An Artificial Neural Network Approach to Predict the Effects of Formulation and Process Variables on Prednisone Release from a Multipartite System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pellets
2.3. Dissolution Test Conditions
2.4. HPLC Analysis
2.5. Scanning Electron Microscopy (SEM)
2.6. Yield
2.7. Response Surface Modelling
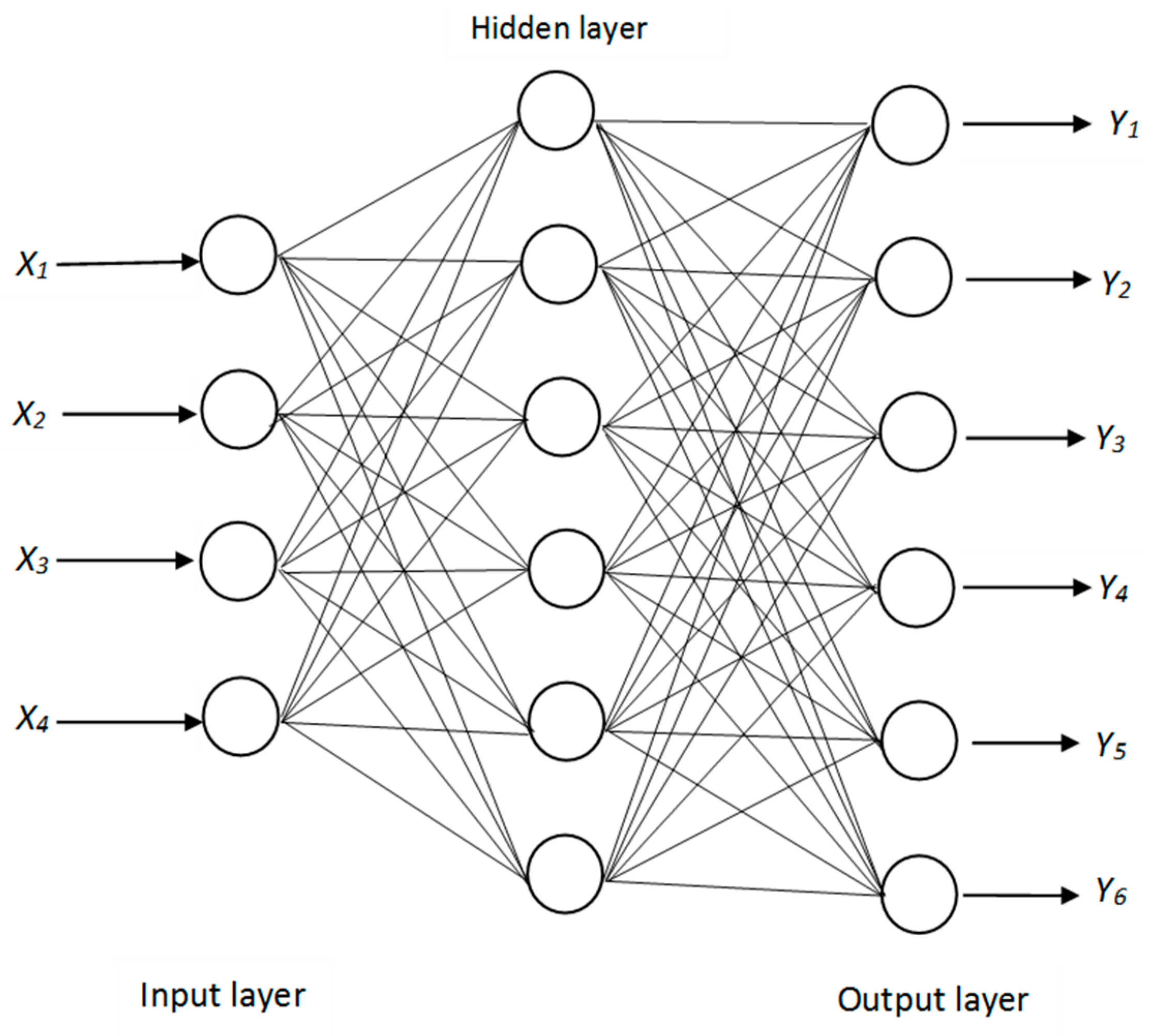
2.8. Artificial Neural Network
3. Results and Discussion
3.1. RSM Modeling
3.2. ANN Modelling
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Shahbazi, M.A.; Mäkilä, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Chuang, M.L. Pneumocystis jirovecii pneumonia in AIDS and non-AIDS immunocompromised patients—an update. J. Infect. Dev. Contries 2018, 12, 824–834. [Google Scholar] [CrossRef]
- Chi, K.N.; Protheroe, A.; Rodríguez-Antolín, A.; Facchini, G.; Suttman, H.; Matsubara, N.; McQuarrie, K. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): An international, randomised phase 3 trial. Lancet Oncol. 2018, 19, 194–206. [Google Scholar] [CrossRef]
- Fleishaker, D.L.; Mukherjee, A.; Whaley, F.S.; Daniel, S.; Zeiher, B.G. Safety and pharmacodynamic dose response of short-term prednisone in healthy adult subjects: A dose ranging, randomized, placebo-controlled, crossover study. BMC Musculoskel. Dis. 2016, 17, 293. [Google Scholar] [CrossRef] [PubMed]
- Reinau, D.; Schwenkglenks, M.; Früh, M.; Signorell, A.; Blozik, E.; Meier, C.R. Glucocorticoids and the risk of peptic ulcer bleeding: Case control analysis based on Swiss claims data. Drug Saf. 2018, 41, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S. Influence of medications on taste and smell. World J. Otorhinolaryngol Head Neck Surg. 2018, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Purkar, P.Y.; Dabir, P.D. A review on colonic drug delivery system. WJPR 2018, 7, 328–347. [Google Scholar]
- CH, A.B.; Sumathi, M.; Varalakshmi, M.; Devi, K.T.; Rao, M.P. Design, formulation and characterization of venlafaxine hydrochloride extended release multi-particulate systems. WJPR 2018, 7, 556–557. [Google Scholar]
- Albertini, B.; Melegari, C.; Bertoni, S.; Dolci, L.S.; Passerini, N. A novel approach for dry powder coating of pellets with ethylcellulose. Part II: Evaluation of caffeine release. AAPS PharmSciTech 2018, 19, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.J.; Ferraz, H.G. Gellan gum and polyvinylpyrrolidone (PVP) as binding agents in extrusion-spheronization pellet formulations. Acta Pharm. 2019, 69, 99–109. [Google Scholar] [CrossRef]
- Alves-Silva, I.; Marreto, R.N.; Gelfuso, G.M.; Sá-Barreto, L.C.; Lima, E.M.; Cunha-Filho, M.S. Preparation of benznidazole pellets for immediate drug delivery using the extrusion spheronization technique. Drug Dev. Ind. Pharm. 2017, 43, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Petrovick, G.F.; Breitkreutz, J. Spheronization of solid lipid extrudates: Elucidation of spheroid formation mechanism. Eur. J. Pharm. Biopharm. 2018, 125, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Alvarenga, V.O.; de Souza Sant’Ana, A.; Santos, J.S.; Granato, D. The use of statistical software in food science and technology: Advantages, limitations and misuses. Food Res. Int. 2015, 75, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esonye, C.; Onukwuli, O.D.; Ofoefule, A.U. Optimization of methyl ester production from Prunus Amygdalus seed oil using response surface methodology and artificial neural networks. Renew. Energ. 2019, 130, 61–72. [Google Scholar] [CrossRef]
- Chaibva, F.; Burton, M.; Walker, R.B. Optimization of salbutamol sulfate dissolution from sustained release matrix formulations using an artificial neural network. Pharmaceutics 2010, 2, 182–198. [Google Scholar] [CrossRef] [PubMed]
- León Blanco, J.M.; González-R, P.L.; Arroyo García, C.M.; Cózar-Bernal, M.J.; Calle Suárez, M.; Canca Ortiz, D.; González Rodríguez, M.L. Artificial neural networks as an alternative tool for minimizing error predictions in manufacturing ultradeformable nanoliposome formulations. Drug Dev. Ind. Pharm. 2018, 44, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Schmidli, H.; van Hoogevest, P.; Leuenberger, H. Basic concepts of artificial neural networks (ANN) modeling in the application to pharmaceutical development. Pharm. Dev. Technol. 1997, 2, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Dissolution Testing of Immediate Release Solid Oral Dosage Forms. Guidance for Industry; Center for Drug Evaluation and Research (CDER), US Government Printing Office: Washington, DC, USA, 1997. [Google Scholar]
- Ma, B.L.; Yang, Y.; Dai, Y.; Li, Q.; Lin, G.; Ma, Y.M. Polyethylene glycol 400 (PEG400) affects the systemic exposure of oral drugs based on multiple mechanisms: Taking berberine as an example. RSC Adv. 2017, 7, 2435–2442. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from oro-dispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.S.; Navari, R.M. Safety of Polysorbate 80 in the Oncology Setting. Adv. Ther. 2018, 35, 754–767. [Google Scholar] [CrossRef] [PubMed]
- U.S. Pharmacopoeia-National Formulary (USP 29 NF 24). Rockville, Md: United States Pharmacopeial Convention, Inc; USP Monographs: Prednisone. 2005. Available online: http://ftp.uspbpep.com/v29240/usp29nf24s0_m68940.html (accessed on 10 October 2018).
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 10 November 2005; pp. 11–12. [Google Scholar]
- Muley, S.; Nandgude, T.; Poddar, S. Extrusion-spheronization a promising pelletization technique: In-depth review. Asian J. Pharm. 2016, 11, 684–699. [Google Scholar] [CrossRef]
- Wang, J.; Kan, S.; Chen, T.; Liu, J. Application of quality by design (QbD) to formulation and processing of naproxen pellets by extrusion–spheronization. Pharm. Dev. Technol. 2015, 20, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.S.; Gogate, P.R.; Joshi, S.M. Ultrasound assisted synthesis of biodiesel from karanja oil by interesterification: Intensification studies and optimization using RSM. Ultrason. Sonochem. 2019, 50, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Nam, S.N.; Cho, H.; Han, J.; Her, N.; Yoon, J. Photocatalytic degradation of acesulfame K: Optimization using the Box–Behnken design (BBD). Process Saf. Environ. 2018, 113, 10–21. [Google Scholar] [CrossRef]
- Alinia, S.; Khamedi, R.; Ahmadi, I. The investigation and optimization of process parameters in warm deep drawing of ASS304 steel using Box–Behnken Design and applying temperature gradient. Exp. Techniques 2018, 42, 645–657. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.P.; Portugal, L.A.; Dos Reis, P.S.; Souza, A.S.; et al. Box–Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Khamanga, S.M.; Walker, R.B. The use of response surface methodology in the evaluation of captopril microparticles manufactured using an oil in oil solvent evaporation technique. J. Microencapsulation 2012, 29, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L.; Karabegović, I.T. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Patel, S.B.; Gohel, J.V. Enhanced solar cell performance by optimization of spray coated CZTS thin film using Taguchi and response surface method. J. Mater. Sci. Mater. Electron. 2018, 29, 5613–5623. [Google Scholar] [CrossRef]
- Sable, P.; Khanvilkar, V.V. Pharmaceutical Applications of Artificial Intelligence. Int. J. Pharma. Res. Health Sci. 2018, 6, 2342–2345. [Google Scholar]
- Jagtap, J.; Kokare, M. Human age classification using facial skin aging features and artificial neural network. Cogn. Syst. Res. 2016, 40, 116–128. [Google Scholar] [CrossRef]
- Munro, K.; Miller, T.H.; Martins, C.P.; Edge, A.M.; Cowan, D.A.; Barron, L.P. Artificial neural network modelling of pharmaceutical residue retention times in wastewater extracts using gradient liquid chromatography-high resolution mass spectrometry data. J. Chromatogr. A 2015, 1396, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Elçiçek, H.; Akdoğan, E.; Karagöz, S. The use of artificial neural network for prediction of dissolution kinetics. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Khunt, D.; Misra, M. Application of quality by design for optimization of spray drying process used in drying of risperidone nanosuspension. Powder Technol. 2019, 342, 156–165. [Google Scholar] [CrossRef]
- Santoro, A.B.; Botton, M.R.; Struchiner, C.J.; Suarez-Kurtz, G. Influence of pharmacogenetic polymorphisms and demographic variables on metformin pharmacokinetics in an admixed Brazilian cohort. Br. J. Clin. Pharmacol. 2018, 84, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Patel, P.; Gupta, A. Novel solid lipid nanocarrier of glibenclamide: A factorial design approach with response surface methodology. Curr. Pharm. Des. 2018, 24, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Goyal, S.; Miranda, G.; Sharma, N. Parametric study of the dry sliding wear behaviour of AA6082-T6/SiC and AA6082-T6/B 4 C composites using RSM. J. Mech. Sci. Technol. 2018, 32, 579–592. [Google Scholar] [CrossRef]
- Khaw, K.W.; Khoo, M.B.; Castagliola, P.; Rahim, M.A. New adaptive control charts for monitoring the multivariate coefficient of variation. Comput. Ind. Eng. 2018, 126, 595–610. [Google Scholar] [CrossRef]
- Tank, D.; Karan, K.; Gajera, B.Y.; Dave, R.H. Investigate the effect of solvents on wet granulation of microcrystalline cellulose using hydroxypropyl methylcellulose as a binder and evaluation of rheological and thermal characteristics of granules. Saudi Pharm. J. 2018, 26, 593–602. [Google Scholar] [CrossRef]
- Kleinebudde, P. The crystallite-gel-model for microcrystalline cellulose in wet-granulation, extrusion, and spheronization. Pharm. Res. 1997, 14, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Beg, A.E.; Ali, H.; Zafar, F.; Ashfaq, M.; Alam, S.; Shafique, S. Effects of superdisintegrants in oral dissolving formulation of cinitapride tablets. Pak. J. Pharm. Sci. 2018, 31, 643–650. [Google Scholar]
- Georgy, K.R.; Ragwa, M.F.; Randa, L.; Ehab, R.B. A new design for a chronological release profile of etodolac from coated bilayer tablets: In-vitro and in-vivo assessment. J. Adv. Res. 2019, 15, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kilor, V.A.; Sapkal, N.P.; Awari, J.G.; Shewale, B.D. Development and characterization of enteric-coated immediate-release pellets of aceclofenac by extrusion-spheronization technique using κ-carrageenan as a pelletizing agent. AAPS PharmSciTech 2010, 11, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Chamsai, B.; Sriamornsak, P. Novel disintegrating microcrystalline cellulose pellets with improved drug dissolution performance. Powder Technol. 2013, 233, 278–285. [Google Scholar] [CrossRef]
- Newton, J.M.; Pinto, M.R.; Podczeck, F. The preparation of pellets containing a surfactant or a mixture of mono-and di-gylcerides by extrusion-spheronization. Eur. J. Pharm. Sci. 2007, 30, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Podczeck, F.; Alessi, P.; Newton, J.M. The preparation of pellets containing non-ionic surfactants by extrusion-spheronization. Int. J. Pharm. 2008, 361, 33–40. [Google Scholar] [CrossRef] [PubMed]





| Run | Microcrystalline Cellulose (X1) % w/w | Sodium Starch Glycolate (X2) % w/w | Spheronization Time (X3) min | Extrusion Speed (X4) rpm | Tween® 80 (T) % w/w | PEG 400 (P) % w/w | Eudragit® RL 30 D (E) % w/w |
|---|---|---|---|---|---|---|---|
| 1 | 60 | 2.0 | 1 | 30 | 12.8 | 6.4 | 12.8 |
| 2 | 60 | 1.0 | 2 | 25 | 13.2 | 6.6 | 13.2 |
| 3 | 60 | 1.0 | 1 | 30 | 13.2 | 6.6 | 13.2 |
| 4 | 70 | 1.0 | 2 | 30 | 9.20 | 4.6 | 9.20 |
| 5 | 70 | 1.5 | 2 | 25 | 9.00 | 4.5 | 9.00 |
| 6 | 50 | 1.5 | 1 | 30 | 17.0 | 8.5 | 17.0 |
| 7 | 60 | 2.0 | 3 | 30 | 12.8 | 6.4 | 12.8 |
| 8 | 60 | 2.0 | 2 | 25 | 12.8 | 6.4 | 12.8 |
| 9 | 60 | 1.5 | 2 | 30 | 13.0 | 6.5 | 13.0 |
| 10 | 60 | 1.5 | 2 | 30 | 13.0 | 6.5 | 13.0 |
| 11 | 70 | 1.5 | 3 | 30 | 9.00 | 4.5 | 9.00 |
| 12 | 50 | 2.0 | 2 | 30 | 16.8 | 8.4 | 16.8 |
| 13 | 60 | 1.5 | 2 | 30 | 13.0 | 6.5 | 13.0 |
| 14 | 60 | 1.5 | 1 | 35 | 13.0 | 6.5 | 13.0 |
| 15 | 60 | 1.5 | 2 | 30 | 13.0 | 6.5 | 13.0 |
| 16 | 60 | 1.5 | 3 | 35 | 13.0 | 6.5 | 13.0 |
| 17 | 60 | 2.0 | 2 | 35 | 12.8 | 6.4 | 12.8 |
| 18 | 60 | 1.0 | 3 | 30 | 13.2 | 6.6 | 13.2 |
| 19 | 50 | 1.0 | 2 | 30 | 17.2 | 8.6 | 17.2 |
| 20 | 50 | 1.5 | 2 | 25 | 17.0 | 8.5 | 17.0 |
| 21 | 50 | 1.5 | 2 | 35 | 17.0 | 8.5 | 17.0 |
| 22 | 50 | 1.5 | 3 | 30 | 17.0 | 8.5 | 17.0 |
| 23 | 70 | 1.5 | 2 | 35 | 9.00 | 4.5 | 9.00 |
| 24 | 70 | 1.5 | 1 | 30 | 9.00 | 4.5 | 9.00 |
| 25 | 60 | 1.5 | 3 | 25 | 13.0 | 6.5 | 13.0 |
| 26 | 70 | 2.0 | 2 | 30 | 8.80 | 4.4 | 8.80 |
| 27 | 60 | 1.5 | 1 | 25 | 13.0 | 6.5 | 13.0 |
| 28 | 60 | 1.0 | 2 | 35 | 13.2 | 6.6 | 13.2 |
| 29 | 60 | 1.5 | 2 | 30 | 13.0 | 6.5 | 13.0 |
| Response | R2 | Adj R2 | Pred R2 | Adeq Precision | CV % | F-Value | p-Value | SD |
|---|---|---|---|---|---|---|---|---|
| Y1 | 0.6594 | 0.3188 | −0.4673 | 5.530 | 9.12 | 1.94 | 0.1144 | 0.11 |
| Y2 | 0.9128 | 0.8256 | 0.5137 | 13.129 | 8.42 | 10.47 | 0.0001 | 5.00 |
| Y3 | 0.8885 | 0.7769 | 0.4885 | 10.775 | 17.33 | 7.97 | 0.0002 | 9.28 |
| Y4 | 0.8606 | 0.7213 | 0.3201 | 10.612 | 13.03 | 6.18 | 0.0008 | 8.97 |
| Y5 | 0.8405 | 0.6811 | 0.1800 | 10.297 | 10.90 | 5.27 | 0.0018 | 8.41 |
| Y6 | 0.8143 | 0.6286 | −0.0071 | 9.671 | 9.35 | 4.39 | 0.0046 | 7.62 |
| Run | Aspect Ratio | Yield % | Drug Release % (15 min) | Drug Release % (30 min) | Drug Release % (45 min) | Drug Release % (60 min) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1EXP | Y1ANN | Y2EXP | Y2ANN | Y3EXP | Y3ANN | Y4EXP | Y4ANN | Y5EXP | Y5ANN | Y6EXP | Y6ANN | |
| 1 | 1.09 | 1.10 | 55.7 | 45.3 | 75.3 | 76.3 | 88.2 | 87.6 | 100.1 | 95.0 | 100.3 | 98.6 |
| 2 | 1.07 | 1.22 | 54.8 | 62.2 | 31.1 | 32.1 | 53.3 | 56.0 | 66.8 | 68.8 | 84.6 | 78.0 |
| 3 | 1.21 | 1.19 | 58.3 | 64.7 | 37.1 | 34.2 | 56.0 | 57.8 | 65.0 | 66.7 | 75.2 | 78.4 |
| 4 | 1.20 | 1.21 | 47.5 | 47.7 | 24.6 | 24.6 | 39.8 | 39.8 | 51.3 | 51.3 | 59.4 | 59.4 |
| 5 | 1.19 | 1.18 | 58.4 | 62.5 | 32.8 | 25.4 | 51.8 | 46.0 | 66.5 | 63.5 | 74.6 | 74.0 |
| 6 | 1.40 | 1.43 | 41.2 | 39.4 | 80.4 | 78.2 | 89.7 | 82.8 | 90.7 | 91.3 | 90.2 | 89.4 |
| 7 | 1.08 | 1.07 | 56.4 | 57.8 | 71.5 | 67.9 | 82.8 | 84.6 | 86.2 | 94.0 | 90.5 | 96.5 |
| 8 | 1.09 | 1.07 | 52.5 | 50.0 | 77.5 | 80.3 | 88.2 | 89.5 | 95.1 | 95.1 | 99.1 | 99.3 |
| 9 | 1.16 | 1.25 | 58.9 | 57.7 | 63.4 | 63.3 | 78.8 | 77.5 | 85.2 | 84.6 | 87.9 | 87.9 |
| 10 | 1.17 | 1.25 | 63.2 | 57.7 | 47.2 | 63.3 | 62.3 | 77.5 | 71.9 | 84.6 | 77.6 | 87.9 |
| 11 | 1.19 | 1.23 | 73.9 | 65.7 | 18.5 | 24.6 | 32.8 | 40.0 | 43.3 | 51.6 | 51.1 | 60.4 |
| 12 | 1.53 | 1.50 | 34.3 | 36.8 | 80.2 | 80.4 | 82.0 | 83.8 | 91.3 | 91.4 | 80.9 | 79.0 |
| 13 | 1.45 | 1.25 | 60.8 | 57.7 | 64.0 | 63.3 | 76.7 | 77.5 | 82.8 | 84.6 | 84.8 | 87.9 |
| 14 | 1.27 | 1.26 | 60.4 | 64.0 | 68.7 | 65.2 | 83.5 | 77.4 | 86.2 | 79.3 | 86.7 | 86.0 |
| 15 | 1.27 | 1.25 | 62.8 | 57.7 | 72.6 | 63.3 | 82.6 | 77.5 | 86.6 | 84.6 | 86.5 | 87.9 |
| 16 | 1.24 | 1.07 | 79.3 | 77.0 | 50.8 | 45.4 | 67.1 | 79.9 | 73.9 | 88.0 | 80.8 | 94.9 |
| 17 | 1.11 | 1.18 | 56.0 | 55.8 | 64.1 | 65.4 | 80.0 | 79.9 | 85.3 | 86.7 | 90.5 | 88.7 |
| 18 | 1.08 | 1.08 | 55.7 | 61.3 | 25.1 | 25.9 | 46.4 | 46.0 | 61.4 | 61.9 | 71.7 | 75.4 |
| 19 | 1.25 | 1.28 | 39.2 | 40.1 | 63.7 | 76.3 | 79.9 | 80.1 | 85.3 | 85.3 | 84.0 | 85.3 |
| 20 | 1.53 | 1.52 | 70.9 | 78.3 | 58.9 | 80.4 | 77.1 | 88.5 | 80.9 | 80.7 | 81.5 | 83.9 |
| 21 | 1.17 | 1.12 | 36.4 | 38.9 | 67.3 | 63.0 | 72.3 | 73.7 | 77.1 | 78.5 | 77.2 | 76.6 |
| 22 | 1.15 | 1.07 | 72.4 | 71.2 | 55.6 | 57.5 | 72.1 | 73.0 | 76.6 | 76.1 | 75.4 | 76.1 |
| 23 | 1.28 | 1.23 | 74.1 | 66.5 | 17.8 | 24.6 | 33.0 | 40.1 | 44.4 | 51.6 | 53.9 | 60.7 |
| 24 | 1.45 | 1.27 | 78.3 | 67.5 | 24.7 | 25.3 | 43.2 | 44.2 | 55.8 | 55.1 | 65.2 | 66.8 |
| 25 | 1.09 | 1.16 | 62.1 | 59.1 | 70.7 | 73.3 | 82.3 | 82.5 | 85.4 | 92.4 | 87.0 | 94.2 |
| 26 | 1.10 | 1.17 | 56.7 | 62.6 | 46.4 | 55.6 | 75.7 | 77.7 | 91.5 | 88.8 | 99.0 | 92.1 |
| 27 | 1.20 | 1.09 | 76.9 | 56.2 | 57.9 | 75.8 | 74.8 | 87.2 | 81.0 | 94.9 | 83.2 | 98.5 |
| 28 | 1.12 | 1.07 | 60.9 | 54.5 | 43.2 | 43.6 | 68.7 | 67.0 | 91.2 | 89.9 | 99.3 | 95.8 |
| 29 | 1.18 | 1.25 | 63.5 | 57.7 | 61.6 | 63.3 | 74.6 | 77.5 | 77.7 | 84.6 | 85.9 | 87.9 |
| Parameter | Aspect Ratio | Yield | Drug Release % (15 min) | Drug Release % (30 min) | Drug Release % (45 min) | Drug Release % (60 min) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1RSM | Y1ANN | Y2RSM | Y2ANN | Y3RSM | Y3ANN | Y4RSM | Y4ANN | Y5RSM | Y5ANN | Y6RSM | Y6ANN | |
| R2 | 0.659 | 0.797 | 0.913 | 0.849 | 0.889 | 0.934 | 0.861 | 0.946 | 0.841 | 0.934 | 0.814 | 0.906 |
| MAE | 0.012 | 0.007 | 24.97 | 41.51 | 86.13 | 55.94 | 80.46 | 33.29 | 70.72 | 32.42 | 58.10 | 31.90 |
| RMSE | 0.110 | 0.081 | 4.997 | 6.443 | 9.281 | 7.479 | 8.970 | 5.770 | 8.410 | 5.694 | 7.622 | 5.648 |
| Material | Concentration % | Function |
|---|---|---|
| Prednisone | 4 | Active ingredient |
| Tween 80 | 12.8 | Surfactant, Solubilizer. |
| Polyethylene glycol 400 | 6.4 | Pore-former, solubilizer, Imparts hydrophilicity. |
| Eudragit® RL 30 D (50 % aqueous dilution) | 12.8 | Improves pellet tensile strength, Imparts hydrophilicity. |
| Comprecel® M102 D+ | 60 | Bulking agent, spheronization aid. |
| Sodium starch glycolate | 2 | Disintegrant. |
| Talc | 1.5 | Glidant, anti-adherent. |
| Magnesium stearate | 0.5 | Lubricant, anti-adherent. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manda, A.; Walker, R.B.; Khamanga, S.M.M. An Artificial Neural Network Approach to Predict the Effects of Formulation and Process Variables on Prednisone Release from a Multipartite System. Pharmaceutics 2019, 11, 109. https://doi.org/10.3390/pharmaceutics11030109
Manda A, Walker RB, Khamanga SMM. An Artificial Neural Network Approach to Predict the Effects of Formulation and Process Variables on Prednisone Release from a Multipartite System. Pharmaceutics. 2019; 11(3):109. https://doi.org/10.3390/pharmaceutics11030109
Chicago/Turabian StyleManda, Arthur, Roderick B. Walker, and Sandile M. M. Khamanga. 2019. "An Artificial Neural Network Approach to Predict the Effects of Formulation and Process Variables on Prednisone Release from a Multipartite System" Pharmaceutics 11, no. 3: 109. https://doi.org/10.3390/pharmaceutics11030109






