MRI of the Colon in the Pharmaceutical Field: The Future before us
Abstract
:1. Introduction
2. Colon Anatomy and Physical Dimensions
3. Colonic Motility
4. Colonic Chyme and Fluid
5. Colon Transit and Luminal Flow
6. Conclusions and Future Outlook
Funding
Conflicts of Interest
References
- Reppas, C.; Karatza, E.; Goumas, C.; Markopoulos, C.; Vertzoni, M. Characterization of Contents of Distal Ileum and Cecum to Which Drugs/Drug Products are Exposed During Bioavailability/Bioequivalence Studies in Healthy Adults. Pharm. Res. 2015, 32, 3338–3349. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Abrahamsson, B.; Brewster, M.; Brouwers, J.; Butler, J.; Carlert, S.; Dickinson, P.A.; Dressman, J.; Holm, R.; Klein, S.; et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2014, 57, 342–366. [Google Scholar] [CrossRef]
- Lennernas, H.; Aarons, L.; Augustijns, P.; Beato, S.; Bolger, M.; Box, K.; Brewster, M.; Butler, J.; Dressman, J.; Holm, R.; et al. Oral biopharmaceutics tools—Time for a new initiative—An introduction to the IMI project OrBiTo. Eur. J. Pharm. Sci. 2014, 57, 292–299. [Google Scholar] [CrossRef]
- Van Meerveld, B.G.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. Handb. Exp. Pharmacol. 2017, 239, 1–16. [Google Scholar] [CrossRef]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.F. Absorption and secretion by the colon. Gastroenterology 1969, 56, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S.; Morton, D. The colon: From banal to brilliant. Toxicol. Pathol. 2014, 42, 67–81. [Google Scholar] [CrossRef]
- Hoad, C.; Clarke, C.; Marciani, L.; Graves, M.J.; Corsetti, M. Will MRI of gastrointestinal function parallel the clinical success of cine cardiac MRI? Br. J. Radiol. 2018, 91, 20180433. [Google Scholar] [CrossRef]
- Tannergren, C.; Bergendal, A.; Lennernas, H.; Abrahamsson, B. Toward an increased understanding of the barriers to colonic drug absorption in humans: Implications for early controlled release candidate assessment. Mol. Pharm. 2009, 6, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L. Assessment of gastrointestinal motor functions by MRI: A comprehensive review. Neurogastroenterol. Motil. 2011, 23, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Leighton, M.P.; Lam, C.; Mehta, S.; Spiller, R.C. Efficacy and mode of action of mesalazine in the treatment of diarrhoea-predominant irritable bowel syndrome (IBS-D): Study protocol for a randomised controlled trial. Trials 2013, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Tozer, P.; Ng, S.C.; Siddiqui, M.R.; Plamondon, S.; Burling, D.; Gupta, A.; Swatton, A.; Tripoli, S.; Vaizey, C.J.; Kamm, M.A.; et al. Long-term MRI-guided combined anti-TNF-alpha and thiopurine therapy for Crohn’s perianal fistulas. Inflamm. Bowel Dis. 2012, 18, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Stoppino, L.P.; Della Valle, N.; Rizzi, S.; Cleopazzo, E.; Centola, A.; Iamele, D.; Bristogiannis, C.; Stoppino, G.; Vinci, R.; Macarini, L. Magnetic resonance enterography changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn’s disease: Correlation with SES-CD and clinical-biological markers. BMC Med. Imaging 2016, 16, 37. [Google Scholar] [CrossRef]
- Inoue, A.; Ohta, S.; Nitta, N.; Yoshimura, M.; Shimizu, T.; Tani, M.; Kushima, R.; Murata, K. MRI can be used to assess advanced T-stage colon carcinoma as well as rectal carcinoma. Jpn. J. Radiol. 2016, 34, 809–819. [Google Scholar] [CrossRef]
- Nerad, E.; Lambregts, D.M.; Kersten, E.L.; Maas, M.; Bakers, F.C.; van den Bosch, H.C.; Grabsch, H.I.; Beets-Tan, R.G.; Lahaye, M.J. MRI for Local Staging of Colon Cancer: Can MRI Become the Optimal Staging Modality for Patients With Colon Cancer? Dis. Colon Rectum 2017, 60, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Koh, F.H.X.; Tan, K.K.; Teo, L.L.S.; Ang, B.W.L.; Thian, Y.L. Prospective comparison between magnetic resonance imaging and computed tomography in colorectal cancer staging. Anz. J. Surg. 2018, 88, E498–E502. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Buhmann, S.; Kirchhoff, C.; Wielage, C.; Mussack, T.; Reiser, M.F.; Lienemann, A. Assessment of large bowel motility by cine magnetic resonance imaging using two different prokinetic agents: A feasibility study. Investig. Radiol. 2005, 40, 689–694. [Google Scholar] [CrossRef]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Undseth, R.; Berstad, A.; Klow, N.E.; Arnljot, K.; Moi, K.S.; Valeur, J. Abnormal accumulation of intestinal fluid following ingestion of an unabsorbable carbohydrate in patients with irritable bowel syndrome: An MRI study. Neurogastroenterol. Motil. 2014, 26, 1686–1693. [Google Scholar] [CrossRef]
- Mark, E.B.; Poulsen, J.L.; Haase, A.M.; Frokjaer, J.B.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Assessment of colorectal length using the electromagnetic capsule tracking system: A comparative validation study in healthy subjects. Colorectal Dis. 2017, 19, O350–O357. [Google Scholar] [CrossRef]
- Hahn, T.; Kozerke, S.; Schwizer, W.; Fried, M.; Boesiger, P.; Steingoetter, A. Visualization and quantification of intestinal transit and motor function by real-time tracking of 19F labeled capsules in humans. Magn. Reson. Med. 2011, 66, 812–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhoff, S.; Nicolaus, M.; Schirra, J.; Reiser, M.F.; Goke, B.; Lienemann, A. Assessment of colon motility using simultaneous manometric and functional cine-MRI analysis: Preliminary results. Abdom. Imaging 2011, 36, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Garsed, K.C.; Hoad, C.L.; Fields, A.; Fordham, I.; Pritchard, S.E.; Placidi, E.; Murray, K.; Chaddock, G.; Costigan, C.; et al. Stimulation of colonic motility by oral PEG electrolyte bowel preparation assessed by MRI: Comparison of split vs. single dose. Neurogastroenterol. Motil. 2014, 26, 1426–1436. [Google Scholar] [CrossRef]
- Hahnemann, M.L.; Nensa, F.; Kinner, S.; Gerken, G.; Lauenstein, T.C. Motility mapping as evaluation tool for bowel motility: Initial results on the development of an automated color-coding algorithm in cine MRI. J. Magn. Reson. Imaging 2015, 41, 354–360. [Google Scholar] [CrossRef]
- Lam, C.; Chaddock, G.; Marciani, L.; Costigan, C.; Paul, J.; Cox, E.; Hoad, C.; Menys, A.; Pritchard, S.; Garsed, K.; et al. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterol. Motil. 2016, 28, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Hoad, C.L.; Menys, A.; Garsed, K.; Marciani, L.; Hamy, V.; Murray, K.; Costigan, C.; Atkinson, D.; Major, G.; Spiller, R.C.; et al. Colon wall motility: Comparison of novel quantitative semi-automatic measurements using cine MRI. Neurogastroenterol. Motil. 2016, 28, 327–335. [Google Scholar] [CrossRef]
- Schiller, C.; Frohlich, C.P.; Giessmann, T.; Siegmund, W.; Monnikes, H.; Hosten, N.; Weitschies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placidi, E.; Marciani, L.; Hoad, C.L.; Napolitano, A.; Garsed, K.C.; Pritchard, S.E.; Cox, E.F.; Costigan, C.; Spiller, R.C.; Gowland, P.A. The effects of loperamide, or loperamide plus simethicone, on the distribution of gut water as assessed by MRI in a mannitol model of secretory diarrhoea. Aliment. Pharmacol. Ther. 2012, 36, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, G.; Murray, K.; Singh, G.; Nowak, A.; Hoad, C.L.; Marciani, L.; Silos-Santiago, A.; Kurtz, C.B.; Johnston, J.M.; Gowland, P.; et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol. Motil. 2018, 30, e13400. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Smith, V.; Dellschaft, N.; Ansell, J.; Hoad, C.; Marciani, L.; Gowland, P.; Spiller, R. Mechanisms underlying effects of kiwifruit on intestinal function shown by MRI in healthy volunteers. Aliment. Pharmacol. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.E.; Marciani, L.; Garsed, K.C.; Hoad, C.L.; Thongborisute, W.; Roberts, E.; Gowland, P.A.; Spiller, R.C. Fasting and postprandial volumes of the undisturbed colon: Normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol. Motil. 2014, 26, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Sandberg, T.H.; Poulsen, J.L.; Gram, M.; Frokjaer, J.B.; Ostergaard, L.R.; Krogh, K.; Brock, C.; Drewes, A.M. Quantification and variability in colonic volume with a novel magnetic resonance imaging method. Neurogastroenterol. Motil. 2015, 27, 1755–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, S.E.; Garsed, K.C.; Hoad, C.L.; Lingaya, M.; Banwait, R.; Thongborisute, W.; Roberts, E.; Costigan, C.; Marciani, L.; Gowland, P.A.; et al. Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterol. Motil. 2015, 27, 542–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandberg, T.H.; Nilsson, M.; Poulsen, J.L.; Gram, M.; Frokjaer, J.B.; Ostergaard, L.R.; Drewes, A.M. A novel semi-automatic segmentation method for volumetric assessment of the colon based on magnetic resonance imaging. Abdom. Imaging 2015, 40, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Coletta, M.; Gates, F.K.; Marciani, L.; Shiwani, H.; Major, G.; Hoad, C.L.; Chaddock, G.; Gowland, P.A.; Spiller, R.C. Effect of bread gluten content on gastrointestinal function: A crossover MRI study on healthy humans. Br. J. Nutr. 2016, 115, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Lam, C.; Rehman, S.; Marciani, L.; Costigan, C.; Hoad, C.L.; Lingaya, M.R.; Banwait, R.; Bawden, S.J.; Gowland, P.A.; et al. Corticotropin-releasing factor increases ascending colon volume after a fructose test meal in healthy humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Poulsen, J.L.; Brock, C.; Sandberg, T.H.; Gram, M.; Frokjaer, J.B.; Krogh, K.; Drewes, A.M. Opioid-induced bowel dysfunction in healthy volunteers assessed with questionnaires and MRI. Eur. J. Gastroenterol. Hepatol. 2016, 28, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Bendezu, R.A.; Mego, M.; Monclus, E.; Merino, X.; Accarino, A.; Malagelada, J.R.; Navazo, I.; Azpiroz, F. Colonic content: Effect of diet, meals, and defecation. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Lam, C.; Chaddock, G.; Marciani Laurea, L.; Costigan, C.; Cox, E.; Hoad, C.; Pritchard, S.; Gowland, P.; Spiller, R. Distinct Abnormalities of Small Bowel and Regional Colonic Volumes in Subtypes of Irritable Bowel Syndrome Revealed by MRI. Am. J. Gastroenterol. 2017, 112, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology 2017, 152, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Hoad, C.L.; Mudie, D.M.; Wright, J.; Heissam, K.; Abrehart, N.; Pritchard, S.E.; Al Atwah, S.; Gowland, P.A.; Garnett, M.C.; et al. Magnetic Resonance Imaging Quantification of Fasted State Colonic Liquid Pockets in Healthy Humans. Mol. Pharm. 2017, 14, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.E.; Paul, J.; Major, G.; Marciani, L.; Gowland, P.A.; Spiller, R.C.; Hoad, C.L. Assessment of motion of colonic contents in the human colon using MRI tagging. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Poulsen, J.L.; Mark, E.B.; Brock, C.; Frokjaer, J.B.; Krogh, K.; Drewes, A.M. Colorectal Transit and Volume During Treatment with Prolonged-release Oxycodone/Naloxone Versus Oxycodone Plus Macrogol 3350. J. Neurogastroenterol. Motil. 2018, 24, 119–127. [Google Scholar] [CrossRef]
- Sloan, T.J.; Jalanka, J.; Major, G.A.D.; Krishnasamy, S.; Pritchard, S.; Abdelrazig, S.; Korpela, K.; Singh, G.; Mulvenna, C.; Hoad, C.L.; et al. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLoS ONE 2018, 13, e0201410. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Smith, V.C.; Major, G.; Ashleigh, L.; Murray, K.; Hoad, C.L.; Marciani, L.; Gowland, P.A.; Spiller, R.C. Insights Into the Different Effects of Food on Intestinal Secretion Using Magnetic Resonance Imaging. J. Parenter. Enter. Nutr. 2018, 42, 1342–1348. [Google Scholar] [CrossRef]
- Buhmann, S.; Kirchhoff, C.; Ladurner, R.; Mussack, T.; Reiser, M.F.; Lienemann, A. Assessment of colonic transit time using MRI: A feasibility study. Eur. Radiol. 2007, 17, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Chaddock, G.; Lam, C.; Hoad, C.L.; Costigan, C.; Cox, E.F.; Placidi, E.; Thexton, I.; Wright, J.; Blackshaw, P.E.; Perkins, A.C.; et al. Novel MRI tests of orocecal transit time and whole gut transit time: Studies in normal subjects. Neurogastroenterol. Motil. 2014, 26, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Savarino, V.; Fox, M.; Di Leo, G.; Furnari, M.; Marabotto, E.; Gemignani, L.; Bruzzone, L.; Moscatelli, A.; De Cassan, C.; et al. Measurement of oro-caecal transit time by magnetic resonance imaging. Eur. Radiol. 2015, 25, 1579–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, M.; Zhou, Z.; Chen, H.; Xiong, F.; Huang, J.; He, H.; Zhang, M.; Su, M.; Gao, X.; Hu, P. Clinical application of a gadolinium-based capsule as an MRI contrast agent in slow transit constipation diagnostics. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.; Hoad, C.L.; Menys, A.; Nowak, A.; Taylor, S.A.; Paparo, S.; Lingaya, M.; Falcone, Y.; Singh, G.; Spiller, R.C.; et al. MRI assessment of the postprandial gastrointestinal motility and peptide response in healthy humans. Neurogastroenterol. Motil. 2018. [Google Scholar] [CrossRef]
- Roth, C.G.; Marzio, D.H.-D.; Guglielmo, F.F. Contributions of Magnetic Resonance Imaging to Gastroenterological Practice: MRIs for GIs. Dig. Dis. Sci. 2018, 63, 1102–1122. [Google Scholar] [CrossRef]
- Camilleri, M. New imaging in neurogastroenterology: An overview. Neurogastroenterol. Motil. 2006, 18, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.K.; Roth, C.G.; Ward, R.J.; deJesus, J.O.; Mitchell, D.G. Optimizing Abdominal MR Imaging: Approaches to Common Problems. RadioGraphics 2010, 30, 185–199. [Google Scholar] [CrossRef]
- Anupindi, S.A.; Podberesky, D.J.; Towbin, A.J.; Courtier, J.; Gee, M.S.; Darge, K.; Dillman, J.R. Pediatric inflammatory bowel disease: Imaging issues with targeted solutions. Abdom. Imaging 2015, 40, 975–992. [Google Scholar] [CrossRef]
- Roh, A.T.; Xiao, Z.; Cheng, J.Y.; Vasanawala, S.S.; Loening, A.M. Conical ultrashort echo time (UTE) MRI in the evaluation of pediatric acute appendicitis. Abdom. Radiol. 2019, 44, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.-S.H.; Cernigliaro, J.G.; Pooley, R.A.; Bridges, M.D.; Giesbrandt, J.G.; Williams, J.C.; Haley, W.E. Detection of different kidney stone types: An ex vivo comparison of ultrashort echo time MRI to reference standard CT. Clin. Imaging 2016, 40, 90–95. [Google Scholar] [CrossRef] [PubMed]
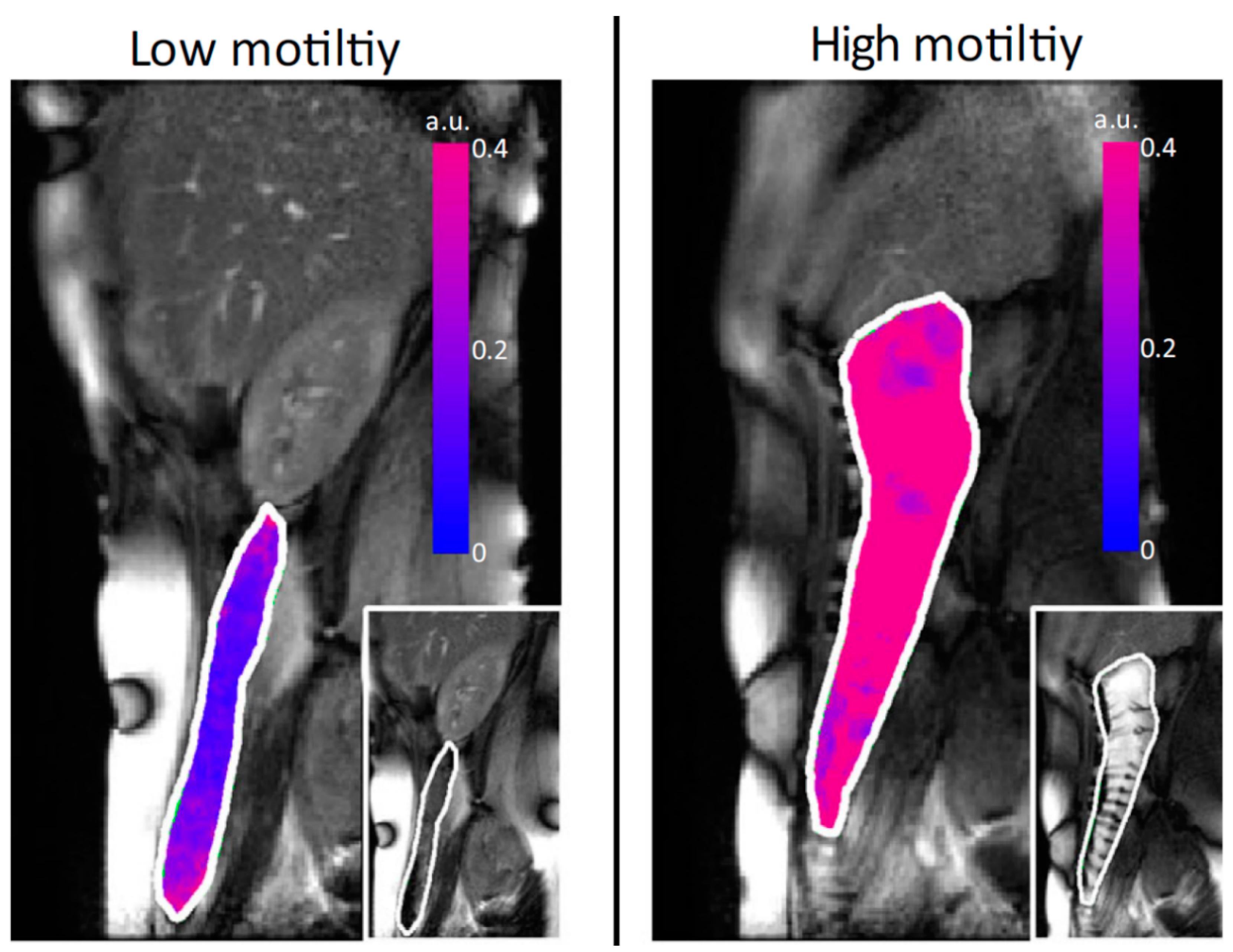

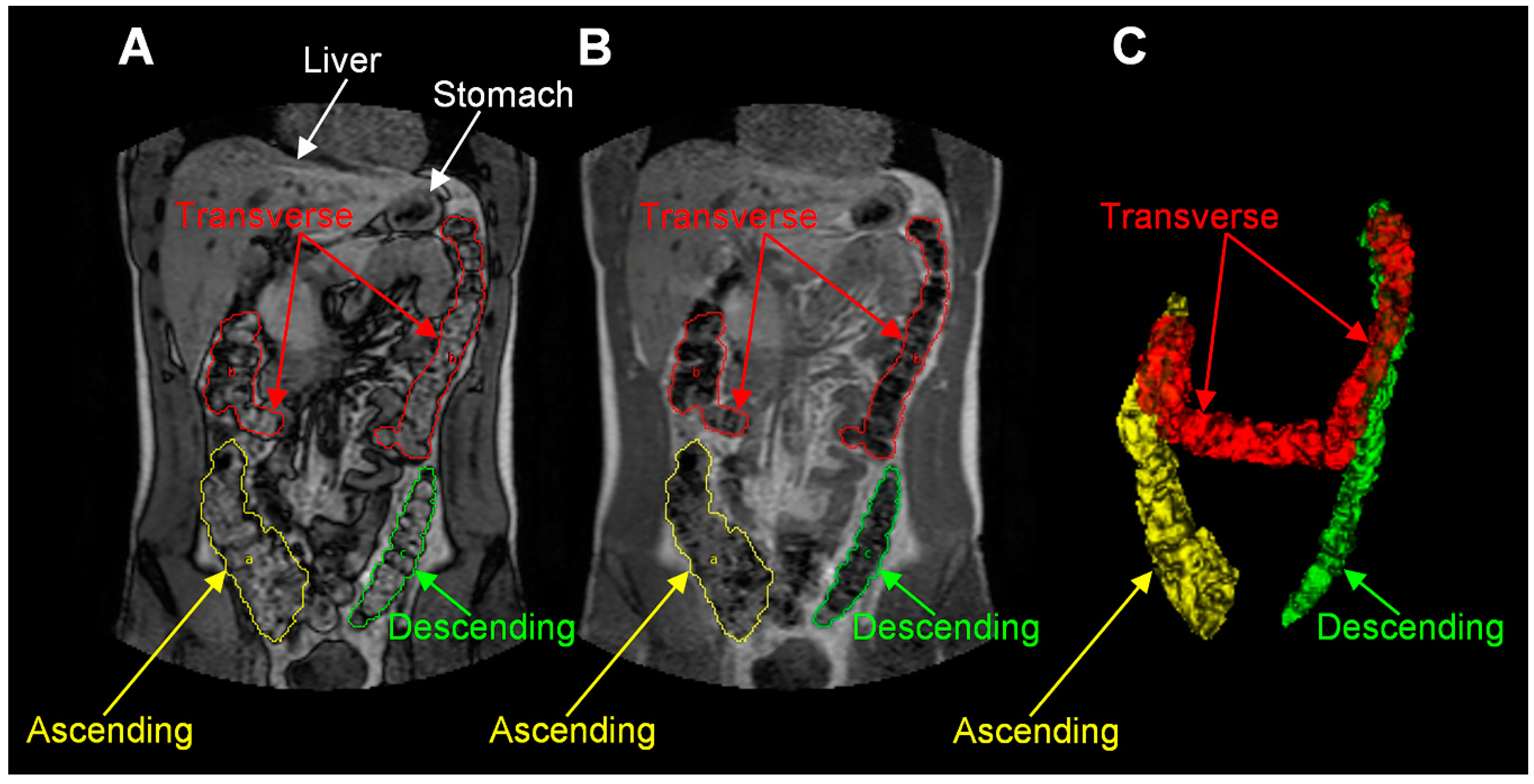

| Reference | Aims | Methods | Outcomes |
|---|---|---|---|
| [29] | Assessment of the intestinal transit by MRI | 12 healthy volunteers were scanned in fasted and fed state and after consumption of gel-filled capsules | Location of the capsules was affected by food consumption (in the large intestine: fasted vs. fed state was 3 vs. 17 capsules respectively, p < 0.01) |
| [48] | Assessment of new MRI technique of estimating intestinal transit with per os capsules containing gadolinium-saline solution | 7 females and 8 males (all healthy) consumed 5 capsules | Mean transit time for female and male volunteers was 41 ± 9 h and 31 ± 10 h respectively |
| [23] | Application of 19F and 1H MRI on intestinal transit | 2 healthy subjects consumed perfluoro-[15]-crown-5-ether capsules: 1 each on scanning day 1 and 2 each on scanning day 2 | Single capsule tracking: total transit lasted 27 h and 32 h for subjects A and B respectively (mean capsule velocity was 1.0 mm/s and 1.0 mm/s respectively). Capsule found outside the stomach 170 min and 220 min respectively Dual capsule tracking: capsules located out of the stomach 210 min after ingestion |
| [49] | Validate MRI technique towards OCTT3 and WGT1 measurements | 21 healthy subjects OCTT3 estimated by the arrival of the head of the meal into the beginning of the large bowel with MRI and by LUBT4 WGT1 estimated by MRI marker capsules and ROMs5 | MRI measurement of OCTT3 was (median(IQR)) 225 (180–270) min and of WGT1 was 28 (4–50) h |
| [25] | Investigation of the effect of oral PEG electrolyte in two dosing regimens on colonic motility | 12 healthy subjects consumed the split dose (1 L before the first scanning day and 1 L on the scanning day) and the other 12 healthy volunteers the single dose (2 L on the first scanning day) Each volunteer ingested MRI marker pills the day before the MRI transit scan (days 8, 14, 28) | No differences due to dosing regimens as Mean position score of split vs. single dose at Day 8: 6.2 ± 0.4 vs. 5.4 ± 0.6, p = 0.2527, Day 14: 5.8 ± 0.4 vs. 5.5 ± 0.5, p = 0.6076, Day 28: 6.1 ± 0.5 vs. 6.6 ± 0.3, p = 0.3327 No differences between the days regardless dosing: Day 8 vs. 14: p = 0.7750 Day 8 vs. 28: p = 0.2350 |
| [50] | Evaluation of MRI techniques of OCTT3 assessment towards LHBT6 in healthy volunteers | 28 healthy volunteers were recruited OCTT3 was assessed by the arrival of the head of the lactulose ingestion (10 g/125 mL) | OCTT3 by MRI measurements was (median (IQR)) 135 (120–150) min |
| [27] | MRI investigation of the effect of PEG electrolyte as a laxative on the colonic environment | 24 patients with functional constipation and 24 with IBS-C participated in this study. They has to consume 5 MRI marker pills before the scanning day and 1 L of PEG electrolyte after the baseline scan on the study day | WAPS2 for FC (3.6 (2.5–4.2)) was higher than the IBS-C (2.0 (1.5–3.2)), p = 0.01 |
| [41] | Distinguish subgroups of IBS based on MRI markers | 91 volunteers took part (34 healthy, 30 with IBS-D, 16 with IBS-C, and 11 IBS-M as mixed. IBS-M and IBS-D were listed as IBS-nonC) | WGT1 for IBS-C, healthy volunteers and IBS-D was 69 (51–111) h, 34 (4–63) h and 34 (17–78) h respectively and OCTT3 was 203 (154–266) min, 188 (135–262) min and 165 (116–244) min respectively |
| [44] | Study the ascending colonic transit in healthy and constipated subjects | 11 healthy and 11 constipated subjects were scanned fasted and after ingestion of 500 mL of macrogol and consumption MR markers | WAPS2 between healthy and patients was (median (IQR)) 0.6 (0–1) and 2.6 (1.4–3.6) respectively, p = 0.0011 |
| [51] | Evaluation of the applicability of gadolinium filled MRI capsules towards radio-opaque markers (ROMs5) on colon transit time (CTT) | 7 constipated and 9 healthy subjects ingested 5 gadolinium-based capsules as MRI markers and 20 ROMs5 | MRI measurements revealed that CTTs in healthy and constipated were 30.9 ± 15.9 h and 74.1 ± 7.2 h respectively, p < 0.05 Patients had higher CTTs than the healthy ones |
| [52] | Establishment of an MRI technique for bowel motion and transit assessment | Baseline and fed state MRI scanning of 15 healthy subjects Meal: chicken or mushroom soup Each subject consumed 5 MRI capsules of Gadoteric acid the day before the study day | WAPS2 (24 h) = 1.0 (0–3.8) WGT1 (hours) = 33 hr |
| [31] | Evaluation of psyllium consumption on colonic environment of healthy and constipated volunteers | 9 healthy subjects received maltodextrin (placebo) and psyllium 10.5 g and 21 g for 6 days randomly and 20 constipated subjects ingested maltodextrin and 21 g of psyllium in the same way On treatment day 5, each volunteer ingested 5 MRI marker capsules with gadoteric acid | WGT1 was higher in healthy than patients (p < 0.05) Controls: WAPS224 showed no differences as (median (IQR)) it was 1.0 (0.1–2.2) on maltodextrin, 1.4 (0.2–2.1) on 10.5 g of psyllium and 0.6 (0–1.9) on 21 g of psyllium Patients decreased from 4.2 (3.2–5.3) on maltodextrin to 2.0 (1.5–4.0) on psyllium (p = 0.067) |
| [32] | Evaluation of intestinal volumes and function on kiwifruit consumption | 2 kiwifruits or maltodextrin (control) 2 times per day for 3 days in the fasted and fed state | WGT for kiwifruit was (median (IQR)) 0.8 (0–1.4) and for control 1.0 (0.5–3.1), p = 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, S.; Marciani, L. MRI of the Colon in the Pharmaceutical Field: The Future before us. Pharmaceutics 2019, 11, 146. https://doi.org/10.3390/pharmaceutics11040146
Sulaiman S, Marciani L. MRI of the Colon in the Pharmaceutical Field: The Future before us. Pharmaceutics. 2019; 11(4):146. https://doi.org/10.3390/pharmaceutics11040146
Chicago/Turabian StyleSulaiman, Sarah, and Luca Marciani. 2019. "MRI of the Colon in the Pharmaceutical Field: The Future before us" Pharmaceutics 11, no. 4: 146. https://doi.org/10.3390/pharmaceutics11040146
APA StyleSulaiman, S., & Marciani, L. (2019). MRI of the Colon in the Pharmaceutical Field: The Future before us. Pharmaceutics, 11(4), 146. https://doi.org/10.3390/pharmaceutics11040146






