Application of Pharmacometrics in Pharmacotherapy: Open-Source Software for Vancomycin Therapeutic Drug Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Dataset
2.3. Population PK Modeling
2.4. Covariate Evaluation
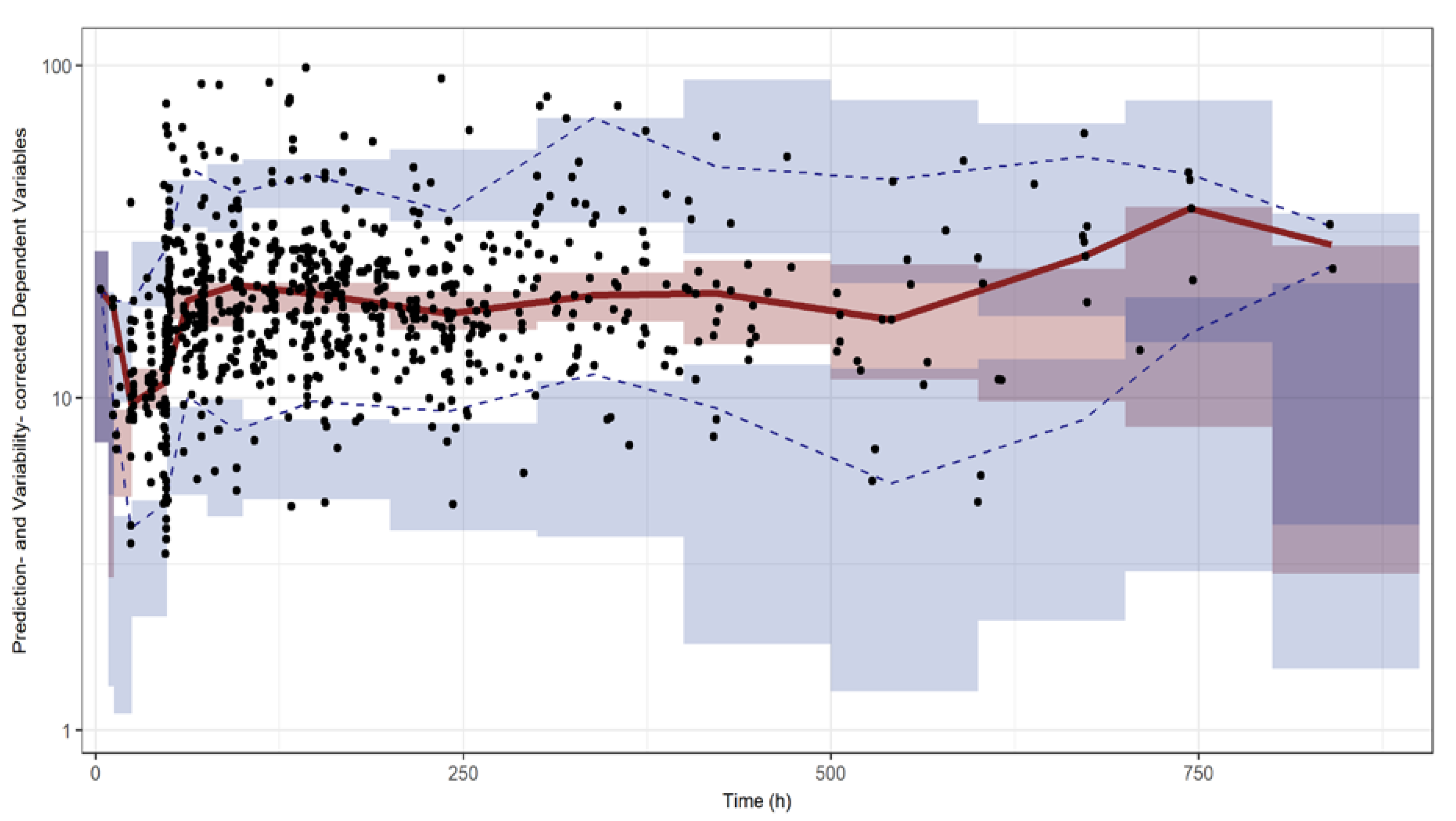
2.5. Model Evaluation and Simulation
2.6. R Shiny Application for VCM TDM
3. Results
3.1. Population PK Modeling
3.2. Model Assessment and Simulation
3.3. R Shiny Application for VCM TDM
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [PubMed]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America; the American Society of Health-System Pharmacists; and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Johannsson, B.; Beekmann, S.E.; Srinivasan, A.; Hersh, A.L.; Laxminarayan, R.; Polgreen, P.M. Improving antimicrobial stewardship: The evolution of programmatic strategies and barriers. Infect. Control. Hosp. Epidemiol. 2011, 32, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Matzke, G.R.; Zhanel, G.G.; Guay, D.R. Clinical pharmacokinetics of vancomycin. Clin. Pharmacokinet. 1986, 11, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Marsot, A.; Boulamery, A.; Bruguerolle, B.; Simon, N. Vancomycin: A review of population pharmacokinetic analyses. Clin. Pharmacokinet. 2012, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wong-Beringer, A.; Joo, J.; Tse, E.; Beringer, P. Vancomycin-associated nephrotoxicity: A critical appraisal of risk with high-dose therapy. Int. J. Antimicrob. Agents. 2011, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.K.; Li, C.; Zhai, S.D. Guidelines for therapeutic drug monitoring of vancomycin: A systematic review. PLoS ONE 2014, 9, e99044. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, S.; Khalili, H.; Dashti-Khavidaki, S.; Mohammadpour, A. Vancomycin-induced nephrotoxicity: Mechanism; incidence; risk factors and special populations. A literature review. Eur. J. Clin. Pharmacol. 2012, 68, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; An, H.; Shin, K.H.; Shin, D.; Lee, S.H.; Kim, J.H.; Cho, S.H.; Kang, H.R.; Jang, I.J.; Yu, K.S.; Lim, K.S. Trough concentration over 12.1 mg/L is a major risk factor of vancomycin-related nephrotoxicity in patients with therapeutic drug monitoring. Ther. Drug. Monit. 2014, 36, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Pai, M.P.; Rodvold, K.A.; Lomaestro, B.; Drusano, G.L.; Lodise, T.P. Vancomycin: We can’t get there from here. Clin. Infect. Dis. 2011, 52, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Neely, M.; Rodvold, K.A.; Lodise, T.P. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv. Drug. Deliv. Rev. 2014, 77, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.G.; Kim, H.K.; Roe, E.K.; Lee, S.Y.; Ahn, B.S.; Kim, J.H.; Park, M.S.; Yoon, H.J.; Kim, J.M. Therateutic Drug Monitoring of Vancomycin in Korean Patients. Infect. Chemother. 2004, 36, 311–318. [Google Scholar]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. The AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Bae, K.S.; Houk, B.E.; Savic, R.M.; Karlsson, M.O. Standard Error of Empirical Bayes Estimate in NONMEM(R) VI. Korean. J. Physiol. Pharmacol. 2012, 16, 97–106. [Google Scholar] [CrossRef] [PubMed]
- A Step-By-Step Guide to Prediction Corrected Visual Predictive Checks (VPC) of NONMEM Models. Available online: https://www.pmxsolutions.com/2018/09/21/a-step-by-step-guide-to-prediction-corrected-visual-predictive-checks-vpc-of-nonmem-models/ (accessed on 25 March 2019).
- Kim, M.G.; Yim, D.S.; Bae, K.S. R-based reproduction of the estimation process hidden behind NONMEM® Part 1: first-order approximation method. Transl. Clin. Pharmacol. 2015, 23, 1–7. [Google Scholar] [CrossRef]



| Variables (Unit) | Mean (Range) |
|---|---|
| Age (year) | 63 (21–98) |
| Sex (male/female) | 139/81 |
| Weight (kg) | 61.6 (30.0–126.7) |
| Serum creatinine, Scr (mg/dL) | 1.7 (0.20–13.3) |
| Creatinine clearance, CLCR (mL/min) 1 | 77.0 (4.57–279) |
| Application of continuous renal replacement therapy (CRRT) | 9 |
| Patients who received hemodialysis (HD) | 20 |
| Parameter | Description | Estimate | %RSE | Bootstrap Median (95% CI) |
|---|---|---|---|---|
| Structural model | ||||
| (L/h) | Clearance in patients not receiving CRRT nor HD treatment | |||
| θ1 | 2.82 | 4.18 | 2.80 (2.56–3.04) | |
| θ2 | 0.836 | 6.89 | 0.837 (0.717–0.971) | |
| CLCRRT (L/h) | CL in patients with CRRT | 0.716 | 11.0 | 0.733 (0.437–1.72) |
| CLHD (L/h) | CL in patients with HD | 0.334 | 11.9 | 0.335 (0.142–0.452) |
| V1 (L) | Volume of central compartment | 31.8 | 7.01 | 32.8 (25.6–42.8) |
| Q (L/h) | Intercompartmental clearance | 11.7 | 7.42 | 11.3 (6.93–13.8) |
| ) (L) | Volume of peripheral compartment | |||
| θ3 | 75.4 | 7.91 | 75.7 (58.6–94.9) | |
| Inter-individual variability | ||||
| ωCL (%) | Interindividual variability of CL | 99.2 | 6.55 | 101 (83.4–116) |
| ωV2 (%) | Interindividual variability of V2 | 49.2 | 3.08 | 48.8 (40.5–57.4) |
| Residual error | ||||
| σprop | Proportional error | 0.253 | 2.91 | 0.250 (0.222–0.281) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.H.; Yim, D.-S.; Lee, H.; Park, A.-R.; Kwon, J.-E.; Sumiko, H.; Han, S. Application of Pharmacometrics in Pharmacotherapy: Open-Source Software for Vancomycin Therapeutic Drug Management. Pharmaceutics 2019, 11, 224. https://doi.org/10.3390/pharmaceutics11050224
Bae SH, Yim D-S, Lee H, Park A-R, Kwon J-E, Sumiko H, Han S. Application of Pharmacometrics in Pharmacotherapy: Open-Source Software for Vancomycin Therapeutic Drug Management. Pharmaceutics. 2019; 11(5):224. https://doi.org/10.3390/pharmaceutics11050224
Chicago/Turabian StyleBae, Soo Hyeon, Dong-Seok Yim, Hyemi Lee, Ae-Ryoung Park, Ji-Eun Kwon, Hirata Sumiko, and Seunghoon Han. 2019. "Application of Pharmacometrics in Pharmacotherapy: Open-Source Software for Vancomycin Therapeutic Drug Management" Pharmaceutics 11, no. 5: 224. https://doi.org/10.3390/pharmaceutics11050224
APA StyleBae, S. H., Yim, D.-S., Lee, H., Park, A.-R., Kwon, J.-E., Sumiko, H., & Han, S. (2019). Application of Pharmacometrics in Pharmacotherapy: Open-Source Software for Vancomycin Therapeutic Drug Management. Pharmaceutics, 11(5), 224. https://doi.org/10.3390/pharmaceutics11050224





