Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery
Abstract
:1. Introduction
A Brief Note on Deep Eutectic Solvents (DES)
2. Properties of ILs
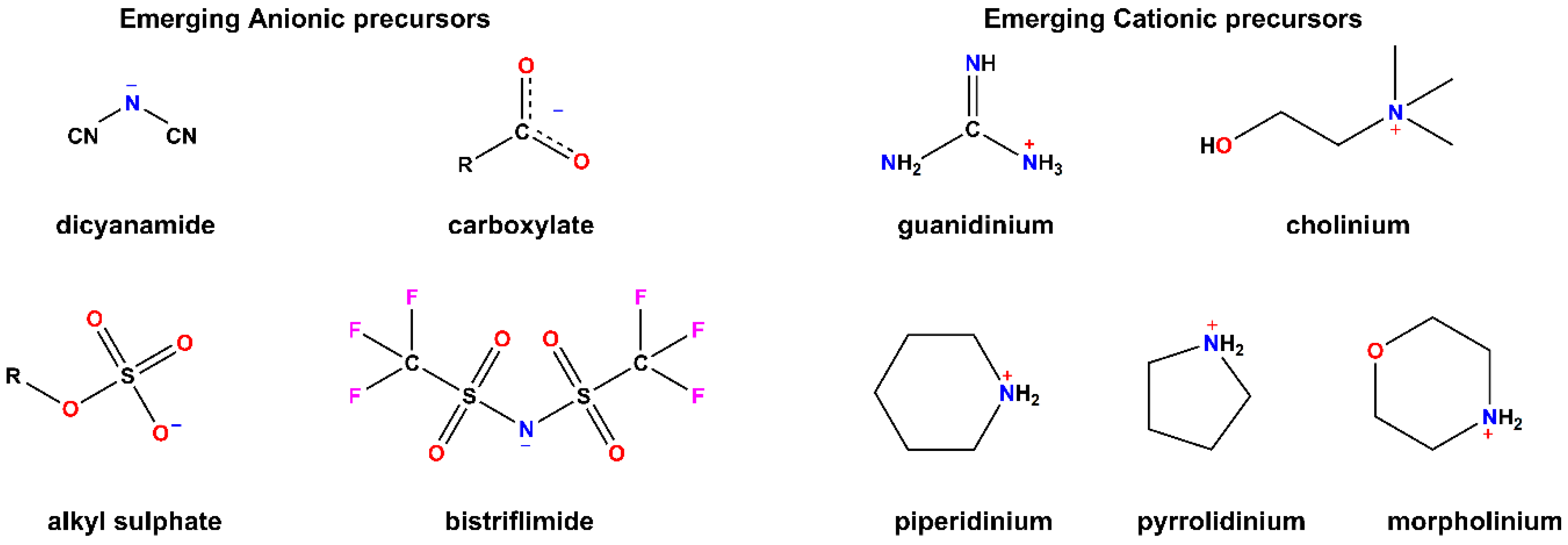
2.1. Chemical Traits
2.2. Physical Properties
2.3. Interactions of ILs with Biomolecules and Membranes
3. Current Challenges in Transdermal Drug Delivery
Current Limitations of Chemical Permeation Enhancers (CPEs)
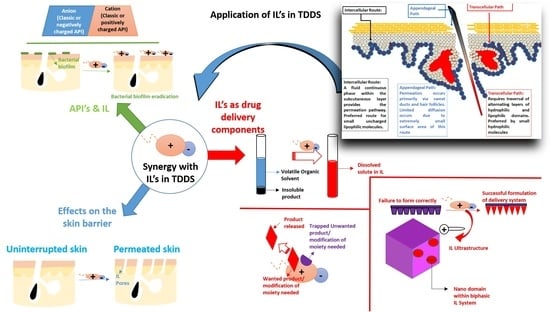
4. ILs Meeting the Needs in Transdermal Drug Delivery
4.1. ILs as Skin Permeation Enhancers
4.2. Evidence of Successful Synergy between Combinations of ILs and Chemical Enhancers
4.3. ILs in Drug Dissolution for Improving Bioavailability in TDDS
4.3.1. Drug Moiety and Delivery System Modification
4.3.2. Forming a Complex of IL with the API
4.3.3. Dissolving the API in the IL (Solvation)
4.4. ILs as Active Pharmaceutical Ingredients (APIs) in TDDS
5. Proposed/Future Opportunities for Synergism in TDDS
- 1)
- The use of ILs in a pre-treatment cycle: By pre-treating with ILs, the skin barrier properties are decreased. This in turn would allow more of the active compounds being employed with physical permeation to penetrate.
- 2)
- Using ILs in the permeation system: ILs are a diverse medium in which permeation systems can be made and modified. They allow incorporation into systems by acting as electrolyte solutions, coating media, and media in which delivery systems can be synthesized. Care must be taken to ensure that unwanted products do not form when changing the manufacturing process.
- 3)
- The use of ILs as a bioactive: Certain drug molecules can be derivatized as ILs and can be incorporated into other permeation systems. This will improve permeation and remove unwanted polymorphs. Using prodrug platforms in this method may enhance permeation; however, the need for the molecule to be activated may lead to longer therapeutic lead times.
- 4)
- Synergistic combination: A primary method of improving permeation with chemical enhancers is the combination use of ILs with another chemical enhancer, which can lead to synergistic or additive penetration. They can be combined in a single formulation where the IL can be the primary solvent, co-solvent, or surfactant, or can be employed as a second permeation enhancer. The risk to this strategy is that the irritation may be much more apparent during patient use.
- 5)
- Altered favorable environment for drug molecules: The use of ILs as a medium for solubilizing drug molecules can be varied to be more favorable. This strategy may not be applicable to a large number of drug molecules, as most are synthesized as crystalline solids as opposed to liquids. These classes of drug molecules can therefore be easily incorporated into the ILs.
- 6)
- Diverse activity profile: ILs can act as a number of formulation components. They can act as vehicles solubilizing drug molecules, surfactants, micelles, and permeation enhancers, and they can replace aqueous or oily phases in formulations. This diverse portfolio makes them ideal for incorporation into many formulations. However, a lack of long-term toxicity studies limits the widespread use of ILs.
- 7)
- Modification of embedded substrates: The use of ILs to enhance drug profiles has sound evidence. These are often favorable for drug delivery where drug molecular profiles are altered to enhance permeation and systemic absorption. The long-term stability and safety of these altered drug molecules is largely unknown and, therefore, requires much work before this strategy can reach clinical applications.
6. Conclusions
Funding
Conflicts of Interest
References
- Gadilohar, B.L.; Shankarling, G.S. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liquids 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Bermudez, M.D.; Jimenez, A.E.; Sanes, J.; Carrion, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Das, R.N.; Roy, K. Advances in qspr/qstr models of ionic liquids for the design of greener solvents of the future. Mol. Divers. 2013, 17, 151–196. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Amiril, S.A.S.; Rahim, E.A.; Syahrullail, S. A review on ionic liquids as sustainable lubricants in manufacturing and engineering: Recent research, performance, and applications. J. Clean. Prod. 2017, 168, 1571–1589. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale studies on ionic liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef]
- Lim, G.S.; Jaenicke, S.; Klahn, M. How the spontaneous insertion of amphiphilic imidazolium-based cations changes biological membranes: A molecular simulation study. Phys. Chem. Chem. Phys. 2015, 17, 29171–29183. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Roy, S.; Mukherjee, D.; Maiti, T.K.; Sarkar, N. Unveiling the interaction between fatty-acid-modified membrane and hydrophilic imidazolium-based ionic liquid: Understanding the mechanism of ionic liquid cytotoxicity. J. Phys. Chem. B 2017, 121, 8162–8170. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Prudencio, C.; Noronha, J.P.; Petrovski, Z. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Dobler, D.; Schmidts, T.; Klingenhofer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Chemistry: Develop ionic liquid drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulailaa, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Commun. 2010, 46, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, M.; Moniruzzaman, M.; Goto, M. Recent advances in exploiting ionic liquids for biomolecules: Solubility, stability and applications. Biotechnol. J. 2016, 11, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Kaur, R.; Kaur, R. An ionic liquid-in-water microemulsion as a potential carrier for topical delivery of poorly water soluble drug: Development, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2015, 495, 913–923. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering-promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorovic, Z.B.; Dokic-Stojanovic, D.R.; Stamenkovic, O.S.; Veljkovic, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Kudlak, B.; Owczarek, K.; Namiesnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 11975–11992. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are ionic liquids chemically stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilibria 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 3, 7–25. [Google Scholar] [CrossRef]
- Boudalis, A.K.; Rogez, G.; Heinrich, B.; Raptis, R.G.; Turek, P. Towards ionic liquids with tailored magnetic properties: Bmim(+) salts of ferro- and antiferromagnetic cu triangles. Dalton Trans. 2017, 46, 12263–12273. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef]
- Visser, A.E.; Reichert, W.M.; Swatloski, R.P.; Willauer, H.D.; Huddleston, J.G.; Rogers, R.D. Characterization of hydrophilic and hydrophobic ionic liquids: Alternatives to volatile organic compounds for liquid-liquid separations. In Ionic Liquids; American Chemical Society: Washington, DC, USA, 2002; Volume 818, pp. 289–308. [Google Scholar]
- Cao, Y.; Yao, S.; Wang, X.; Peng, Q.; Song, H. The physical and chemical properties of ionic liquids and its application in extraction. In Handbook of Ionic Liquids: Properties, Applications and Hazards; Mun, J., Sim, H., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 145–172. [Google Scholar]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.; Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef]
- Bhargava, B.L.; Klein, M.L. Initial stages of aggregation in aqueous solutions of ionic liquids: Molecular dynamics studies. J. Phys. Chem. B 2009, 113, 9499–9505. [Google Scholar] [CrossRef]
- Miskiewicz, A.; Ceranowicz, P.; Szymczak, M.; Bartus, K.; Kowalczyk, P. The use of liquids ionic fluids as pharmaceutically active substances helpful in combating nosocomial infections induced by klebsiella pneumoniae new delhi strain, acinetobacter baumannii and enterococcus species. Int. J. Mol. Sci. 2018, 19, 2779. [Google Scholar] [CrossRef]
- Gao, W.W.; Zhang, F.X.; Zhang, G.X.; Zhou, C.H. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Mantz, R.A.; Trulove, P. Viscosity and Density of Ionic Liquids; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2003; Volume 2, pp. 56–68. [Google Scholar]
- Katritzky, A.R.; Lomaka, A.; Petrukhin, R.; Jain, R.; Karelson, M.; Visser, A.E.; Rogers, R.D. Qspr correlation of the melting point for pyridinium bromides, potential ionic liquids. J. Chem. Inf. Comput. Sci. 2002, 42, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Takekiyo, T.; Yoshimura, Y. Suppression and dissolution of amyloid aggregates using ionic liquids. Biophys. Rev. 2018, 10, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.V.S.; Benedetto, A. Ionic liquids in protein amyloidogenesis: A brief screenshot of the state-of-the-art. Biophys. Rev. 2018, 10, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A. Room-temperature ionic liquids meet bio-membranes: The state-of-the-art. Biophys. Rev. 2017, 9, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, K.; Ma, H.; Banerjee, A.; Tanner, E.; Nangia, S.; Mitragotri, S. Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids; American Chemical Society: Washington, DC, USA, 2018; Volume 4. [Google Scholar]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Richter, C.; Ruhling, A.; Drucker, P.; Siegmund, D.; Metzler-Nolte, N.; Glorius, F.; Galla, H.J. A remarkably simple class of imidazolium-based lipids and their biological properties. Chemistry 2015, 21, 15123–15126. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Richter, C.; Ruhling, A.; Huwel, S.; Glorius, F.; Galla, H.J. Anti-tumor activity and cytotoxicity in vitro of novel 4,5-dialkylimidazolium surfactants. Biochem. Biophys. Res. Commun. 2015, 467, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Bodo, E.; Gontrani, L.; Ballone, P.; Caminiti, R. Amino acid anions in organic ionic compounds. An ab initio study of selected ion pairs. J. Phys. Chem. B 2014, 118, 2471–2486. [Google Scholar] [CrossRef]
- Evans, K.O. Supported phospholipid membrane interactions with 1-butyl-3-methylimidazolium chloride. J. Phys. Chem. B 2008, 112, 8558–8562. [Google Scholar] [CrossRef]
- Jing, B.; Lan, N.; Qiu, J.; Zhu, Y. Interaction of ionic liquids with a lipid bilayer: A biophysical study of ionic liquid cytotoxicity. J. Phys. Chem. B 2016, 120, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Heinrich, F.; Gonzalez, M.A.; Fragneto, G.; Watkins, E.; Ballone, P. Structure and stability of phospholipid bilayers hydrated by a room-temperature ionic liquid/water solution: A neutron reflectometry study. J. Phys. Chem. B 2014, 118, 12192–12206. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Bingham, R.J.; Ballone, P. Structure and dynamics of popc bilayers in water solutions of room temperature ionic liquids. J. Chem. Phys. 2015, 142, 124706. [Google Scholar] [CrossRef] [PubMed]
- Drucker, P.; Ruhling, A.; Grill, D.; Wang, D.; Draeger, A.; Gerke, V.; Glorius, F.; Galla, H.J. Imidazolium salts mimicking the structure of natural lipids exploit remarkable properties forming lamellar phases and giant vesicles. Langmuir 2017, 33, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Zhu, D.D.; Liu, X.B.; Chen, B.Z.; Guo, X.D. Microneedles with controlled bubble sizes and drug distributions for efficient transdermal drug delivery. Sci. Rep. 2016, 6, 38755. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Mitragotri, S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat. Biotechnol. 2004, 22, 192–197. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P. Transdermal drug delivery system: An overview. Asian J. Pharm. 2012, 6, 161–170. [Google Scholar] [CrossRef]
- Ita, K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: Two decades of research. J. Drug Deliv. Sci. Technol. 2018, 44, 314–322. [Google Scholar] [CrossRef]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control 2006, 34, S98–S110. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Shimizu, E.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; Kitahara, T.; et al. Characterization of overall ceramide species in human stratum corneum. J. Lipid Res. 2008, 49, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Maibach, H. Dermatological formulations: Percutaneous absorption. By brian w. Barry. Marcel dekker, 270 madison avenue, new york, ny 10016. 1983. 479pp. 16 × 23.5cm. Price $55.00 (2070 higher outside the us. And canada). J. Pharm. Sci. 1984, 73, 573. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef] [PubMed]
- Munch, S.; Wohlrab, J.; Neubert, R.H.H. Dermal and transdermal delivery of pharmaceutically relevant macromolecules. Eur. J. Pharm. Biopharm. 2017, 119, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vlasova, A.; Velasquez, D.E.; Saif, L.J.; Kandasamy, S.; Kochba, E.; Levin, Y.; Jiang, B. Skin vaccination against rotavirus using microneedles: Proof of concept in gnotobiotic piglets. PLoS ONE 2016, 11, e0166038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel chemical permeation enhancers for transdermal drug delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef]
- Arya, J.; Prausnitz, M.R. Microneedle patches for vaccination in developing countries. J. Control. Release 2016, 240, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Latsch, S.; Selzer, T.; Fink, L.; Kreuter, J. Crystallisation of estradiol containing tdds determined by isothermal microcalorimetry, x-ray diffraction, and optical microscopy. Eur. J. Pharm. Biopharm. 2003, 56, 43–52. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Milewski, M.; Yerramreddy, T.R.; Ghosh, P.; Crooks, P.A.; Stinchcomb, A.L. In vitro permeation of a pegylated naltrexone prodrug across microneedle-treated skin. J. Control. Release 2010, 146, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Hikima, T.; Tojo, K. Effect of molecular weight of penetrants on iontophoretic transdermal delivery in vitro. J. Chem. Eng. Jpn. 2006, 39, 360–365. [Google Scholar] [CrossRef]
- Banga, A.K.; Bose, S.; Ghosh, T.K. Iontophoresis and electroporation: Comparisons and contrasts. Int. J. Pharm. 1999, 179, 1–19. [Google Scholar] [CrossRef]
- Kumar, S.K.; Bhowmik, D.; Komala, M. Transdermal sonophoresis technique-an approach for controlled drug delivery. Indian J. Res. Pharm. Biotechnol. 2013, 1, 379–381. [Google Scholar]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Tfayli, A.; Guillard, E.; Manfait, M.; Baillet-Guffroy, A. Molecular interactions of penetration enhancers within ceramides organization: A raman spectroscopy approach. Analyst 2012, 137, 5002–5010. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Dermatological Formulations: Percutaneous Absorption; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, J.; Zhang, D.; Yang, Y.; Zheng, L.; Qu, Y.; Yang, X.; Cui, X. Ionic liquid—Microemulsions assisting in the transdermal delivery of dencichine: Preparation, in-vitro and in-vivo evaluations, and investigation of the permeation mechanism. Int. J. Pharm. 2018, 535, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Shibata, A.; Yamaguchi, T. The molecular assembly of the ionic liquid/aliphatic carboxylic acid/aliphatic amine as effective and safety transdermal permeation enhancers. Eur. J. Pharm. Sci. 2016, 86, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Prausnitz, M.R. Lidocaine-ibuprofen ionic liquid for dermal anesthesia. AICHE J. 2015, 61, 2732–2738. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Mitragotri, S. Therapeutic rnai robed with ionic liquid moieties as a simple, scalable prodrug platform for treating skin disease. J. Control. Release 2016, 242, 80–88. [Google Scholar] [CrossRef]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Chetoni, P.; Chiappe, C.; Sanzone, A.; Tampucci, S. Ionic liquids as potential enhancers for transdermal drug delivery. Int. J. Pharm. 2017, 516, 45–51. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, H.J.; Cui, X.M.; Wang, C.X. Evaluations of imidazolium ionic liquids as novel skin permeation enhancers for drug transdermal delivery. Pharm. Dev. Technol. 2017, 22, 511–520. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal Protein Delivery Using Choline and Geranate (cage) Deep Eutectic Solvent; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 6. [Google Scholar]
- Araki, S.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Ionic liquid-mediated transcutaneous protein delivery with solid-in-oil nanodispersions. MedChemComm 2015, 6, 2124–2128. [Google Scholar] [CrossRef]
- Yoshiura, H.; Tamura, M.; Aso, M.; Kamiya, N.; Goto, M. Ionic Liquid-in-Oil Microemulsions as Potential Carriers for the Transdermal Delivery of Methotrexate; The Society of Chemical Engineers: Tokyo, Japan, 2013; Volume 46, pp. 794–796. [Google Scholar]
- Kandasamy, S.; Moniruzzaman, M.; Sivapragasam, M.; Shamsuddin, M.R.; Mutalib, M.I.A. Formulation and characterization of acetate based ionic liquid in oil microemulsion as a carrier for acyclovir and methotrexate. Sep. Purif. Technol. 2018, 196, 149–156. [Google Scholar] [CrossRef]
- Aboofazeli, R.; Zia, H.; Needham, T.E. Transdermal delivery of nicardipine: An approach to in vitro permeation enhancement. Drug Deliv. 2002, 9, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Jing, B.; Jones, S.E.; Lamberti, G.A.; Zhu, Y.; Shah, J.K.; Maginn, E.J. Molecular mechanisms of ionic liquid cytotoxicity probed by an integrated experimental and computational approach. Sci. Rep. 2016, 6, 19889. [Google Scholar] [CrossRef] [PubMed]
- Frade, R.F.; Afonso, C.A. Impact of ionic liquids in environment and humans: An overview. Hum. Exp. Toxicol. 2010, 29, 1038–1054. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Liu, Q.P.; Smith, T.J.; Li, N.; Zong, M.H. Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef]
- Caparica, R.; Julio, A.; Baby, A.R.; Araujo, M.E.M.; Fernandes, A.S.; Costa, J.G.; Santos de Almeida, T. Choline-amino acid ionic liquids as green functional excipients to enhance drug solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Tian, T.; Gao, Y.; Ji, X.; Li, Z.; Luan, Y. Pharmaceutically active ionic liquid self-assembled vesicles for the application as an efficient drug delivery system. ChemPhysChem 2013, 14, 3454–3457. [Google Scholar] [CrossRef]
- Rao, K.S.; So, S.; Kumar, A. Vesicles and reverse vesicles of an ionic liquid in ionic liquids. Chem. Commun. 2013, 49, 8111–8113. [Google Scholar] [CrossRef]
- Hanna, S.L.; Huang, J.L.; Swinton, A.J.; Caputo, G.A.; Vaden, T.D. Synergistic effects of polymyxin and ionic liquids on lipid vesicle membrane stability and aggregation. Biophys. Chem. 2017, 227, 1–7. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tamura, M.; Tahara, Y.; Kamiya, N.; Goto, M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: Characterization and cytotoxicity evaluation. Int. J. Pharm. 2010, 400, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Feder-Kubis, J.; Tatarczak-Michalewska, M.; Plazinska, A.; Madejska, A.; Swatko-Ossor, M. Natural terpene derivatives as new structural task-specific ionic liquids to enhance the enantiorecognition of acidic enantiomers on teicoplanin-based stationary phase by high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Lee, B.S.; Chi, D.Y. Synergistic effect of two solvents, tert-alcohol and ionic liquid, in one molecule in nucleophilic fluorination. Org. Lett. 2008, 10, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.S.; Aswin, K.; Logaiya, K.; Sudhan, S.P.N. [bmim]bf4 ionic liquid: An efficient reaction medium for the one-pot multi-component synthesis of 2-amino-4, 6-diphenylpyridine-3-carbonitrile derivatives. J. Saudi Chem. Soc. 2016, 20, 517–522. [Google Scholar] [CrossRef]
- Davis, J.H., Jr.; Fox, P.A. From curiosities to commodities: Ionic liquids begin the transition. Chem. Commun. 2003, 11, 1209–1212. [Google Scholar] [CrossRef]
- Nishi, N.; Kawakami, T.; Shigematsu, F.; Yamamoto, M.; Kakiuchi, T. Fluorine-free and hydrophobic room-temperature ionic liquids, tetraalkylammonium bis(2-ethylhexyl)sulfosuccinates, and their ionic liquid–water two-phase properties. Green Chem. 2006, 8, 349–355. [Google Scholar] [CrossRef]
- Cojocaru, O.A.; Bica, K.; Gurau, G.; Narita, A.; McCrary, P.D.; Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Prodrug ionic liquids: Functionalizing neutral active pharmaceutical ingredients to take advantage of the ionic liquid form. MedChemComm 2013, 4, 559–563. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.X.; Han, W.; Yang, X.Y.; Qu, Y.; Cui, X.M.; Yang, Y. Promotion on In Vitro Percutaneous Absorption of Trace Ginsenoside rh1 Using Imidazole Type-Ionic Liquids; Tianjin Chinese Herbal Medicine Magazine: Tianjin, China, 2014; Volume 45, pp. 2917–2923. [Google Scholar]
- Heckenbach, M.E.; Romero, F.N.; Green, M.D.; Halden, R.U. Meta-analysis of ionic liquid literature and toxicology. Chemosphere 2016, 150, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hough-Troutman, W.L.; Smiglak, M.; Griffin, S.; Reichert, W.M.; Mirska, I.; Jodynis-Liebert, J.; Adamska, T.; Nawrot, J.; Stasiewicz, M.; Rogers, R.D.; et al. Ionic liquids with dual biological function: Sweet and anti-microbial, hydrophobic quaternary ammonium-based salts. New J. Chem. 2009, 33, 26–33. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodriguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Bica, K.; Rijksen, C.; Nieuwenhuyzen, M.; Rogers, R.D. In search of pure liquid salt forms of aspirin: Ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 2010, 12, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Rogers, R.D. Confused ionic liquid ions—A “liquification” and dosage strategy for pharmaceutically active salts. Chem. Commun. 2010, 46, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Zavgorodnya, O.; Shamshina, J.L.; Mittenthal, M.; McCrary, P.D.; Rachiero, G.P.; Titi, H.M.; Rogers, R.D. Polyethylene glycol derivatization of the non-active ion in active pharmaceutical ingredient ionic liquids enhances transdermal delivery. New J. Chem. 2017, 41, 1499–1508. [Google Scholar] [CrossRef]
- Miwa, Y.; Hamamoto, H.; Ishida, T. Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid. Eur. J. Pharm. Biopharm. 2016, 102, 92–100. [Google Scholar] [CrossRef] [PubMed]




| Chain Length Alteration | Change on Physio-Chemical Properties of ILs | Reference |
|---|---|---|
| Increase | Increased viscosity | [6,30] |
| Increase enthalpy of vaporization | [6] | |
| Increased aggregation (not necessarily ordered) | [6,30,31] | |
| Increased toxicity (bacterial and marine ecosystems) | [3,32] | |
| Increased surfactant activity | [6] | |
| Chain lengths similar to biological membranes | Increased bioaccumulation (potential for toxicity) | [3,20] |
| Decrease | Increased conductance | [6] |
| Increase in electrostatic forces | [6,30] | |
| Ordered aggregation; depends largely on polarity and geometric packing | [6,31] | |
| Increased lipase catalytic activity | [19,33] | |
| Increased polarity | [6] |
| Permeation Techniques | Example of Technique | References |
|---|---|---|
| Formulation enhancement |
| [69,70,71] |
| Physical permeation techniques |
| [72,73,74,75] |
| Chemical permeation enhancers |
| [57,76,77] |
| Chemical Enhancer | ILs | Synergism Documented | Reference |
|---|---|---|---|
| Lipid vesicles | ILs based on methylimidazolium chloride |
| [97] |
| Surfactants | Dimethyl-imidazolium dimethyl-phosphate |
| [98] |
| Terpenes | Menthoxymethyl-3-methylimidazolium chloride |
| [99] |
| Amines | Amine-based ILs |
| [81] |
| Alcohols | N-tert-Alcohol-substituted imidazole |
| [100] |
| IL-API Formed | Synergism | Efficacy | Reference |
|---|---|---|---|
| Acetyl salicylic acid/salicylate | Improved manufacturing methods Altered side-effect Profile | Solvent-free synthesis Lowered Gastric distress | [14,109,110,111] |
| Lidocaine docusate | Improved therapeutic outcome | Longer duration of action | [107,112] |
| Ranitidine docusate | Improved manufacture outcomes | Improved polymorphic challenges | [107] |
| Didecyldimethylammonium ibuprofenate | Proof of concept | Dual API formation | [107] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. https://doi.org/10.3390/pharmaceutics11020096
Sidat Z, Marimuthu T, Kumar P, du Toit LC, Kondiah PPD, Choonara YE, Pillay V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics. 2019; 11(2):96. https://doi.org/10.3390/pharmaceutics11020096
Chicago/Turabian StyleSidat, Zainul, Thashree Marimuthu, Pradeep Kumar, Lisa C. du Toit, Pierre P. D. Kondiah, Yahya E. Choonara, and Viness Pillay. 2019. "Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery" Pharmaceutics 11, no. 2: 96. https://doi.org/10.3390/pharmaceutics11020096
APA StyleSidat, Z., Marimuthu, T., Kumar, P., du Toit, L. C., Kondiah, P. P. D., Choonara, Y. E., & Pillay, V. (2019). Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics, 11(2), 96. https://doi.org/10.3390/pharmaceutics11020096