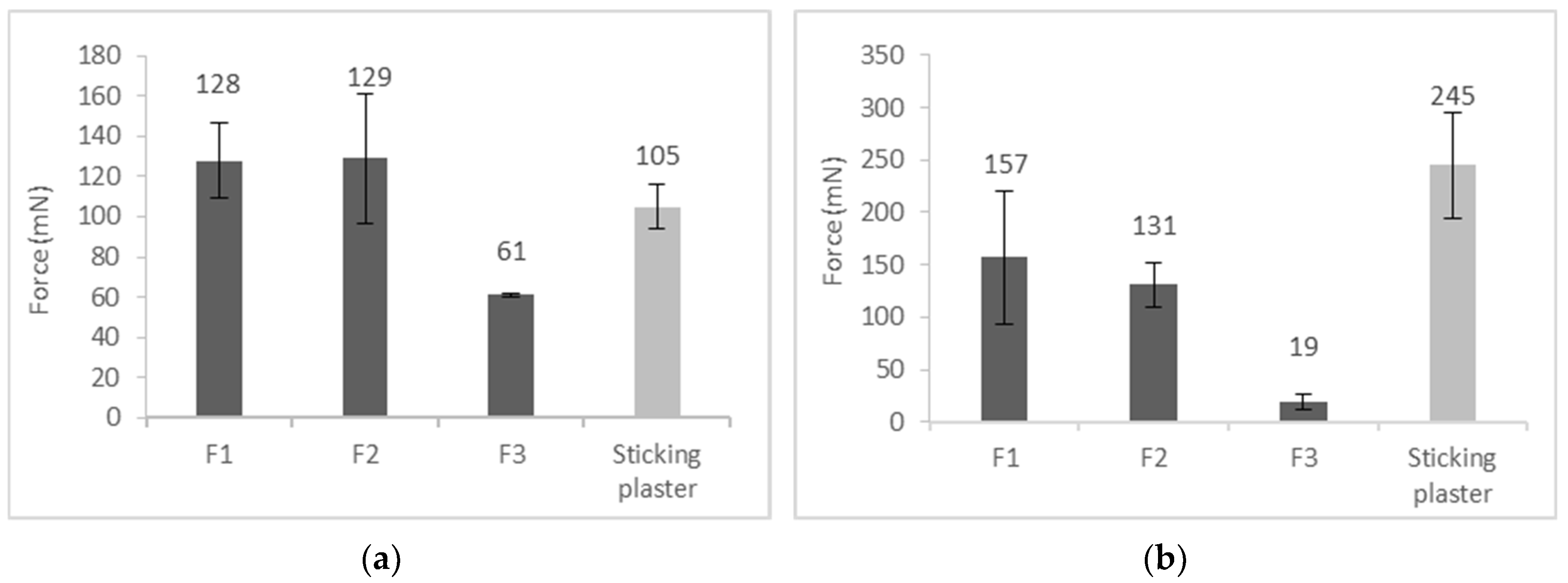
3.3. Skin Adhesion
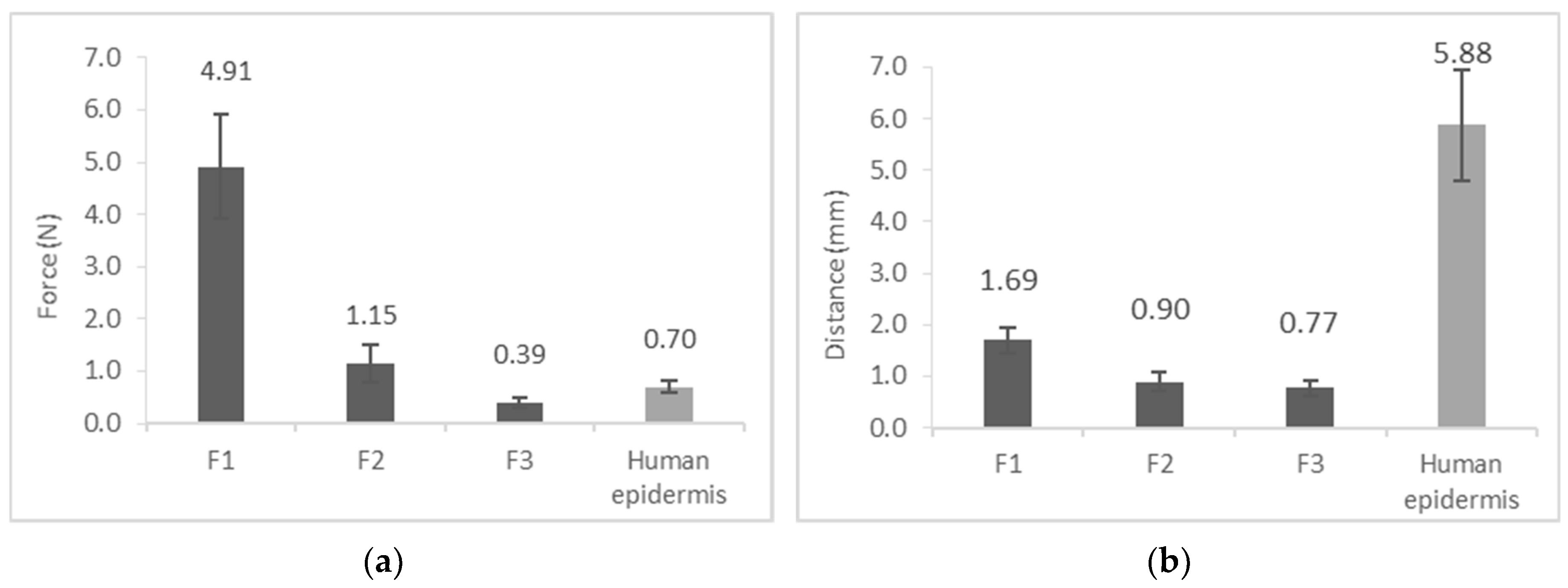
3.4. Film Flexibility (Resilience)
3.5. Film Burst Strength
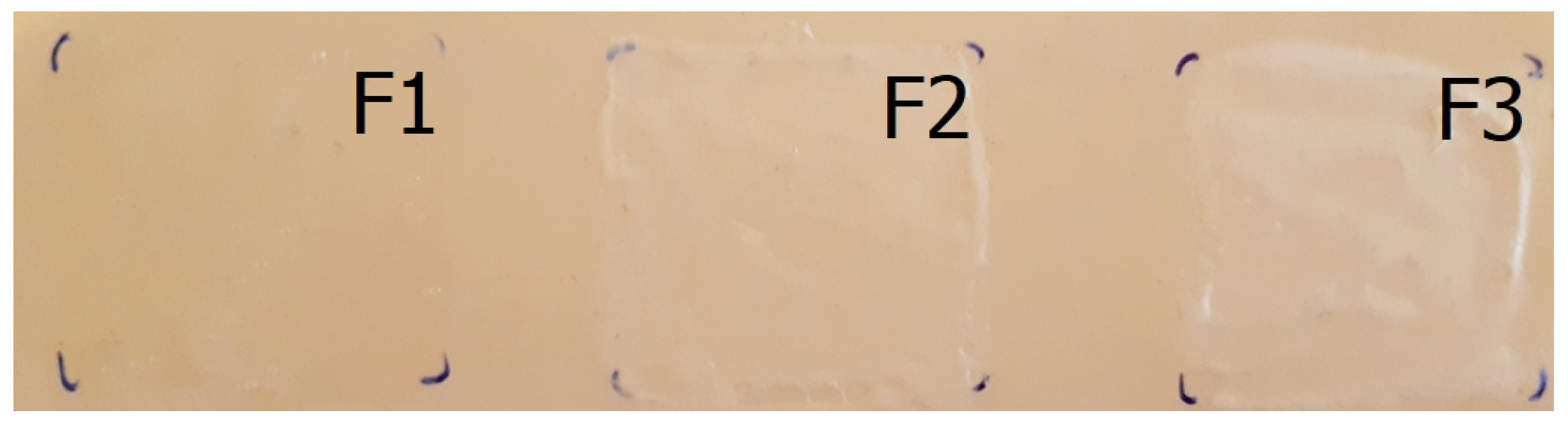
3.6. Film Appearance
3.7. Film Integrity
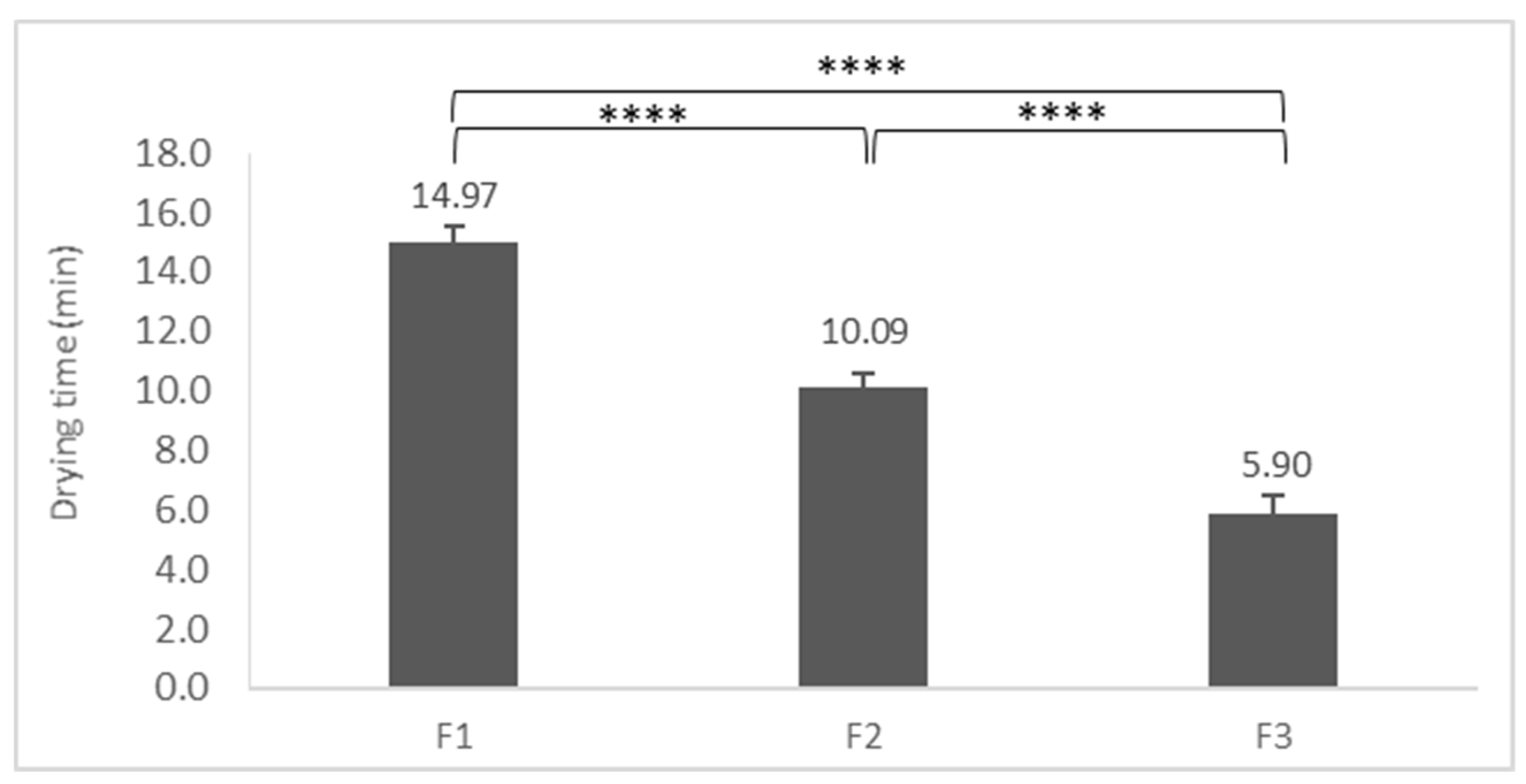
3.8. Drying Time of FFSs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Benítez, J.M.; Montáns, F.J. The mechanical behavior of skin: Structures and models for the finite element analysis. Comput. Struct. 2017, 190, 75–107. [Google Scholar] [CrossRef]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Tran, T.T.D.; Tran, P.H.L. Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Schröder, I.Z. Film Forming Polymeric Solutions as Drug Delivery Systems for the Skin. Ph.D. Thesis, Saarland University, Saarbrücken, Germany, 2007. [Google Scholar]
- McAuley, W.J.; Caserta, F. Film-Forming and Heated Systems. In Novel Delivery Systems for Transdermal and Intradermal Drug Delivery; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 97–124. [Google Scholar]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Frederiksen, K.; Guy, R.H.; Petersson, K. The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opin. Drug Deliv. 2016, 13, 349–360. [Google Scholar] [CrossRef]
- De Paepe, K.; Sieg, A.; Le Meur, M.; Rogiers, V. Silicones as Nonocclusive Topical Agents. Skin Pharmacol. Physiol. 2014, 27, 164–171. [Google Scholar] [CrossRef]
- Aliyar, H.; Schalau, G. Recent developments in silicones for topical and transdermal drug delivery. Ther. Deliv. 2015, 6, 827–839. [Google Scholar] [CrossRef]
- DiSapio, A.J. Silicones as Alternatives to Hydrocarbons in Personal Care Formulations. Available online: https://www.skinident.es/fileadmin/img/spanishpictures/pdf/Silicones_as_Alternatives_to_Hydrocarbons.pdf (accessed on 25 July 2019).
- Visser, J.C.; Dohmen, W.M.C.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W.; Woerdenbag, H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015, 485, 70–76. [Google Scholar] [CrossRef]
- Singh, H.; Khurana, L.K.; Singh, R. Pharmaceutical Development. In Pharmaceutical Medicine and Translational Clinical Research; Academic Press: Cambridge, MA, USA, 2018; pp. 33–46. [Google Scholar]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- International Council for Harmonisation ICH Q8 (R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-19.pdf (accessed on 4 July 2019).
- Gyulai, O.; Kovács, A.; Sovány, T.; Csóka, I.; Aigner, Z. Optimization of the critical parameters of the spherical agglomeration crystallization method by the application of the Quality by Design approach. Materials 2018, 14, 635. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation ICH Q9. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-3.pdf (accessed on 4 July 2019).
- Bakonyi, M.; Berkó, S.; Kovács, A.; Budai-Szűcs, M.; Kis, N.; Erős, G.; Csóka, I.; Csányi, E. Application of quality by design principles in the development and evaluation of semisolid drug carrier systems for the transdermal delivery of lidocaine. J. Drug Deliv. Sci. Technol. 2018, 44, 136–145. [Google Scholar] [CrossRef]
- Charoo, N.A.; Shamsher, A.A.A.; Zidan, A.S.; Rahman, Z. Quality by design approach for formulation development: A case study of dispersible tablets. Int. J. Pharm. 2012, 423, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L. Design Space and QbD Approach for Production of Drug Nanocrystals by Wet Media Milling Techniques. Pharmaceutics 2018, 10, 104. [Google Scholar] [CrossRef]
- Kovács, A.; Berkó, S.; Csányi, E.; Csóka, I. Development of nanostructured lipid carriers containing salicyclic acid for dermal use based on the Quality by Design method. Eur. J. Pharm. Sci. 2017, 99, 246–257. [Google Scholar] [CrossRef]
- Dhat, S.; Pund, S.; Kokare, C.; Sharma, P.; Shrivastava, B. Risk management and statistical multivariate analysis approach for design and optimization of satranidazole nanoparticles. Eur. J. Pharm. Sci. 2017, 96, 273–283. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, D.Y.; Seo, H.W.; Jeong, S.H.; Kim, J.H.; Kim, M.S. Preparation of erythromycin-loaded poly(vinylalcohol) film and investigation of its feasibility as a transdermal delivery carrier. Tissue Eng. Regen. Med. 2014, 11, 211–216. [Google Scholar] [CrossRef]
- Hiorth, M.; Nilsen, S.; Tho, I. Bioadhesive Mini-Tablets for Vaginal Drug Delivery. Pharmaceutics 2014, 6, 494–511. [Google Scholar] [CrossRef]
- Texture Analysis in Action Peel Testing. Available online: https://textureanalysisprofessionals.blogspot.com/2015/10/texture-analysis-in-action-peel-testing.html (accessed on 2 July 2019).
- Texture Analysis in Action: The Film Support Rig. Available online: https://textureanalysisprofessionals.blogspot.com/2015/02/texture-analysis-in-action-film-support.html (accessed on 2 July 2019).
- Kligman, A.M.; Christophers, E. Preparation of Isolated Sheets of Human Stratum Corneum. Arch. Dermatol. 1963, 88, 702–705. [Google Scholar] [CrossRef]
- Saravanan, J.; Elango, K.; Chellakumari, S.D.; Arulkumar, K. Formulation and evaluation of film forming gel of diclofenac diethylamine. World J. Pharm. Pharm. Sci. 2018, 7, 1106–1117. [Google Scholar]
- Klykken, P.; Servinski, M.; Thomas, X. Silicone Film-Forming Technologies for Health Care Applications. Available online: https://pdfs.semanticscholar.org/53f4/f961044f0a08c2425d187f1a08eba874f124.pdf (accessed on 2 July 2019).
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Raza, K.; Katare, O.P. Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv. 2016, 23, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Yeom, S.B.; Choi, D.H. Scale-Up Strategy in Quality by Design Approach for Pharmaceutical Blending Process with Discrete Element Method Simulation. Pharmaceutics 2019, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Tahsin, M.; Karaman, S.; Dogan, M.; Yetim, H.; Kayacier, A. Characterization of O/W model system meat emulsions using shear creep and creep recovery tests based on mechanical simulation models and their correlation with texture profile analysis ( TPA ) parameters. J. Food Eng. 2012, 108, 327–336. [Google Scholar]
- Dow Corning® ST-Cyclomethicone 5-NF, Product Information, Healthcare Solution. Available online: https://www.dupont.com/content/dam/Dupont2.0/Products/transportation/Literature/Downloaded-TDS/0901b803809b7844.pdf (accessed on 3 July 2019).
- Stable Micro Systems-Applications. Available online: https://www.stablemicrosystems.com/TextureAnalysisApplications.html (accessed on 3 July 2019).





| Composition | Function of the Excipient | F1 | F2 | F3 |
|---|---|---|---|---|
| Purified water with methyl parahydroxybenzoate | Solvent with preservatives | + | + | + |
| Ethanol 96% | Drying | + | + | + |
| PVA | Film forming, Viscosity enhancing | + | + | + |
| Xanthan gum | Film forming, Viscosity enhancing | + | + | - |
| Dimethiconol Blend 20 | Film forming | - | + | - |
| ST-Elastomer 10 | Film forming | - | + | - |
| 7-3101 Elastomer Blend HIP Emulsion | Film forming | - | - | + |
| ST-Cyclometicone 5NF | Drying excipient | - | + | + |
| QTPP | Target | Justification |
|---|---|---|
| Route of administration | Dermal | Dermal delivery is an opportunity to achieve a high concentration of the drugs on the application site and to avoid systemic side effects. Furthermore, it is a non-invasive and painless administration route, resulting in high patient compliance. |
| Dosage form | Semisolid in situ film-forming system | Suitable for dermal and transdermal application, it has a longer residence time and prevents smearing. Dermatological treatment may require less frequent dosing of the FFS [7]. |
| Site of activity | Deeper layer of the skin | The dermal drug delivery system ensures penetration through the skin as deep as the therapeutic effects require. |
| Appearance of semisolid system | Transparent or white, homogeneous | To ensure the aesthetic appearance of the film, the semisolid system has to show similar properties. To increase patient compliance [7]. |
| Stability (physical, chemical) | Homogeneous; in the formulation there is no visible sign of instability | To ensure applicability, stability is a critical point of formulation. Appearance change, phase separation, pH change and viscosity change are stability issues, which can inhibit usability. |
| Silicone content | Film formation, fast-drying, silky touch | To change the mechanical and drying properties of the film favorably. Silky on the skin, but neither shiny nor greasy [29]. |
| Packaging material type | Well closing Appropriate for the dosage form | The system includes volatile components. The packaging material type is important to keep these volatile components in the formulation [15]. |
| Mechanical properties of film for skin application | Flexible, highly adherent, resistant film | In order to ensure that the composition achieves the desired effect, the in situ formed film needs to have suitable mechanical properties, such as to stay on the skin constantly, to have flexible movement similar to that of the skin and to be easily removable in one piece [28]. |
| CQA | Target | Justification |
|---|---|---|
| Semisolid System Properties | ||
| Physical properties (color, odor, appearance) | Translucent or white appearance, homogeneous, clear and odorless smell | To increase patient compliance. |
| Viscosity | Optimal spreadability on the skin (range: 50,000–150,000 mPas) | Rheological attributes, e.g., viscosity, to influence the application of the formulation on the skin and the stability of the semisolid system [28]. |
| Homogeneity | Homogenous distribution of the components in the formulation | Homogeneity ensures stability and aesthetic appearance. During application the uniformity of dosage units has to be maintained. |
| pH | Optimal pH of transdermal formulation (range: 4–8 pH) | For the safety and efficacy of the product. |
| Skin feeling | Not sticky, not greasy, silky touch on the skin | Formulations containing silicones influence skin feeling. They are slightly slippery and silky on the skin. Due to the semi-occlusive effect of silicones, the skin could be softer and well-hydrated [29]. |
| Drying time | Optimal drying time for comfortable use (within 10 min) | The optimal drying time is a critical point of comfortable use. The formulation has to dry fast to avoid smearing [28]. |
| Stability (physical, chemical) | No visible sign of instability at the time of preparation and after one month (at room temperature) | The physical and chemical stability of the semisolid system is essential to form a homogeneous, aesthetic appearance, and these properties ensure the mechanical attributes of the film for skin application. |
| Stability (microbiological) | Meets the requirements of the pharmacopoeia for dermally-applied systems | The safety of the FFS is a requirement for marketing authorization. |
| Film properties | ||
| Film appearance | Translucent, homogeneous, compact film | To increase patient compliance (almost invisible, not shiny, easy to remove) [7]. |
| Film burst strength | Compact film structure approaches the properties of the heat-separated human epidermis (range: under 5 N) | The film has to be strong enough to form a compact film on the skin and not to tear when the skin is moving. |
| Skin adhesion | Approach the adhesion of an adhesive plaster to the skin. (Mean peel range: 100–500 mN) | Good adhesion ensures the residence time on the skin for the appropriate exposure time. |
| Film flexibility | Approach the properties of the heat-separated human epidermis (range: above 25%) | To follow the skin moving, thereby avoiding the separation from the skin surface [28]. |
| Film integrity | Compact film on the skin surface | To provide aesthetic appearance and easy removability. It can be pulled down completely [28]. |
| QTPPs | Impact | CQAs | CPPs and CMAs | Occurrence |
|---|---|---|---|---|
| Route of administration | High | Physical properties | Mixing rate | Medium |
| Dosage form | High | Viscosity | Mixing time | Low |
| Site of activity | Medium | Homogeneity | Type of mixer | Medium |
| Appearance of semisolid system | Medium | pH | Temperature of technology | High |
| Stability | High | Skin feeling | Type of technology | High |
| Silicone content | High | Drying time | Viscosity enhancing excipients | Medium |
| Type of packaging material | Medium | Stability (physical, chemical) | Preservatives | Low |
| Mechanical properties of film for skin application | High | Stability (microbiol.) | Drying excipients | High |
| Film appearance | Film-forming excipients | High | ||
| Film burst strength | Type of silicones | High | ||
| Skin adhesion | ||||
| Film flexibility | ||||
| Film integrity |
| QTPP | Route of Administration (H) | Dosage Form (H) | Site of Activity (M) | Appearance of Semisolid System (M) | Stability (H) | Silicone Content (H) | Type of Packaging Material (M) | Mechanical Properties of Film for Skin Application (H) | |
|---|---|---|---|---|---|---|---|---|---|
| CQAs | |||||||||
| Physical properties | 5% | Low | Low | Low | High | High | Medium | Medium | Low |
| Viscosity | 6% | Medium | Medium | Low | Medium | Medium | Medium | Medium | High |
| Homogeneity | 6% | Low | High | Low | High | High | Medium | Low | Low |
| pH | 5% | High | Medium | Medium | Low | Medium | Low | Low | Low |
| Skin feeling | 5% | Medium | Medium | Low | Low | Low | High | Low | Medium |
| Drying time | 9% | Medium | High | High | Low | Medium | High | Medium | High |
| Stability (physical, chemical) | 7% | Low | High | Low | Medium | High | Medium | Medium | Medium |
| Stability (microbiol.) | 5% | Low | High | Low | Medium | High | Low | Low | Low |
| Film appearance | 10% | Medium | High | Low | Medium | High | High | Low | High |
| Film burst strength | 11% | High | High | Low | Low | High | High | Low | High |
| Skin adhesion | 11% | High | High | Medium | Low | High | High | Low | High |
| Film flexibility | 11% | High | High | Medium | Low | High | High | Low | High |
| Film integrity | 9% | Low | High | Medium | Low | High | High | Low | High |
| CMAs, CPPs | Mixing Rate (M) CPP | Mixing Time (L) CPP | Type of Mixer (M) CPP | Temperature of Technology (H) CPP | Type of Technology (H) CPP | Viscosity Enhancing Excipients (M) CMA | Preserv-Atives (L) CMA | Drying Excipients (H) CMA | Film-Forming Excipients (H) CMA | Type of Silicones (H) CMA | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CQAs | |||||||||||
| Physical properties | 5% | High | Medium | High | High | High | High | Low | Medium | High | Medium |
| Viscosity | 6% | High | Medium | Medium | High | High | High | Medium | Medium | Medium | Medium |
| Homogeneity | 6% | High | High | High | High | High | High | Low | Medium | High | High |
| pH | 5% | Low | Low | Low | Low | Low | High | Low | Low | Low | Low |
| Skin feeling | 5% | Low | Low | Low | Low | Low | Medium | Low | High | High | High |
| Drying time | 9% | Low | Low | Low | Low | Low | Medium | Low | High | Medium | High |
| Stability (physical, chemical) | 7% | High | High | High | High | High | High | Low | Medium | High | High |
| Stability (microbiol.) | 5% | Low | Low | Low | Low | Low | Low | High | Low | Low | Low |
| Film appearance | 10% | Low | Low | Low | Low | Medium | Medium | Low | High | High | High |
| Film burst strength | 11% | Low | Low | Low | Low | Low | Medium | Low | High | High | High |
| Skin adhesion | 11% | Low | Low | Low | Low | Low | Medium | Low | High | High | High |
| Film flexibility | 11% | Low | Low | Low | Low | Low | Medium | Low | High | High | High |
| Film integrity | 9% | Low | Low | Low | Low | Low | Medium | Low | High | High | High |
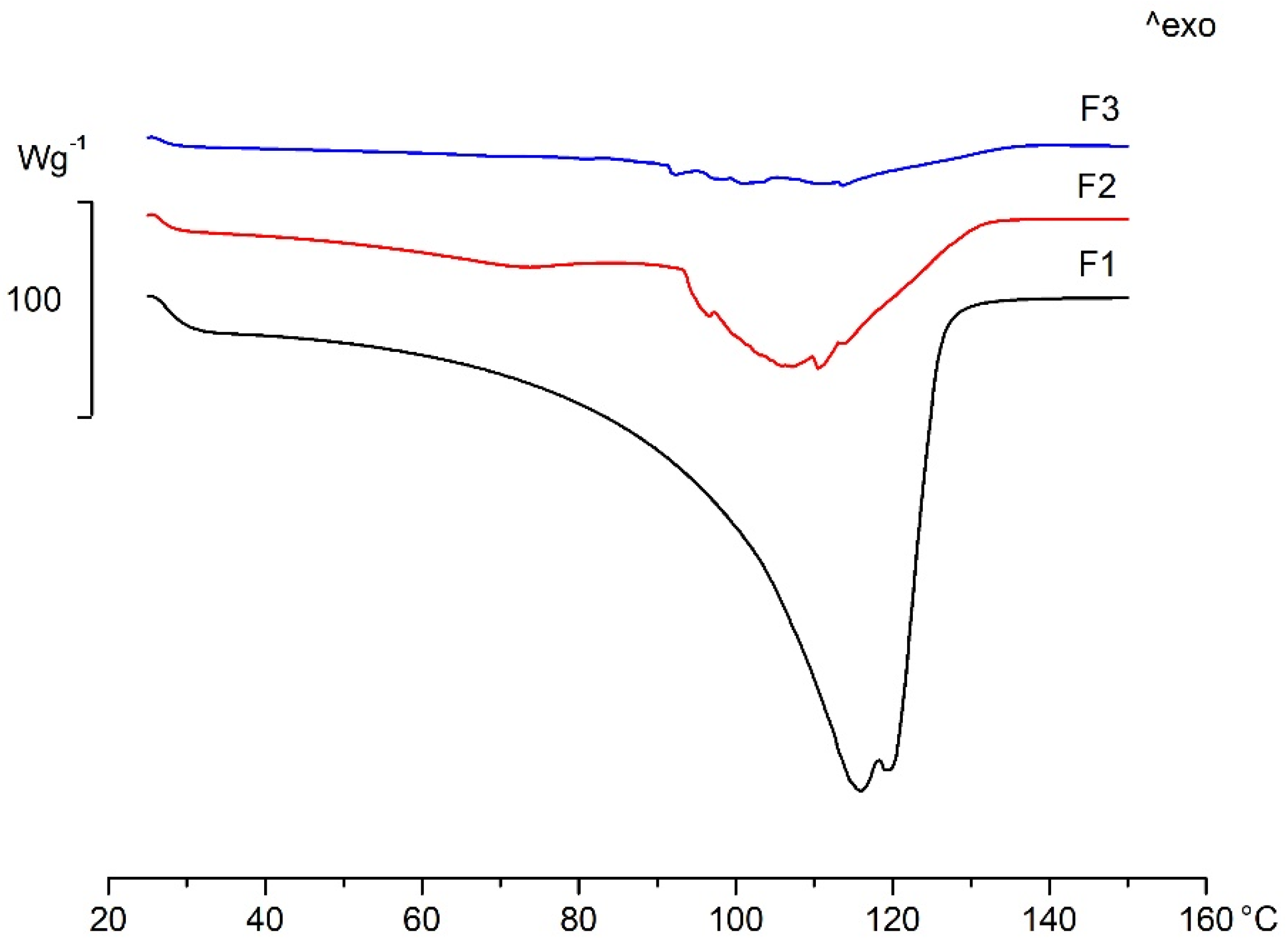
| FFS | Onset (°C) | Peak (°C) | Endset (°C) |
|---|---|---|---|
| F1 | 93.71 | 107.01 | 140.21 |
| F2 | 59.16 | 71.70 | 80.02 |
| 91.57 | 107.62 | 113.15 | |
| F3 | 90.67 | 91.54 | 93.77 |
| 109.34 | 112.62 | 120.91 |
| Formulation | F1 | F2 | F3 | |
|---|---|---|---|---|
| Investigation | ||||
| Skin adhesion | ✔ | ✔ | ✖ | |
| Film flexibility | ✔ | ✔ | ✖ | |
| Film burst strength | ✔ | ✔ | ✔ | |
| Film appearance | ✔ | ✔ | ✔ | |
| Film integrity | ✔ | ✔ | ✔ | |
| Drying time | ✖ | ✔ | ✔✔ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, N.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Csányi, E.; Csóka, I.; Berkó, S. Investigation of Silicone-Containing Semisolid in Situ Film-Forming Systems Using QbD Tools. Pharmaceutics 2019, 11, 660. https://doi.org/10.3390/pharmaceutics11120660
Kis N, Kovács A, Budai-Szűcs M, Gácsi A, Csányi E, Csóka I, Berkó S. Investigation of Silicone-Containing Semisolid in Situ Film-Forming Systems Using QbD Tools. Pharmaceutics. 2019; 11(12):660. https://doi.org/10.3390/pharmaceutics11120660
Chicago/Turabian StyleKis, Nikolett, Anita Kovács, Mária Budai-Szűcs, Attila Gácsi, Erzsébet Csányi, Ildikó Csóka, and Szilvia Berkó. 2019. "Investigation of Silicone-Containing Semisolid in Situ Film-Forming Systems Using QbD Tools" Pharmaceutics 11, no. 12: 660. https://doi.org/10.3390/pharmaceutics11120660
APA StyleKis, N., Kovács, A., Budai-Szűcs, M., Gácsi, A., Csányi, E., Csóka, I., & Berkó, S. (2019). Investigation of Silicone-Containing Semisolid in Situ Film-Forming Systems Using QbD Tools. Pharmaceutics, 11(12), 660. https://doi.org/10.3390/pharmaceutics11120660






