Current State and Future Perspectives on Gastroretentive Drug Delivery Systems
Abstract
:1. Introduction
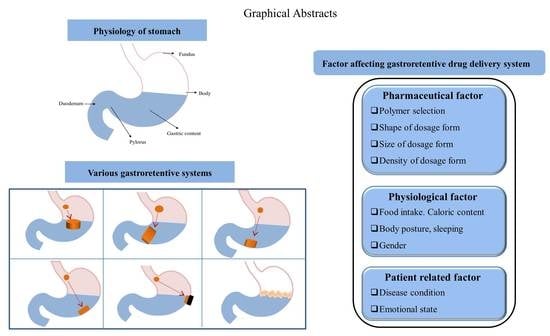
2. Physiology of Stomach
3. Application of GRDDS
4. Critical Factors Affecting GRDDS Efficacy
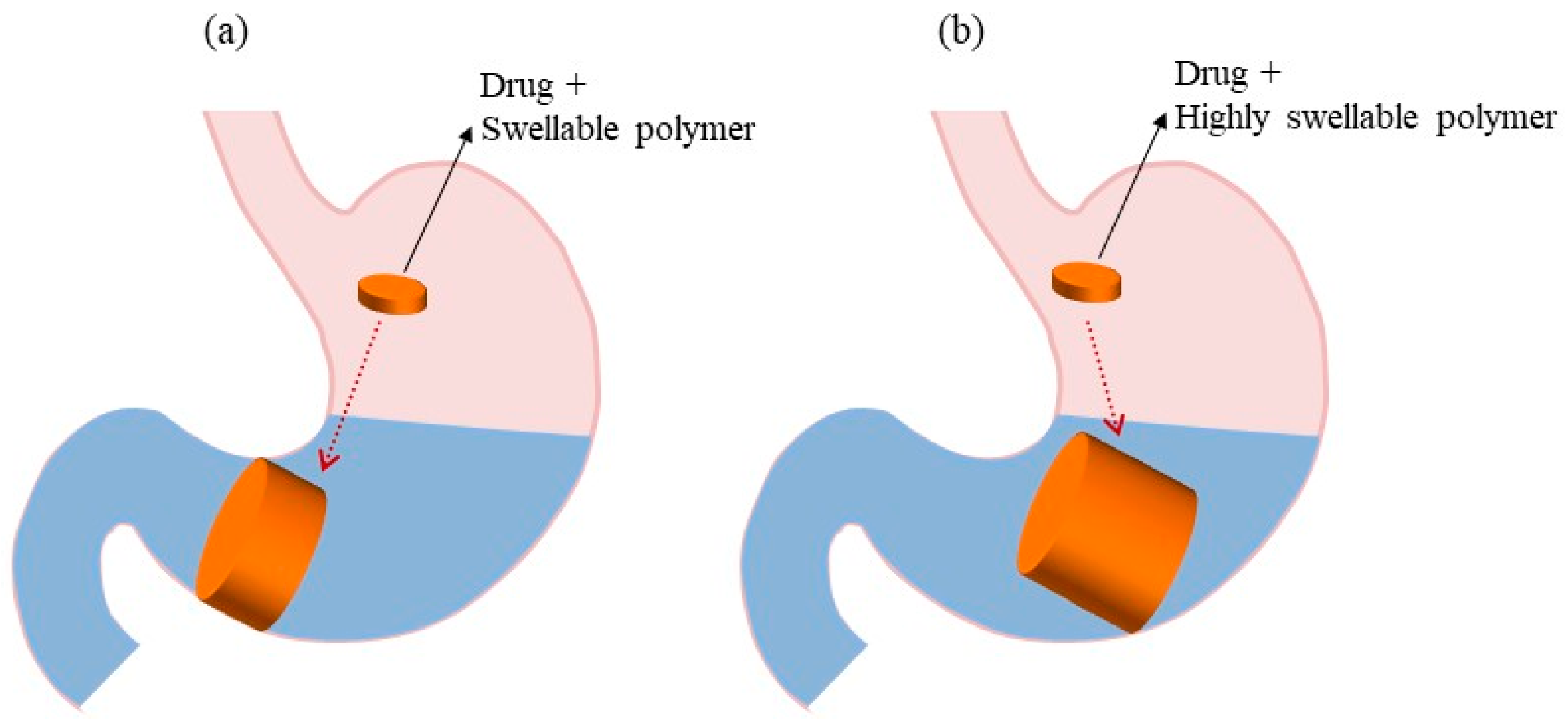
4.1. Pharmacutical Factors
4.2. Physiological Factors
4.3. Patient-Related Factors
5. Current Pharmaceutical Technologies of GRDDS
5.1. Low-Density Systems
5.1.1. Non-Effervescent Floating Systems
5.1.2. Effervescent Floating Systems
5.2. High-Density Systems
5.3. Expandable Systems
5.4. Superporous Hydrogel Systems
5.5. Bioadhesive/Mucoadhesive Systems
5.6. Raft-Forming Systems
5.7. Magnetic Systems
5.8. Ion-Exchange Resin Systems
6. Evaluation Parameters of GRDDS
6.1. In Vitro Evaluation Parameters
6.2. In Vivo Evaluation Parameters
7. Future Perspectives of GRDDS
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [PubMed]
- Rouge, N.; Buri, P.; Doelker, E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996, 136, 117–139. [Google Scholar] [CrossRef]
- Fujimori, J.; Machida, Y.; Tanaka, S.; Nagai, T. Effect of magnetically controlled gastric residence of sustained release tablets on bioavailability of acetaminophen. Int. J. Pharm. 1995, 119, 47–55. [Google Scholar] [CrossRef]
- Hwang, K.-M.; Cho, C.-H.; Tung, N.-T.; Kim, J.-Y.; Rhee, Y.-S.; Park, E.-S. Release kinetics of highly porous floating tablets containing cilostazol. Eur. J. Pharm. Biopharm. 2017, 115, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, K.-M.; Park, Y.S.; Nguyen, T.-T.; Park, E.-S. Preparation and evaluation of non-effervescent gastroretentive tablets containing pregabalin for once-daily administration and dose proportional pharmacokinetics. Int. J. Pharm. 2018, 550, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Klausner, E.A.; Lavy, E.; Friedman, M.; Hoffman, A. Expandable gastroretentive dosage forms. J. Control. Release 2003, 90, 143–162. [Google Scholar] [CrossRef]
- Sarkar, D.; Nandi, G.; Changder, A.; Hudati, P.; Sarkar, S.; Ghosh, L.K. Sustained release gastroretentive tablet of metformin hydrochloride based on poly (acrylic acid)-grafted-gellan. Int. J. Biol. Macromol. 2017, 96, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Sarparanta, M.P.; Bimbo, L.M.; Mäkilä, E.M.; Salonen, J.J.; Laaksonen, P.H.; Helariutta, A.K.; Linder, M.B.; Hirvonen, J.T.; Laaksonen, T.J.; Santos, H.A. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials 2012, 33, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Jeong, S. Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach. Pharmaceutics 2018, 10, 161. [Google Scholar] [CrossRef]
- Chavanpatil, M.D.; Jain, P.; Chaudhari, S.; Shear, R.; Vavia, P.R. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006, 316, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, W. Pharmacokinetics of verapamil and norverapamil from controlled release floating pellets in humans. Eur. J. Pharm. Biopharm. 2002, 53, 29–35. [Google Scholar] [CrossRef]
- Bardonnet, P.; Faivre, V.; Pugh, W.; Piffaretti, J.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef] [PubMed]
- El-Zahaby, S.A.; Kassem, A.A.; El-Kamel, A.H. Design and evaluation of gastroretentive levofloxacin floating mini-tablets-in-capsule system for eradication of Helicobacter pylori. Saudi Pharm. J 2014, 22, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, I.; Quirino-Barreda, T.; Villafuerte-Robles, L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008, 362, 37–43. [Google Scholar] [CrossRef]
- Sethi, S.; Mangla, B.; Kamboj, S.; Rana, V. A QbD approach for the fabrication of immediate and prolong buoyant cinnarizine tablet using polyacrylamide-g-corn fibre gum. Int. J. Biol. Macromol. 2018, 117, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Kulkarni, G.T. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: Where do we stand? Durg Deliv. 2016, 23, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Khutliwala, T.A.; Zala, B.S. Raft forming system—An upcoming approach of gastroretentive drug delivery system. J. Control. Release 2013, 168, 151–165. [Google Scholar] [CrossRef]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharm. Sci. 2016, 11, 575–584. [Google Scholar] [CrossRef]
- Prinderre, P.; Sauzet, C.; Fuxen, C. Advances in gastro retentive drug-delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1189–1203. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Park, H.; Park, K. Gastric retentive drug-delivery systems. Crit. Rev. Ther. Drug 1998, 15. [Google Scholar] [CrossRef]
- Cvijic, S.; Ibric, S.; Parojcic, J.; Djuris, J. An in vitro—In silico approach for the formulation and characterization of ranitidine gastroretentive delivery systems. J. Drug Deliv. Sci. Technol. 2018, 45, 1–10. [Google Scholar] [CrossRef]
- Hooda, A.; Nanda, A.; Jain, M.; Kumar, V.; Rathee, P. Optimization and evaluation of gastroretentive ranitidine HCl microspheres by using design expert software. Int. J. Biol. Macromol. 2012, 51, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Odeku, O.; Aderogba, A.; Ajala, T.; Akin-Anjani, O.; Okunlola, A. Formulation of floating metronidazole microspheres using cassava starch (Manihot esculenta) as polymer. J. Pharm. Investig. 2017, 47, 445–451. [Google Scholar] [CrossRef]
- Diós, P.; Nagy, S.; Pál, S.; Pernecker, T.; Kocsis, B.; Budán, F.; Horváth, I.; Szigeti, K.; Bölcskei, K.; Máthé, D.; et al. Preformulation studies and optimization of sodium alginate based floating drug delivery system for eradication of Helicobacter pylori. Eur. J. Pharm. Biopharm. 2015, 96, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Jangdey, M.S. Lectin conjugated gastroretentive multiparticulate delivery system of clarithromycin for the effective treatment of Helicobacter pylori. Mol. Pharm. 2008, 6, 295–304. [Google Scholar] [CrossRef]
- Hardikar, S.; Bhosale, A. Formulation and evaluation of gastro retentive tablets of clarithromycin prepared by using novel polymer blend. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 147–157. [Google Scholar] [CrossRef]
- Inukai, K.; Takiyama, K.; Noguchi, S.; Iwao, Y.; Itai, S. Effect of gel formation on the dissolution behavior of clarithromycin tablets. Int. J. Pharm. 2017, 521, 33–39. [Google Scholar] [CrossRef]
- Mostafavi, A.; Emami, J.; Varshosaz, J.; Davies, N.M.; Rezazadeh, M. Development of a prolonged-release gastroretentive tablet formulation of ciprofloxacin hydrochloride: Pharmacokinetic characterization in healthy human volunteers. Int. J. Pharm. 2011, 409, 128–136. [Google Scholar] [CrossRef]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharm. 2018, 549, 370–379. [Google Scholar] [CrossRef]
- Kagan, L.; Lapidot, N.; Afargan, M.; Kirmayer, D.; Moor, E.; Mardor, Y.; Friedman, M.; Hoffman, A. Gastroretentive Accordion Pill: Enhancement of riboflavin bioavailability in humans. J. Control. Release 2006, 113, 208–215. [Google Scholar] [CrossRef]
- Qin, C.; Wu, M.; Xu, S.; Wang, X.; Shi, W.; Dong, Y.; Yang, L.; He, W.; Han, X.; Yin, L. Design and optimization of gastro-floating sustained-release tablet of pregabalin: In vitro and in vivo evaluation. Int. J. Pharm. 2018, 545, 37–44. [Google Scholar] [CrossRef]
- Ngwuluka, N.C.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. An optimized gastroretentive nanosystem for the delivery of levodopa. Int. J. Pharm. 2015, 494, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.-O.; Kim, J.-Y.; Ha, J.-M.; Chi, S.-C.; Rhee, Y.-S.; Park, C.-W.; Park, E.-S. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur. J. Pharm. Biopharm. 2013, 83, 460–467. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Zhang, R.; Wu, Z.; Yin, L. Gastro-floating bilayer tablets for the sustained release of metformin and immediate release of pioglitazone: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2014, 476, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Talele, G.S. Gastroretentive mucoadhesive tablet of lafutidine for controlled release and enhanced bioavailability. Durg Deliv. 2015, 22, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Kansal, S.; Garg, G.; Awasthi, R.; Singodia, D.; Kulkarni, G.T. Gastroretentive dosage forms: A review with special emphasis on floating drug delivery systems. Durg Deliv. 2011, 18, 97–110. [Google Scholar] [CrossRef]
- Kotreka, U.; Adeyeye, M.C. Gastroretentive floating drug-delivery systems: A critical review. Crit. Rev. Ther. Drug 2011, 28. [Google Scholar] [CrossRef]
- Talukder, R.; Fassihi, R. Gastroretentive delivery systems: A mini review. Drug Dev. Ind. Pharm. 2004, 30, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sharma, S. Gastroretentive drug delivery systems. Expert opin. Drug Deliv. 2006, 3, 217–233. [Google Scholar]
- Salessiotis, N. Measurement of the diameter of the pylorus in man: Part I. Experimental project for clinical application. Am. J. Surg. 1972, 124, 331–333. [Google Scholar] [CrossRef]
- Timmermans, J.; Moes, A.J. How well do floating dosage forms float? Int. J. Pharm. 1990, 62, 207–216. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kumar, A.; Pathak, K. Osmotically regulated floating asymmetric membrane capsule for controlled site-specific delivery of ranitidine hydrochloride: Optimization by central composite design. AAPS PharmSciTech 2012, 13, 1492–1501. [Google Scholar] [CrossRef]
- Ali, J.; Arora, S.; Ahuja, A.; Babbar, A.K.; Sharma, R.K.; Khar, R.K.; Baboota, S. Formulation and development of hydrodynamically balanced system for metformin: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2007, 67, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Newton, J.; Short, M. Gastrointestinal transit of pellets of differing size and density. Int. J. Pharm. 1993, 100, 81–92. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Ali, J.; Ahuja, A.; Khar, R.K.; Baboota, S. Floating drug delivery systems: A review. AAPS PharmSciTech 2005, 6, E372–E390. [Google Scholar] [CrossRef] [PubMed]
- Shaha, S.; Patel, J.; Pundarikakshudu, K.; Patel, N. An overview of a gastro-retentive floating drug delivery system. Asian J. Pharm. Sci. 2009, 4, 65–80. [Google Scholar]
- Calbet, J.A.; MacLean, D.A. Role of caloric content on gastric emptying in humans. J. Physiol. 1997, 498 Pt 2, 553–559. [Google Scholar] [CrossRef]
- Juvonen, K.R.; Purhonen, A.-K.; Salmenkallio-Marttila, M.; Lahteenmaki, L.; Laaksonen, D.E.; Herzig, K.-H.; Uusitupa, M.I.; Poutanen, K.S.; Karhunen, L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate. PLoS ONE 2013, 8, e67482. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, G. Progress in controlled gastroretentive delivery systems. Trop. J. Pharm. Res. 2008, 7, 1055–1066. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Debreceni, T.L.; Burgstad, C.M.; Wishart, J.M.; Bellon, M.; Rayner, C.K.; Wittert, G.A.; Horowitz, M. Effects of posture and meal volume on gastric emptying, intestinal transit, oral glucose tolerance, blood pressure and gastrointestinal symptoms after Roux-en-Y gastric bypass. Obes. Surg. 2015, 25, 1392–1400. [Google Scholar] [CrossRef]
- Wang, Y.T.; Mohammed, S.D.; Farmer, A.D.; Wang, D.; Zarate, N.; Hobson, A.R.; Hellström, P.M.; Semler, J.R.; Kuo, B.; Rao, S.S. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment. Pharmacol. Ther. 2015, 42, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Barnett, C. Fasting gastric pH and its relationship to true hypochlorhydria in humans. Dig. Dis. Sci. 1991, 36, 866–869. [Google Scholar] [CrossRef]
- Mojaverian, P.; Vlasses, P.H.; Kellner, P.E.; Rocci, M.L. Effects of gender, posture, and age on gastric residence time of an indigestible solid: Pharmaceutical considerations. Pharm. Res. 1988, 5, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Krygowska-Wajs, A.; Cheshire, W.P.; Wszolek, Z.K.; Hubalewska-Dydejczyk, A.; Jasinska-Myga, B.; Farrer, M.J.; Moskala, M.; Sowa-Staszczak, A. Evaluation of gastric emptying in familial and sporadic Parkinson disease. Parkinsonism Relat. D. 2009, 15, 692–696. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Kalantzis, C.; Papadopoulos, A.; Apostolopoulos, P.; Rokkas, T.; Kalantzis, N.; Ladas, S. Video-capsule endoscopy gastric and small bowel transit time and completeness of the examination in patients with diabetes mellitus. Dig. Liver Dis. 2007, 39, 575–580. [Google Scholar] [CrossRef]
- N Abduljabbar, H.; M Badr-Eldin, S.; M Aldawsari, H. Gastroretentive ranitidine hydrochloride tablets with combined floating and bioadhesive properties: Factorial design analysis, in vitro evaluation and in vivo abdominal X-ray imaging. Curr. Drug Deliv. 2015, 12, 578–590. [Google Scholar] [CrossRef]
- Choi, B.; Park, H.; Hwang, S.; Park, J. Preparation of alginate beads for floating drug delivery system: Effects of CO2 gas-forming agents. Int. J. Pharm. 2002, 239, 81–91. [Google Scholar] [CrossRef]
- Rossi, A.; Conti, C.; Colombo, G.; Castrati, L.; Scarpignato, C.; Barata, P.; Sandri, G.; Caramella, C.; Bettini, R.; Buttini, F. Floating modular drug delivery systems with buoyancy independent of release mechanisms to sustain amoxicillin and clarithromycin intra-gastric concentrations. Drug Dev. Ind. Pharm. 2016, 42, 332–339. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Ho, H.-O.; Lee, T.-Y.; Sheu, M.-T. Physical characterizations and sustained release profiling of gastroretentive drug delivery systems with improved floating and swelling capabilities. Int. J. Pharm. 2013, 441, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Abouelatta, S.M.; Aboelwafa, A.A.; El-Gazayerly, O.N. Gastroretentive raft liquid delivery system as a new approach to release extension for carrier-mediated drug. Durg Deliv. 2018, 25, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.A.H.A.; Kassem, A.A.; El-Massik, M.A.E.; Boraie, N.A. Development of gastroretentive metronidazole floating raft system for targeting Helicobacter pylori. Int. J. Pharm. 2015, 486, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Nagpal, M. Comparison of various generations of superporous hydrogels based on chitosan-acrylamide and in vitro drug release. ISRN Pharm. 2013, 2013, 624841. [Google Scholar] [CrossRef]
- Sheth, P.; Tossounian, J. The hydrodynamically balanced system (HBS™): A novel drug delivery system for oral use. Drug Dev. Ind. Pharm. 1984, 10, 313–339. [Google Scholar] [CrossRef]
- Reddy, L.H.V.; Murthy, R. Floating dosage systems in drug delivery. Crit. Rev. Ther. Drug 2002, 19, 553–585. [Google Scholar] [CrossRef]
- Hilton, A.; Deasy, P. In vitro and in vivo evaluation of an oral sustained-release floating dosage form of amoxycillin trihydrate. Int. J. Pharm. 1992, 86, 79–88. [Google Scholar] [CrossRef]
- Michaels, A. Drug Delivery Device with Self Actuated Mechanism for Retaining Device in Selected Area. U.S. Patent 3,786,813, 22 January 1974. [Google Scholar]
- Kawashima, Y.; Niwa, T.; Takeuchi, H.; Hino, T.; Itoh, Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J. Pharm. Sci. 1992, 81, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.A.; Carter, P.; Elkordy, A.A. Influence of calcium carbonate and sodium carbonate gassing agents on pentoxifylline floating tablets properties. Powder Technol. 2017, 322, 65–74. [Google Scholar] [CrossRef]
- Baumgartner, S.; Kristl, J.; Vrečer, F.; Vodopivec, P.; Zorko, B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int. J. Pharm. 2000, 195, 125–135. [Google Scholar] [CrossRef]
- Tadros, M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010, 74, 332–339. [Google Scholar] [CrossRef]
- Moes, A. Gastric retention systems for oral drug delivery. In Business Briefing: Pharmatech; Business Briefings Ltd.: Singapore, 2003; pp. 157–159. [Google Scholar]
- Sivaneswari, S.; Karthikeyan, E.; Chandana, P. Novel expandable gastro retentive system by unfolding mechanism of levetiracetam using simple lattice design–Formulation optimization and in vitro evaluation. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 63–72. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Robinson, J.R. Bioadhesive polymers as platforms for oral-controlled drug delivery: Method to study bioadhesion. Int. J. Pharm. 1984, 19, 107–127. [Google Scholar] [CrossRef]
- Wang, J.; Tauchi, Y.; Deguchi, Y.; Morimoto, K.; Tabata, Y.; Ikada, Y. Positively charged gelatin microspheres as gastric mucoadhesive drug delivery system for eradication of H. pylori. Durg Deliv. 2000, 7, 237–243. [Google Scholar]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Recent advancements in mucoadhesive floating drug delivery systems: A mini-review. J. Drug Deliv. Sci. Technol. 2016, 31, 65–71. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; El-Naaj, I.A.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; De, P.K.; De, A.; Ojha, S.; De, R.; Mukhopadhyay, A.K.; Samanta, A. Floating mucoadhesive alginate beads of amoxicillin trihydrate: A facile approach for H. pylori eradication. Int. J. Biol. Macromol. 2016, 89, 622–631. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Design of a gastroretentive mucoadhesive dosage form of furosemide for controlled release. Acta Pharm. Sin. B 2012, 2, 509–517. [Google Scholar] [CrossRef]
- Bera, H.; Kandukuri, S.G.; Nayak, A.K.; Boddupalli, S. Alginate-sterculia gum gel-coated oil-entrapped alginate beads for gastroretentive risperidone delivery. Carbohydr. Polym. 2015, 120, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Nappinnai, M.; Sivaneswari, S. Formulation optimization and characterization of gastroretentive cefpodoxime proxetil mucoadhesive microspheres using 32 factorial design. J. Pharm. Res. 2013, 7, 304–309. [Google Scholar] [CrossRef]
- Pund, S.; Joshi, A.; Vasu, K.; Nivsarkar, M.; Shishoo, C. Gastroretentive delivery of rifampicin: In vitro mucoadhesion and in vivo gamma scintigraphy. Int. J. Pharm. 2011, 411, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Li, J.; Qiao, H.; Di, L. Rheological and mucoadhesive properties of polysaccharide from Bletilla striata with potential use in pharmaceutics as bio-adhesive excipient. Int. J. Biol. Macromol. 2018, 120, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Fabregas, J.; Claramunt, J.; Cucala, J.; Pous, R.; Siles, A. “In-Vitro” Testing of an Antacid Formulation with Prolonged Gastric Residence Time (Almagate Flot-Coat®). Drug Dev. Ind. Pharm. 1994, 20, 1199–1212. [Google Scholar] [CrossRef]
- Hampson, F.C.; Jolliffe, I.G.; Bakhtyari, A.; Taylor, G.; Sykes, J.; Johnstone, L.M.; Dettmar, P.W. Alginate–antacid combinations: Raft formation and gastric retention studies. Drug Dev. Ind. Pharm. 2010, 36, 614–623. [Google Scholar] [CrossRef]
- Murphy, C.S.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Gastroretentive drug delivery systems: Current developments in novel system design and evaluation. Curr. Drug Deliv. 2009, 6, 451–460. [Google Scholar] [CrossRef]
- Ito, R.; Machida, Y.; Sannan, T.; Nagai, T. Magnetic granules: A novel system for specific drug delivery to esophageal mucosa in oral administration. Int. J. Pharm. 1990, 61, 109–117. [Google Scholar] [CrossRef]
- Gaur, P.K.; Mishra, S.; Bhardwaj, S.; Puri, D.; Kumar, S.S. Ion Exchange Resins in Gastroretentive Drug Delivery: Characteristics, Selection, Formulation and Applications. J. Pharm. Sci. Pharmacol. 2014, 1, 304–312. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, K. Drug loading and release properties of ion-exchange resin complexes as a drug delivery matrix. Int. J. Pharm. 2008, 361, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Berhane, N.H.; Haghighi, K.; Park, K. Drug Release Properties of Polymer Coated Ion-Exchange Resin Complexes: Experimental and Theoretical Evaluation. J. Pharm. Sci. 2007, 96, 618–632. [Google Scholar] [CrossRef]
- Anand, V.; Kandarapu, R.; Garg, S. Ion-exchange resins: Carrying drug delivery forward. Drug Discov. Today 2001, 6, 905–914. [Google Scholar] [CrossRef]
- Eisenächer, F.; Garbacz, G.; Mäder, K. Physiological relevant in vitro evaluation of polymer coats for gastroretentive floating tablets. Eur. J. Pharm. Biopharm. 2014, 88, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Strübing, S.; Abboud, T.; Contri, R.V.; Metz, H.; Mäder, K. New insights on poly(vinyl acetate)-based coated floating tablets: Characterisation of hydration and CO2 generation by benchtop MRI and its relation to drug release and floating strength. Eur. J. Pharm. Biopharm. 2008, 69, 708–717. [Google Scholar]
- Chen, J.; Blevins, W.E.; Park, H.; Park, K. Gastric retention properties of superporous hydrogel composites. J. Control. Release 2000, 64, 39–51. [Google Scholar] [CrossRef]
- Kashyap, N.; Viswanad, B.; Sharma, G.; Bhardwaj, V.; Ramarao, P.; Kumar, M.R. Design and evaluation of biodegradable, biosensitive in situ gelling system for pulsatile delivery of insulin. Biomaterials 2007, 28, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Nagpal, K.; Singh, S.; Mishra, D. Unfolding type gastroretentive film of Cinnarizine based on ethyl cellulose and hydroxypropylmethyl cellulose. Int. J. Biol. Macromol. 2014, 64, 347–352. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, K. Development of sustained release fast-disintegrating tablets using various polymer-coated ion-exchange resin complexes. Int. J. Pharm. 2008, 353, 195–204. [Google Scholar] [CrossRef]
- Torres, D.; Boado, L.; Blanco, D.; Vila-Jato, J.L. Comparison between aqueous and non-aqueous solvent evaporation methods for microencapsulation of drug–resin complexes. Int. J. Pharm. 1998, 173, 171–182. [Google Scholar] [CrossRef]
- Farag, Y.; Nairn, J.G. Rate of release of organic carboxylic acids from ion-exchange resins. J. Pharm. Sci. 1988, 77, 872–875. [Google Scholar] [CrossRef] [PubMed]
- El-said, I.A.; Aboelwafa, A.A.; Khalil, R.M.; ElGazayerly, O.N. Baclofen novel gastroretentive extended release gellan gum superporous hydrogel hybrid system: In vitro and in vivo evaluation. Durg Deliv. 2016, 23, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, G.; Udupa, N. Biodegradable Injectable Implant Systems for Long Term Drug Delivery Using Poly (Lactic-co-glycolic) Acid Copolymers. J. Pharm. Pharmacol. 1996, 48, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Badve, S.S.; Sher, P.; Korde, A.; Pawar, A.P. Development of hollow/porous calcium pectinate beads for floating-pulsatile drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.P.; Patel, A.V.; Patel, R.B.; Patel, M.; Patel, K.; Patel, N. Gastroretentive drug delivery systems: A review. Int. J. Pharm. World Res. 2011, 2, 1–24. [Google Scholar]





| Phase | Comments | Duration |
|---|---|---|
| Phase 1 | Quiescent period with rare contractions. | 30–60 min |
| Phase 2 | Intermittent action potentials and contraction that gradually increases in intensity and frequency as the phase progresses. | 20–40 min |
| Phase 3 | Short periods of intense, large, regular contractions. This phase is termed as “housekeeper wave” as it enables all undigested materials to be swept out of the stomach and down to the small intestine. | 10–20 min |
| Phase 4 | Occurs between phase 3 and phase 1 of two consecutive cycles in a brief transitional phase. | 0–5 min |
| Bioavailability Challenges | Drug | Therapeutic Indications | References |
|---|---|---|---|
| Local activity | Ranitidine, Amoxicillin, Levofloxacin, Metronidazole | Peptic ulcer and reflux esophagitis, eradication of H. pylori | [1,13,21,22,23,24] |
| Plasma fluctuations | Ciprofloxacin, Clarithromycin | Urinary tract, respiratory, and GI infections | [1,25,26,27,28] |
| Low solubility at alkaline pH | Ofloxacin | Urinary tract, respiratory, and GI infections | [1,10] |
| Cinnarizine | Nausea, vertigo, and motion sickness | [15] | |
| Narrow absorption window | Riboflavin | Essential nutrients, mouth ulcer and sore throat | [29,30] |
| Cilostazol | Inhibits platelet aggregation | [4] | |
| Pregabalin | Fibromyalgia, diabetic peripheral neuropathy, post-herpetic neuralgia, and adjunctive therapy for partial onset seizures | [5,31] | |
| Short half-life, narrow absorption window | Levodopa | Parkinson’s disease | [32] |
| Metformin | Type II diabetes mellitus | [7,9,33,34] | |
| Poor absorption from lower GIT | Atenolol | Hypertension | [1] |
| Lafutidine | Gastric and duodenal ulcers | [35] | |
| Unstable at alkaline pH | Verapamil, Captopril | Hypertension | [1,11,14] |
| Delivery Systems | Brand Name | Active Ingredient | Manufacturing Company |
|---|---|---|---|
| Bioadhesive tablets | Xifaxan® | Rifampicin | Lupin, India |
| Bilayer floating capsule | Cytotec® | Misoprostol | Pfizer, UK |
| Coated multi-layer & swelling system | Baclofen GRS® | Baclofen | Sun Pharma, India |
| Colloidal gel forming floating system | Conviron® | Ferrous sulphate | Ranbaxy, India |
| Effervescent floating system | Zanocin OD® | Ofloxacin | Ranbaxy, India |
| Riomet OD® | Metformin hydrochloride | Ranbaxy, India | |
| Cifran OD® | Ciprofloxacin | Ranbaxy, India | |
| Effervescent floating liquid alginate preparation | Liquid Gaviscon® | Alginic acid and sodium bicarbonate | Reckitt Benckiser Healthcare, UK |
| Effervescent and swelling based floating system | Prazopress XL® | Prazosin hydrochloride | Sun Pharma, Japan |
| Erodible matrix based system | Cipro XR® | Ciprofloxacin hydrochloride and betaine | Bayer, USA |
| Expandable system (unfolding) | Accordion Pill® | Carbidopa/levodopa | Intec Pharma, Israel |
| Raft forming system | Topalkan® | Aluminum magnesium | Pierre Fabre Medicament, France |
| Almagate FlatCoat® | Aluminium-magnesium antacid | Pierre Fabre Medicament, France | |
| Floating system—controlled release capsule | Madopar HBS® | Levodopa and benserzide | Roche, UK |
| Prolopa HBS® | Levodopa and benserzide hydrochloride | Roche, UK | |
| Valrelease® | Diazepam | Roche, UK | |
| Foam based floating system | Inon Ace Tables® | Simethicone | Sato Pharma, Japan |
| Gastroretention with osmotic system | Coreg CR® | Carvedilol | GlaxoSmithKline, UK |
| Minextab Floating®—floating and swelling system | Metformin HCl | Metformin hydrochloride | Galanix, France |
| Cafeclor LP | Cefaclor | Galanix, France | |
| Tramadol LP | Tramadol | Galanix, France | |
| Polymer based swelling technology: AcuFormTM | Gabapentin GR | Gabapentin | Depomed, USA |
| proQuin XR | Ciprofloxacin | Depomed, USA | |
| Glumetza | Metformin hydrochloride | Depomed, USA | |
| Metfromin GRTM | Metformin hydrochloride | Depomed, USA |
| Gastroretentive Approach | Mechanism | References |
|---|---|---|
| Low-density systems/ floating systems | System causes buoyancy in gastric fluid. Density of pellets/tablets is lower than the density of stomach fluid. | [1,17,20,47] |
| High density systems | Uses the density of dosage form as a strategy to produce the retention mechanism. Sinking system remains at the bottom of the stomach, where the density of the dosage form is greater than the gastric fluid. | [1] |
| Expandable systems | Expansion of the dosage form occurs by swelling or unfolding in the stomach. Swelling usually occurs because of diffusion. Unfolding takes place due to mechanical shape memory. | [6,12,61] |
| Bioadhesive systems | A very complex process with several mechanisms, including electrical theory, adsorption, wetting, diffusion, and fracture theories. The interaction between the negatively charged mucosal surface and positively charged polymers might facilitate the bioadhesive process. | [12,45] |
| Raft forming systems | The polymer in presence of mono or di valent cations, absorbs water, swells and forms in situ gel layers, which float above gastric fluid and termed as raft. | [62,63] |
| Super-porous hydrogel systems | Swells up to 100 times due to water update by capillary wetting through numerous pores. | [12,64] |
| Magnetic systems | Consists of the small internal magnet mixed with the drug. Its position inside the stomach is controlled by an extracorporeal magnet. | [16] |
| Ion-exchange resin systems | Drug is loaded into the resin to form the resin loaded drug complex, which can be combined with floating delivery or bioadhesive systems. | [16] |
| Theories | Mechanisms of Mucoadhesion |
|---|---|
| Wettability | Bioadhesive polymers penetrate and develop intimate contact with the mucous layers. |
| Diffusion | Physical entanglement of mucin strands and flexible polymer chains. Influenced by molecular weight, cross-linking density, chain flexibility, and expansion capacity of both networks. |
| Adsorption | Bioadhesion is due to primary forces (ionic, covalent, and metallic) and secondary forces (van der Waals, hydrophobic and hydrogen bonds) between surfaces. |
| Electronic | Attractive electrostatic forces between the glycoprotein mucin network and the bioadhesive material. |
| Fracture | Detachment force needed to separate the mucus and polymer reflects the force of the adhesive binding. |
| GRDDS | Evaluation | Comment | References |
|---|---|---|---|
| Low-density system, raft-forming system | Floating Lag Time (FLT), total floating time (TFT), floating strength | The test is carried out in a simulated gastric fluid (SGF) at 37 °C. The time between introduction of dosage form and its buoyancy on the SGF (FLT) and the time during which the dosage form remains buoyant (TFT) were measured. The floating strength is measured using specifically designed basket holder connected with analytical balance. The reduction of weight on the analytical balance over time determines the floating strength. | [9,17,19,31,97,98] |
| Superporous hydrogel system, expandable system | Swelling studies | The test is carried out by placing the weighed amount of dosage form into the swelling medium (0.01 N HCl) and weight, diameter, and length of swollen samples are measured at predetermined time point. | [61,99] |
| Raft-forming and Mucoadhesion systems | Viscosity and Rheology | Viscosity of polymer affects the consistency of the dosage form upon contact with the gastric fluid. Brookfield/Ostwald’s viscometer and texture analyzer are commonly used. | [100] |
| Expandable system | In vitro unfolding study | The test is carried out by placing the folded dosage form into the dissolution medium and examining its unfolding behavior in different time interval. | [101] |
| Ion-exchange resin system | Particle size, ion exchange capacity, moisture content | Particle size analysis is carried out using a sieve shaker, laser diffraction, and coulter counter analyzer. The ion exchange capacity depends upon the functional group available for crosslinking. Moisture content can be measured with Karl Fischer. | [96,102,103,104] |
| Applicable for all GRDDS | In vitro drug release | The test is carried out in SGF at a predefined time interval (generally 0 to 12 h) using USP type-II apparatus at 50 rpm and maintained at 37 °C. | [9,27,72,80] |
| Gel strength | The high gel strength is desirable for better mechanical integrity. | [9,105] | |
| Drug-excipient interaction study | It can be studied by using FT-IR spectroscopy, Differential scanning calorimetry, and High Performance Liquid Chromatography. | [17,106] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics 2019, 11, 193. https://doi.org/10.3390/pharmaceutics11040193
Tripathi J, Thapa P, Maharjan R, Jeong SH. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics. 2019; 11(4):193. https://doi.org/10.3390/pharmaceutics11040193
Chicago/Turabian StyleTripathi, Julu, Prakash Thapa, Ravi Maharjan, and Seong Hoon Jeong. 2019. "Current State and Future Perspectives on Gastroretentive Drug Delivery Systems" Pharmaceutics 11, no. 4: 193. https://doi.org/10.3390/pharmaceutics11040193
APA StyleTripathi, J., Thapa, P., Maharjan, R., & Jeong, S. H. (2019). Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics, 11(4), 193. https://doi.org/10.3390/pharmaceutics11040193