Combined Modality Therapy Based on Hybrid Gold Nanostars Coated with Temperature Sensitive Liposomes to Overcome Paclitaxel-Resistance in Hepatic Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the DG-PEG-Cys-9R Complex
2.2.1. DG-PEG Synthesis
2.2.2. Synthesis of DG-PEG-Cys Complex
2.2.3. Synthesis of the DG-PEG-Cys-9R Complex
2.3. Preparation of siCOX-2(9R/DG-GNS)
2.3.1. Preparation of GNS
2.3.2. Preparation of 9R/DG-GNS
2.3.3. Preparation of siCOX-2(9R/DG-GNS)
2.4. Preparation of PTX-TSL-siCOX-2(9R/DG-GNS)
2.5. Characterization of PTX-TSL-siCOX-2(9R/DG-GNS)
2.5.1. Characterization of DG-PEG-Cys-9R Complex
2.5.2. Physicochemical Property of Hybrid Gold Nanostars
2.6. Cell Experiments
2.6.1. Cell Biocompatibility
2.6.2. In Vitro Cellular Uptake Assays
2.6.3. Analysis of In Vitro Gene Silencing
2.6.4. Cell Growth and Anti-Tumor Drug Resistance
2.6.5. Cell Apoptosis Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of DG-PEG-Cys-9R, 9R/DG-GNS and TSL-9R/DG-GNS
3.2. Particle Size, Zeta Potentials, and Morphology of the Nanocarriers
3.3. Spectral Identification and Photothermal Effects of the Nanocarriers
3.4. Analysis of the siCOX-2 Encapsulation
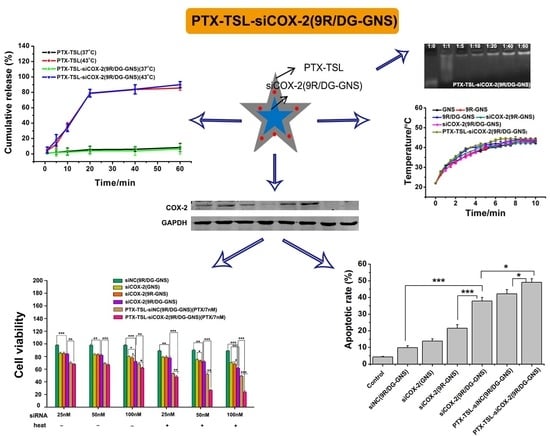
3.5. Detection of Drug Loading and Release Capacity of PTX-TSL
3.6. Stability of the Co-Delivery System PTX-TSL (siCOX-2(9R/DG-GNS))
3.7. In Vitro Formulation Compatibility
3.8. In Vitro Cellular Uptake
3.9. Gene Silencing Efficiency of PTX-TSL-siCOX-2(9R/DG-GNS)
3.10. Effects of PTX-TSL-siCOX-2(9R/DG-GNS) on Cancer Cell Growth
3.11. Cell Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowinsky, E.K. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 1997, 48, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Wientjes, M.G.; Schuller, D.E.; Au, J.L. Pharmacodynamics of Taxol in human head and neck tumors. Cancer Res. 1996, 56, 2086–2093. [Google Scholar] [PubMed]
- Parekh, H.; Wiesen, K.; Simpkins, H. Acquisition of taxol resistance via P-glycoprotein- and non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma cells. Biochem. Pharmacol. 1997, 53, 461–470. [Google Scholar] [CrossRef]
- Huang, M.; Liu, G. The study of innate drug resistance of human hepatocellular carcinoma Bel cell line. Cancer Lett. 1999, 135, 97–105. [Google Scholar] [CrossRef]
- Chabner, B.A.; Wilson, W. Reversal of multidrug resistance. J. Clin. Oncol. 1991, 9, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Cheng, S.C.; Xie, H.; Xie, Y. Chemosensitivity of Human Hepatocellular Carcinoma Cell Line QGY-7703 is Related to Bcl-2 Protein Levels. Tumour Biol. 1999, 20, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Xu, W.; Shi, T.; Yang, R.; Yang, X.; Wang, S.; Pan, W. Targeted delivery of docetaxel to the metastatic lymph nodes: A comparison study between nanoliposomes and activated carbon nanoparticles. Asian J. Pharm. Sci. 2015, 10, 64–72. [Google Scholar] [CrossRef]
- Jang, S.H.; Wientjes, M.G.; Au, J.L. Kinetics of P-Glycoprotein-Mediated Efflux of Paclitaxel. J. Pharmacol. Exp. Ther. 2001, 298, 1236–1242. [Google Scholar]
- Kondratov, R.V.; Komarov, P.G.; Becker, Y.; Ewenson, A.; Gudkov, A.V. Small molecules that dramatically alter multidrug resistance phenotype by modulating the substrate specificity of P-glycoprotein. Proc. Natl. Acad. Sci. USA 2001, 98, 14078–14083. [Google Scholar] [CrossRef]
- Penson, R.T.; Oliva, E.; Skates, S.J.; Glyptis, T.; Fuller, A.F.; Goodman, A.; Seiden, M.V. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004, 93, 98–106. [Google Scholar] [CrossRef]
- Orr, G.A.; Verdier-Pinard, P.; Mcdaid, H.; Horwitz, S.B. Mechanisms of Taxol resistance related to microtubules. Oncogene 2003, 22, 7280–7295. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, Y.; Matsuoka, J.; Gunduz, M.; Imada, T.; Ono, R.; Ito, M.; Motoki, T.; Yamatsuji, T.; Shirakawa, Y.; Takaoka, M.; et al. Resistance to paclitaxel therapy is related with Bcl-2 expression through an estrogen receptor mediated pathway in breast cancer. Int. J. Oncol. 2009, 34, 313–319. [Google Scholar] [PubMed]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Ueno, N.T.; Xia, W. Cationic liposome mediated EIA gene trenser to human breast and ovarian cancer cells and its biologie effects a phase I clinical trial. Clin. Oncol. 2001, 19, 3422–3433. [Google Scholar] [CrossRef] [PubMed]
- Ishida, O.; Maruyama, K.; Yanagie, H.; Eriguchi, M.; Iwatsuru, M. Targeting Chemotherapy to Solid Tumors with Long-circulating Thermosensitive Liposomes and Local Hyperthermia. Jpn. J. Cancer Res. 2000, 91, 118–126. [Google Scholar] [CrossRef]
- Lindner, L.H.; Eichhorn, M.E.; Eibl, H.; Teichert, N.; Schmitt-Sody, M.; Issels, R.D.; Dellian, M. Novel Temperature-Sensitive Liposomes with Prolonged Circulation Time. Clin. Cancer Res. 2004, 10, 2168–2178. [Google Scholar] [CrossRef]
- Ono, A.; Takeuchi, K.; Sukenari, A.; Suzuki, T.; Adachi, I.; Ueno, M. Reconsideration of Drug Release from Temperature-Sensitive Liposomes. Biol. Pharm. Bull. 2002, 25, 97–101. [Google Scholar] [CrossRef][Green Version]
- Wells, J.; Sen, A.; Hui, S.W. Localized delivery to CT-26 tumors in mice using thermosensitive liposomes. Int. J. Pharm. 2003, 261, 105–114. [Google Scholar] [CrossRef]
- Mulik, R.; Kulkarni, V.; Murthy, R.S. Chitosan-Based Thermosensitive Hydrogel Containing Liposomes for Sustained Delivery of Cytarabine. Drug. Dev. Ind. Pharm. 2009, 35, 49–56. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, D.; Li, J.; Wang, Y.; Guo, J.X.; Chen, Z.P.; Cai, B.C.; Yang, T. Influence of lipid composition on the phase transition temperature of liposomes composed of both DPPC and HSPC. Drug. Dev. Ind. Pharm. 2013, 39, 197–204. [Google Scholar] [CrossRef]
- Kono, K. Thermosensitive polymer-modified liposomes. Adv. Drug Deliv. Rev. 2001, 3, 307–319. [Google Scholar] [CrossRef]
- Needham, D.; Dewhirst, M.W. The Development and Testing of a New Temperature-sensitive Drug Delivery System for the Treatment of Solid Tumors. Adv. Drug Deliv. Rev. 2001, 53, 285–305. [Google Scholar] [CrossRef]
- Karino, T.; Koga, S.; Maeta, M. Experimental studies of the effects of local hyperthermia on blood flow, oxygen pressure and pH in tumors. Jpn. J. Surg. 1988, 18, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.H.; Wu, N.Z.; Hong, K.; Huang, S.K.; Dewhirst, M.W.; Papahadjopoulos, D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 1177–1187. [Google Scholar] [CrossRef]
- Sau, S.; Agarwalla, P.; Mukherjee, S.; Bag, I.; Sreedhar, B.; Pal-Bhadra, M.; Patra, C.R.; Banerjee, R. Cancer cell-selective promoter recognition accompanies antitumor effect by glucocorticoid receptor-targeted gold nanoparticle. Nanoscale 2014, 6, 6745–6754. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell. Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Sau, T.K.; Rogach, A.L.; Jäckel, F.; Klar, T.A.; Feldmann, J. Properties and Applications of Colloidal Nonspherical Noble Metal Nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Wang, S.; Peng, H.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Biomedical Applications: Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3009. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomedicine 2012, 8, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ai, K.; Ozaki, Y. Environmentally Friendly Synthesis of Highly Monodisperse Biocompatible Gold Nanoparticles with Urchin-like Shape. Langmuir 2008, 24, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT Peptide-Functionalized Gold Nanostars: Enhanced Intracellular Delivery and Efficient NIR Photothermal Therapy Using Ultralow Irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [PubMed]
- Nergiz, S.Z.; Gandra, N.; Tadepalli, S.; Singamaneni, S. Multifunctional Hybrid Nanopatches of Graphene Oxide and Gold Nanostars for Ultraefficient Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2014, 6, 16395–16402. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- Eschwège, P.; de Ledinghen, V.; Camilli, T.; Kulkarni, S.; Dalbagni, G.; Droupy, S.; Jardin, A.; Benoît, G.; Weksler, B.B. Arachidonic acid and prostaglandins, inflammation and oncology. Presse Med. 2001, 30, 508–510. [Google Scholar]
- Williams, C.S.; Mann, M.; Dubois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef]
- Harris, R.E.; Namboodiri, K.K.; Farrar, W.B. Nonsteroidal Antiinflammatory Drugs and Breast Cancer. Epidemiology 1996, 7, 203–205. [Google Scholar] [CrossRef]
- Sappayatosok, K.; Maneerat, Y.; Swasdison, S.; Viriyavejakul, P.; Dhanuthai, K.; Zwang, J.; Chaisri, U. Expression of pro-inflammatory protein, iNOS, VEGF and COX-2 in oral squamous cell carcinoma (OSCC), relationship with angiogenesis and their clinico-pathological correlation. Med. Oral Patol. Oral Cir. Bucal 2009, 14, E319–E324. [Google Scholar]
- Raspollini, M.R.; Amunni, G.; Villanucci, A.; Boddi, V.; Taddei, G.L. Increased cyclooxygenase-2 (COX-2) and P-glycoprotein-170 (MDR1) expression is associated with chemotherapy resistance and poor prognosis. Int. J. Gynecol. Cancer 2005, 15, 255–260. [Google Scholar] [CrossRef]
- Sheng, H.; Shao, J.; Morrow, J.D.; Beauchamp, R.D.; DuBois, R.N. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998, 58, 362–366. [Google Scholar] [PubMed]
- Oshima, M.; Dinchuk, J.E.; Kargman, S.L.; Oshima, H.; Hancock, B.; Kwong, E.; Trzaskos, J.M.; Evans, J.F.; Taketo, M.M. Suppression of Intestinal Polyposis in ApcΔ716 Knockout Mice by Inhibition of Cyclooxygenase 2 (COX-2). Cell 1996, 87, 803–809. [Google Scholar] [CrossRef]
- Tsujii, M.; Kawano, S.; DuBois, R.N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc. Natl. Acad. Sci. USA 1997, 94, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, J.L.; Koki, A.; Seibert, K. COX-2 Inhibitors: A New Class of Antiangiogenic Agents. Ann. N. Y. Acad. Sci. 1999, 889, 84–86. [Google Scholar] [CrossRef]
- Tsujii, M.; Kawano, S.; Tsuji, S.; Sawaoka, H.; Hori, M.; DuBois, R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998, 93, 705–716. [Google Scholar] [CrossRef]
- Huang, D.S.; Shen, K.Z.; Wei, J.F.; Liang, T.B.; Zheng, S.S.; Xie, H.Y. Specific COX-2 inhibitor NS398 induces apoptosis in human liver cancer cell line HepG2 through BCL-2. World J. Gastroenterol 2005, 11, 204–207. [Google Scholar] [CrossRef]
- McCarty, M.F.; Block, K.I. Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: An example of proapoptotic signal modulation therapy. Integr. Cancer Ther. 2006, 5, 252–268. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, E.; Pyo, H.; Lim, S.J. Cyclooxygenase-independent down-regulation of multidrug resistance-associated protein-1 expression by celecoxib in human lung cancer cells. Mol. Cancer Ther. 2005, 4, 1358–1363. [Google Scholar] [CrossRef]
- Liu, G.; Gao, N.; Zhou, Y.; Nie, J.; Cheng, W.; Luo, M.; Mei, L.; Zeng, X.; Deng, W. Polydopamine-Based “Four-in-One” Versatile Nanoplatforms for Targeted Dual Chemo and Photothermal Synergistic Cancer Therapy. Pharmaceutics 2019, 11, 507. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, M.; Gong, P.; Deng, J.Z.; Yi, H.Q.; Zhang, P.F.; Zhang, Y.J.; Liu, P.; Ma, Y.F.; Cai, L.T. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials 2013, 34, 3431–3438. [Google Scholar] [CrossRef]
- Pitchaimani, A.; Nguyen, T.D.T.; Aryal, S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 2018, 160, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Tezgel, O.; Szarpak-Jankowska, A.; Arnould, A.; Auzély-Velty, R.; Texier, I. Chitosan-lipid nanoparticles (CS-LNPs): Application to siRNA delivery. J. Colloid Interface Sci. 2018, 510, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Q.; Zhang, Z.; Gong, T.; Sun, X. Cationic bovine serum albumin based self-assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancer. Small 2014, 10, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Zhang, S.Y.; Ling, Y.; Meng, G.L.; Yang, Y.; Zhang, W. pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery. J. Control. Release 2015, 220, 529–544. [Google Scholar] [CrossRef]
- Xiong, X.B.; Lavasanifar, A. Traceable Multifunctional Micellar Nanocarriers for Cancer-Targeted Co-delivery of MDR-1 siRNA and Doxorubicin. ACS Nano 2011, 5, 5202–5213. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- López-Noriega, A.; Ruiz-Hernández, E.; Quinlan, E.; Storm, G.; Hennink, W.E.; O’Brien, F.J. Thermally triggered release of a pro-osteogenic peptide from a functionalized collagen-based scaffold using thermosensitive liposomes. J. Control Release 2014, 187, 158–166. [Google Scholar] [CrossRef]
- Simons, A.L.; Ahmad, I.M.; Mattson, D.M.; Dornfeld, K.J.; Spitz, D.R. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007, 67, 3364–3370. [Google Scholar] [CrossRef]
- Simons, A.L.; Fath, M.A.; Mattson, D.M.; Smith, B.J.; Walsh, S.A.; Graham, M.M.; Hichwa, R.D.; Buatti, J.M.; Dornfeld, K.; Spitz, D.R. Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-D-glucose correlates with increased 18 F-FDG uptake as determined by PET imaging. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1222–1230. [Google Scholar] [CrossRef]
- Maschek, G.; Savaraj, N.; Priebe, W.; Braunschweiger, P.; Hamilton, K.; Tidmarsh, G.F.; Young, L.R.D.; Lampidis, T.J. 2-Deoxy-D-glucose Increases the Efficacy of Adriamycin and Paclitaxel in Human Osteosarcoma and Non-Small Cell Lung Cancers in Vivo. Cancer Res. 2004, 64, 31–34. [Google Scholar] [CrossRef]
- Ponce, A.M.; Vujaskovic, Z.; Yuan, F.; Needham, D.; Dewhirst, M.W. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperth. 2006, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J. Technology of hyperthermic intraperitoneal chemotherapy in the United States, Europe, China, Japan, and Korea. Cancer J. 2009, 15, 249–254. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.L.; Fairbairn, D.W.; Smith, M.J.; Poe, B.S. Critical parameters influencing hyperthermia-induced apoptosis in human lymphoid cell lines. Apoptosis 1998, 3, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Nakasu, S.; Baba, K.; Nakajima, M.; Matsuda, M. Hyperthermia induces translocation of apoptosis-inducing factor (AIF) and apoptosis in human glioma cell lines. J. Neurooncol. 2004, 70, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Oliver, L.; Lebreton-De Coster, C.; Moreau, C.; Lecabellec, M.T.; Michel, L.; Vallette, F.M.; Dubertret, L.; Coulomb, B. Infrared Radiation Affects the Mitochondrial Pathway of Apoptosis in Human Fibroblasts. J. Investig. Dermatol. 2004, 123, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Kapse-Mistry, S.; Govender, T.; Srivastava, R.; Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 2014, 5, 159. [Google Scholar] [PubMed]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.X.; Zink, J.I.; et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nature Commun. 2018, 9, 1074. [Google Scholar] [CrossRef]
- Xu, C.; Chen, F.; Valdovinos, H.F.; Jiang, D.; Goel, S.; Yu, B.; Sun, H.; Barnhart, T.E.; Moon, J.J.; Cai, W.B. Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy. Biomaterials 2018, 165, 56–65. [Google Scholar] [CrossRef]
- You, Y.H.; Lin, Y.F.; Nirosha, B.; Chang, H.T.; Huang, Y.F. Polydopamine-coated gold nanostar for combined antitumor and antiangiogenic therapy in multidrug-resistant breast cancer. Nanotheranostics 2019, 3, 266–283. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Yang, Y.; Yu, Y.; Zhang, Y.H.; Zhu, D.H.; Yu, X.P.; Ouyang, X.X.; Xie, Z.Y.; Zhao, Y.L. Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment. Front. Bioeng. Biotechnol. 2019, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, X.Q.; Zhang, M.Z.; Song, Y.Y.; Cheng, K.; An, J.; Zhang, X.S.; Xuan, Y.; Liu, B.; Zhao, Y.D. In vivo Imaging-Guided Nanoplatform for Tumor Targeting Delivery and Combined Chemo-, Gene- and Photothermal Therapy. Theranostics 2018, 8, 5662–5675. [Google Scholar] [CrossRef] [PubMed]








| Sample | Hydrodynamic Diameter (nm) | Polydispersity | Zeta Potential (mV) |
|---|---|---|---|
| GNS | 57.23 ± 3.42 | 0.23 ± 0.02 | 0.12 ± 0.17 |
| 9R-GNS | 89.41 ± 5.53 | 0.25 ± 0.02 | 19.79 ± 0.16 |
| 9R/DG-GNS | 199.12 ±3.91 | 0.21 ± 0.03 | 10.85 ± 0.25 |
| siCOX-2(9R-GNS) | 203.26 ± 6.21 | 0.19 ± 0.02 | 0.26 ± 0.27 |
| siCOX-2(9R/DG-GNS) | 231.48 ± 5.27 | 0.17 ± 0.03 | 0.16 ± 0.62 |
| PTX-TSL | 93.56 ± 5.17 | 0.13 ± 0.01 | −1.78 ± 0.41 |
| PTX-TSL-siCOX-2(9R/DG-GNS) | 293.93 ± 3.21 | 0.12 ± 0.04 | −2.47 ± 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Han, W.; Gan, Y.; Li, Q.; Li, X.; Shao, L.; Zhu, D.; Guo, H. Combined Modality Therapy Based on Hybrid Gold Nanostars Coated with Temperature Sensitive Liposomes to Overcome Paclitaxel-Resistance in Hepatic Carcinoma. Pharmaceutics 2019, 11, 683. https://doi.org/10.3390/pharmaceutics11120683
Zhu H, Han W, Gan Y, Li Q, Li X, Shao L, Zhu D, Guo H. Combined Modality Therapy Based on Hybrid Gold Nanostars Coated with Temperature Sensitive Liposomes to Overcome Paclitaxel-Resistance in Hepatic Carcinoma. Pharmaceutics. 2019; 11(12):683. https://doi.org/10.3390/pharmaceutics11120683
Chicago/Turabian StyleZhu, Hongyan, Weili Han, Ye Gan, Qiaofeng Li, Xiaolan Li, Lanlan Shao, Dan Zhu, and Hongwei Guo. 2019. "Combined Modality Therapy Based on Hybrid Gold Nanostars Coated with Temperature Sensitive Liposomes to Overcome Paclitaxel-Resistance in Hepatic Carcinoma" Pharmaceutics 11, no. 12: 683. https://doi.org/10.3390/pharmaceutics11120683
APA StyleZhu, H., Han, W., Gan, Y., Li, Q., Li, X., Shao, L., Zhu, D., & Guo, H. (2019). Combined Modality Therapy Based on Hybrid Gold Nanostars Coated with Temperature Sensitive Liposomes to Overcome Paclitaxel-Resistance in Hepatic Carcinoma. Pharmaceutics, 11(12), 683. https://doi.org/10.3390/pharmaceutics11120683