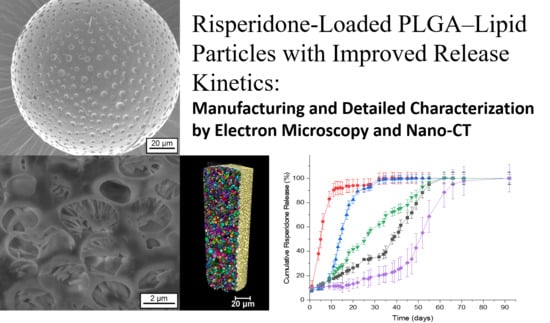
Risperidone-Loaded PLGA–Lipid Particles with Improved Release Kinetics: Manufacturing and Detailed Characterization by Electron Microscopy and Nano-CT
Abstract
1. Introduction
- (i)
- development of risperidone loaded PLGA–lipid microcapsules (MCs) and PLGA–lipid microgels (MGs) with an optimized release profile (no lag time, constant release rate)
- (ii)
- characterization of the three-dimensional inner structure of risperidone-loaded MCs and MGs by using focused ion beam (FIB) preparation and three-dimensional X-ray imaging (nano-CT).
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PLGA Microcapsules (MC) and PLGA Microgels (MG)
2.2.2. Lyophilization
2.2.3. Static Light Scattering Measurements
2.2.4. Powder XRD
2.2.5. High-Performance Liquid Chromatography (HPLC)
2.2.6. Drug Loading and Encapsulation Efficiency (EE)
2.2.7. In Vitro Drug Release Assay
2.2.8. Scanning Electron Microscopy and Focused Ion Beam Preparation
2.2.9. Nano-CT
3. Results and Discussion
3.1. Preparation of MCs and MGs
3.2. Characterization
3.2.1. Particle Size Measurements
3.2.2. Scanning Electron Microscopy and Focused Ion Beam Investigations
4. X-Ray Nano-Imaging (Nano-CT)
5. Drug Release
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable formulations of poly(lactic acid) and its copolymers in clinical use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.V.; Goldstein, D.A. Dexamethasone intravitreal implant (allergan) for the treatment of noninfectious uveitis. Expert Opin. Orphan Drugs 2014, 2, 301–310. [Google Scholar] [CrossRef]
- Hänggi, D.; Etminan, N.; Macdonald, R.L.L.; Steiger, H.J.H.J.; Mayer, S.A.S.A.; Aldrich, F.; Diringer, M.N.M.N.; Hoh, B.L.B.L.; Mocco, J.; Strange, P.; et al. NEWTON: Nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage. Neurocrit. Care 2015, 23, 274–284. [Google Scholar] [CrossRef]
- Plontke, S.K.; Glien, A.; Rahne, T.; Mäder, K.; Salt, A.N. Controlled release dexamethasone implants in the round window niche for salvage treatment of idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 2014, 35, 1168–1171. [Google Scholar] [CrossRef]
- Mäder, K.; Lehner, E.; Liebau, A.; Plontke, S.K.S.K. Controlled drug release to the inner ear: Concepts, materials, mechanisms, and performance. Hear. Res. 2018, 368, 49–66. [Google Scholar] [CrossRef]
- Citrome, L. Long-acting injectable antipsychotics update: Lengthening the dosing interval and expanding the diagnostic indications. Expert Rev. Neurother. 2017, 17, 1029–1043. [Google Scholar] [CrossRef]
- Chou, Y.H.; Chu, P.C.; Wu, S.W.; Lee, J.C.; Lee, Y.H.; Sun, I.W.; Chang, C.L.; Huang, C.L.; Liu, I.C.; Tsai, C.F.; et al. A systemic review and experts’ consensus for long-acting injectable antipsychot. Clin. Psychopharmacol. Neurosci. 2015, 13, 121–128. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021346s015lbl.pdf (accessed on 4 December 2019).
- Rawat, A.; Bhardwaj, U.; Burgess, D.J. Comparison of in vitro-in vivo release of Risperdal® Consta® microspheres. Int. J. Pharm. 2012, 434, 115–121. [Google Scholar] [CrossRef]
- Shen, J.; Choi, S.; Qu, W.; Wang, Y.; Burgess, D.J. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J. Control. Release 2015, 218, 2–12. [Google Scholar] [CrossRef]
- Andhariya, J.V.; Shen, J.; Wang, Y.; Choi, S.; Burgess, D.J. Effect of minor manufacturing changes on stability of compositionally equivalent PLGA microspheres. Int. J. Pharm. 2019, 566, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, M.; Zhang, W.; Yang, Z.; Xu, S.; Shang, Q. Influence of drying processes on the structures, morphology and in vitro release profiles of risperidone-loaded PLGA microspheres. J. Microencapsul. 2019, 36, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Turek, A.; Borecka, A.; Janeczek, H.; Sobota, M.; Kasperczyk, J. Formulation of delivery systems with risperidone based on biodegradable terpolymers. Int. J. Pharm. 2018, 548, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, L.; Fan, C.; Yu, K.; Liu, X.; Zhao, X.; Wang, D.; Liu, W.; Su, Z.; Sun, F.; et al. Development of near zero-order release PLGA-based microspheres of a novel antipsychotic. Int. J. Pharm. 2017, 516, 32–38. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.; Faraj, J.; Deluca, P.; D’Souza, S.; Faraj, J.; Deluca, P. Microsphere delivery of Risperidone as an alternative to combination therapy. Eur. J. Pharm. Biopharm. 2013, 85, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, M.; Engert, J.; Winter, G. Long-term release and stability of pharmaceutical proteins delivered from solid lipid implants. Eur. J. Pharm. Biopharm. 2017, 117, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Fameau, A.-L.; Houinsou-Houssou, B.; Novales, B.; Navailles, L.; Nallet, F.; Douliez, J.-P. 12-Hydroxystearic acid lipid tubes under various experimental conditions. J. Colloid Interface Sci. 2010, 341, 38–47. [Google Scholar] [CrossRef]
- Co, E.; Marangoni, A.G. The formation of a 12-hydroxystearic acid/vegetable oil organogel under shear and thermal fields. J. Am. Oil Chem. Soc. 2013, 90, 529–544. [Google Scholar] [CrossRef]
- Mäder, K.; Windorf, M.; Kutza, J. Injizierbare Depotformulierungen zur Kontrollierten Wirkstofffreisetzung. WO2015062571, 29 October 2014. [Google Scholar]
- Lampp, L.; Rogozhnikova, O.Y.; Trukhin, D.V.; Tormyshev, V.M.; Bowman, M.K.; Devasahayam, N.; Krishna, M.C.; Mäder, K.; Imming, P. A radical containing injectable in-situ-oleogel and emulgel for prolonged in-vivo oxygen measurements with CW EPR. Free Radic. Biol. Med. 2019, 130, 120–127. [Google Scholar] [CrossRef]
- Garner, J.; Skidmore, S.; Park, H.; Park, K.; Choi, S.; Wang, Y. A protocol for assay of poly(lactide-co-glycolide) in clinical products. Int. J. Pharm. 2015, 495, 87–92. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Y.; Yuan, W.; Wu, F.; Su, J.; Jin, T. Effect of bases with different solubility on the release behavior of risperidone loaded PLGA microspheres. Colloids Surf. B Biointerfaces 2011, 86, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.zeiss.com/content/dam/Microscopy/us/download/pdf/technical-notes/x-ray-microscopy/en_44_013_027_tech-note_ultra-nanotomography.pdf (accessed on 4 October 2019).
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Barrow, W.W. Microsphere technology for chemotherapy of mycobacterial infections. Curr. Pharm. Des. 2004, 10, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Dawes, G.J.S.; Fratila-Apachitei, L.E.; Mulia, K.; Apachitei, I.; Witkamp, G.J.; Duszczyk, J. Size effect of PLGA spheres on drug loading efficiency and release profiles. J. Mater. Sci. Mater. Med. 2009, 20, 1089–1094. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- D’Souza, S.; Faraj, J.A.; Giovagnoli, S.; DeLuca, P.P. Development of risperidone PLGA microspheres. J. Drug Deliv. 2014, 620464. [Google Scholar] [CrossRef]
- Krochmal, B.; Diller, D.; Dolitzky, B.-Z.; Aronhime, J. Preparation of Risperidone. WO2002012200A9, 8 August 2001. [Google Scholar]
- Bagratashvili, V.N.; Bogorodskiy, S.E.; Egorov, A.M.; Krotova, L.I.; Mironov, A.V.; Parenago, O.O.; Pokrovskiy, O.I.; Ustinovich, K.B.; Chizhov, P.S.; Prokopchuk, D.I.; et al. Polymorphism of risperidone in supercritical fluid processes of micronization and encapsulation into aliphatic polyesters. Russ. J. Phys. Chem. B 2017, 11, 1163–1172. [Google Scholar] [CrossRef]
- Karabas, I.; Orkoula, M.G.; Kontoyannis, C.G. Analysis and stability of polymorphs in tablets: The case of Risperidone. Talanta 2007, 71, 1382–1386. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Weitkamp, T.; Bunk, O.; David, C. Phase retrieval and differential phase-contrast imaging with low-brilliance X-ray sources. Nat. Phys. 2006, 2, 258–261. [Google Scholar] [CrossRef]
- Kaulich, B.; Thibault, P.; Gianoncelli, A.; Kiskinova, M. Transmission and emission x-ray microscopy: Operation modes, contrast mechanisms and applications. J. Phys. Condens. Matter 2011, 23, 083002. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.J.; Gao, D.; Gureyev, T.E.; Stevenson, A.W.; Wilkins, S.W. Phase-contrast imaging of weakly absorbing materials using hard X-rays. Nature 1995, 373, 595–598. [Google Scholar] [CrossRef]
- Núñez, J.A.; Goring, A.; Hesse, E.; Thurner, P.J.; Schneider, P.; Clarkin, C.E. Simultaneous visualisation of calcified bone microstructure and intracortical vasculature using synchrotron X-ray phase contrast-enhanced tomography. Sci. Rep. 2017, 7, 13289. [Google Scholar] [CrossRef] [PubMed]
- Olivo, A.; Castelli, E. X-ray phase contrast imaging: From synchrotrons to conventional sources. Riv. Nuovo Cim. 2014, 37, 467–508. [Google Scholar]











| Sample | PLGA (mg) | Risperidone [mg] | MCT (mg) | IPM (mg) | HS (mg) | % Encapsul. Efficiency [+/−% SD] |
|---|---|---|---|---|---|---|
| MC-IPM | 100 | 50 | 0 | 100 | 0 | 92.62 [0.94] |
| MG-IPM | 100 | 50 | 0 | 90 | 10 | 100.82 [5.50] |
| MG-MCT | 100 | 50 | 90 | 0 | 10 | 96.05 [3.98] |
| MC-MCT | 100 | 50 | 100 | 0 | 0 | 92.79 [3.23] |
| Sample | D(0.1) (µm) | D(0.5) (µm) | D(0.9) (µm) | Mean D(4,3) (µm) | Span | Uniformity |
|---|---|---|---|---|---|---|
| Risperdal Consta® | 50.7 | 89.1 | 154.5 | 96.9 | 1.165 | 0.36 |
| MC-IPM | 126.1 | 172.8 | 236.3 | 178.1 | 0.637 | 0.202 |
| MG-IPM | 116.4 | 157.2 | 212.6 | 161.2 | 0.612 | 0.193 |
| MG-MCT | 115.3 | 155.9 | 211.8 | 160.2 | 0.620 | 0.196 |
| MC-MCT | 123.7 | 169.5 | 230.8 | 174.4 | 0.631 | 0.197 |
| Sample | Volume ISS | ISS Diameter | ISS Sphericity |
|---|---|---|---|
| Risperdal Consta® | 13% | 0.8 ± 0.2 μm | 0.95 ± 0.02 |
| MG-MCT | 35% | 2.0 ± 0.9 μm | 0.8 ± 0.2 |
| MC-MCT | 46% | 2.2 ± 0.8 μm | 0.7 ± 0.1 |
| MC-IPM | 39% | 1.9 ± 1.3 μm | 0.8 ± 0.2 |
| MG-IPM | 43% | 2.3 ± 1.0 μm | 0.8 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janich, C.; Friedmann, A.; Martins de Souza e Silva, J.; Santos de Oliveira, C.; Souza, L.E.d.; Rujescu, D.; Hildebrandt, C.; Beck-Broichsitter, M.; Schmelzer, C.E.H.; Mäder, K. Risperidone-Loaded PLGA–Lipid Particles with Improved Release Kinetics: Manufacturing and Detailed Characterization by Electron Microscopy and Nano-CT. Pharmaceutics 2019, 11, 665. https://doi.org/10.3390/pharmaceutics11120665
Janich C, Friedmann A, Martins de Souza e Silva J, Santos de Oliveira C, Souza LEd, Rujescu D, Hildebrandt C, Beck-Broichsitter M, Schmelzer CEH, Mäder K. Risperidone-Loaded PLGA–Lipid Particles with Improved Release Kinetics: Manufacturing and Detailed Characterization by Electron Microscopy and Nano-CT. Pharmaceutics. 2019; 11(12):665. https://doi.org/10.3390/pharmaceutics11120665
Chicago/Turabian StyleJanich, Christopher, Andrea Friedmann, Juliana Martins de Souza e Silva, Cristine Santos de Oliveira, Ligia E. de Souza, Dan Rujescu, Christian Hildebrandt, Moritz Beck-Broichsitter, Christian E. H. Schmelzer, and Karsten Mäder. 2019. "Risperidone-Loaded PLGA–Lipid Particles with Improved Release Kinetics: Manufacturing and Detailed Characterization by Electron Microscopy and Nano-CT" Pharmaceutics 11, no. 12: 665. https://doi.org/10.3390/pharmaceutics11120665
APA StyleJanich, C., Friedmann, A., Martins de Souza e Silva, J., Santos de Oliveira, C., Souza, L. E. d., Rujescu, D., Hildebrandt, C., Beck-Broichsitter, M., Schmelzer, C. E. H., & Mäder, K. (2019). Risperidone-Loaded PLGA–Lipid Particles with Improved Release Kinetics: Manufacturing and Detailed Characterization by Electron Microscopy and Nano-CT. Pharmaceutics, 11(12), 665. https://doi.org/10.3390/pharmaceutics11120665