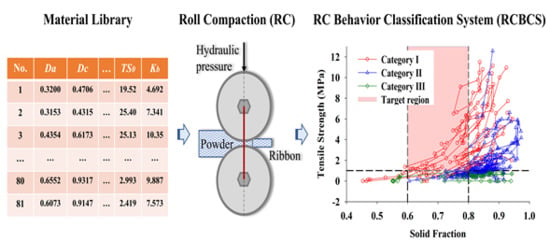
Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction
Abstract
Share and Cite
Yu, J.; Xu, B.; Zhang, K.; Shi, C.; Zhang, Z.; Fu, J.; Qiao, Y. Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction. Pharmaceutics 2019, 11, 662. https://doi.org/10.3390/pharmaceutics11120662
Yu J, Xu B, Zhang K, Shi C, Zhang Z, Fu J, Qiao Y. Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction. Pharmaceutics. 2019; 11(12):662. https://doi.org/10.3390/pharmaceutics11120662
Chicago/Turabian StyleYu, Jiaqi, Bing Xu, Kunfeng Zhang, Chenfeng Shi, Zhiqiang Zhang, Jing Fu, and Yanjiang Qiao. 2019. "Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction" Pharmaceutics 11, no. 12: 662. https://doi.org/10.3390/pharmaceutics11120662
APA StyleYu, J., Xu, B., Zhang, K., Shi, C., Zhang, Z., Fu, J., & Qiao, Y. (2019). Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction. Pharmaceutics, 11(12), 662. https://doi.org/10.3390/pharmaceutics11120662