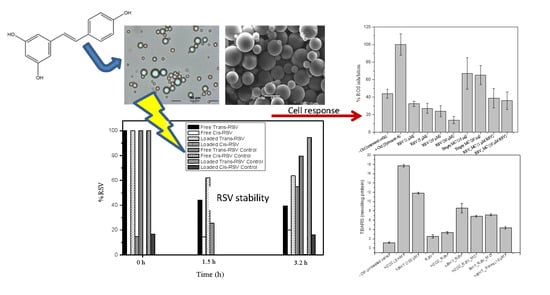
Development, Characterization, and In Vitro Evaluation of Resveratrol-Loaded Poly-(ε-caprolactone) Microcapsules Prepared by Ultrasonic Atomization for Intra-Articular Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Microcapsules
2.2. Experimental Design
2.3. Characterization of Microcapsules
2.3.1. Determination of Drug Encapsulation Content
2.3.2. Particle Size Analysis
2.3.3. SEM
2.3.4. DSC
2.3.5. Photodegradation
2.3.6. ABTS Radical Scavenging Activity
2.3.7. In Vitro Release Profile
2.4. Cell Culture and Treatments
2.5. Cell Proliferation Assay
2.6. Determination of NO Production
2.7. Determination of Intracellular Reactive Oxygen Species
2.8. Phagocytosis Assay
2.9. Lipid Peroxidation Thiobarbituric Acid Assay
2.10. Statistical Analysis of the Data
3. Results and Discussion
3.1. Experimental Design
3.2. Preparation of Microcapsules
3.3. Characterization of Microcapsules
3.3.1. Encapsulation Efficiency, Particle Size and Morphology (SEM)
3.3.2. Characterization of the Physical State of RSV in Microcapsules (DSC)
3.3.3. Photodegradation and Stability
3.3.4. ABTS Radical Scavenging Activity of RSV
3.3.5. In Vitro Release Rate
3.4. Cell Culture Assays
In Vitro Cell Viability
3.5. Determination of NO Production
3.6. ROS Production
3.7. Phagocytosis Assay
3.8. Lipid Peroxidation in Cell Culture
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Datta, S.; Kundu, S.; Ghosh, P.; De, S.; Ghosh, A.; Chatterjee, M. Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin. Rheumatol. 2014, 33, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of resveratrol in inflammatory arthritis. Inflammation 2007, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Camont, L.; Cottart, C.-H.; Rhayem, Y.; Nivet-Antoine, V.; Djelidi, R.; Collin, F.; Beaudeux, J.-L.; Bonnefont-Rousselot, D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal. Chim. Acta 2009, 634, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Roggero, J.-P. Study of the ultraviolet irradiation of resveratrol and wine. J. Food Compost. Anal. 2000, 13, 93–97. [Google Scholar] [CrossRef]
- Marier, J.F.; Vachon, P.; Gritsas, A.; Zhang, J.; Moreau, J.P.; Ducharme, M.P. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002, 302, 369–373. [Google Scholar] [CrossRef]
- Tiku, M.L.; Shah, R.; Allison, G.T. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation: Possible role in cartilage aging and the phathogenesis of osteoarthritis. J. Biol. Chem. 2000, 275, 20069–20076. [Google Scholar] [CrossRef]
- Shah, R.; Raska, K.; Tiku, M.L. The presence of molecular markers of in vivo lipid peroxidation in osteoarthritic cartilage: A pathogenic role in osteoarthritis. Arthritis Rheum. 2005, 52, 2799–2807. [Google Scholar] [CrossRef]
- Morquette, B.; Shi, Q.; Lavigne, P.; Ranger, P.; Fernandes, J.C.; Benderdour, M. Production of lipid peroxidation products in osteoarthritic tissues: New evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis Rheum. 2006, 54, 271–281. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.R.; Guterres, S.S. Poly(ε-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Sanna, V.; Roggio, A.M.; Pala, N.; Marceddu, S.; Lubinu, G.; Mariani, A.; Sechi, M. Effect of chitosan concentration on PLGA microcapsules for controlled release and stability of resveratrol. Int. J. Biol. Macromol. 2015, 72, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Dimer, F.A.; Ortiz, M.; Pohlmann, A.R.; Guterres, S.S. Inhalable resveratrol microparticles produced by vibrational atomization spray drying for treating pulmonary arterial hypertension. J. Drug Deliv. Sci. Technol. 2015, 29, 152–158. [Google Scholar] [CrossRef]
- Park, J.H.; Ye, M.; Yeo, Y.; Lee, W.-K.; Paul, C.; Park, K. Reservoir-Type microcapsules prepared by the solvent exchange method: Effect of formulation parameters on microencapsulation of lysozyme. Mol. Pharm. 2006, 3, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gallego, M.R.; Nielsen, L.F.; Jorgensen, L.; Møller, E.H.; Nielsen, H.M. Design and characterization of core–shell mPEG–PLGA composite microparticles for development of cell–scaffold constructs. Eur. J. Pharm. Biopharm. 2013, 85, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Graves, R.A.; Poole, D.; Moiseyev, R.; Bostanian, L.A.; Mandal, T.K. Encapsulation of indomethacin using coaxial ultrasonic atomization followed by solvent evaporation. Drug Dev. Ind. Pharm. 2008, 34, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Oh, J.M.; Hyun, H.; Kim, K.S.; Lee, S.H.; Kim, Y.H.; Park, K.; Lee, H.B.; Kim, M.S. Insulin-loaded microcapsules for in vivo delivery. Mol. Pharm. 2009, 6, 353–365. [Google Scholar] [CrossRef]
- Kim, B.S.; Oh, J.M.; Kim, K.S.; Seo, K.S.; Cho, J.S.; Khang, G.; Lee, H.B.; Park, K.; Kim, M.S. BSA-FITC-loaded microcapsules for in vivo delivery. Biomaterials 2009, 30, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Pamujula, S.; Graves, R.A.; Moiseyev, R.; Bostanian, L.A.; Kishore, V.; Mandal, T.K. Preparation of polylactide-co-glycolide and chitosan hybrid microcapsules of amifostine using coaxial ultrasonic atomizer with solvent evaporation. J. Pharm. Pharmacol. 2008, 60, 283–289. [Google Scholar] [CrossRef]
- Choi, D.H.; Park, C.H.; Kim, I.H.; Chun, H.J.; Park, K.; Han, D.K. Fabrication of core–shell microcapsules using PLGA and alginate for dual growth factor delivery system. J. Control. Release 2010, 147, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-W.; Um, S.-H.; Rhee, S.-H. Preparation of fluoride-loaded microcapsules for anticariogenic bacterial growth using a coaxial ultrasonic atomizer. J. Biomed. Mater. Res. Part B 2018, 106, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pradal, J.; Maudens, P.; Gabay, C.; Seemayer, C.A.; Jordan, O.; Allémann, E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int. J. Pharm. 2016, 498, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Fraga, F.; Blanco-Méndez, J.; Luzardo-Alvarez, A.; Núñez, E.R.; Pérez, S. Mechanism and activation energies in different blends of poly-(ε-caprolactone) with Polyethyleneglycol (PEG) and Polyvinyl Alcohol (PVA). Mater. Focus 2014, 3, 131–144. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, M.; Ye, J.-H.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Photo-induced chemical reaction of trans-resveratrol. Food Chem. 2015, 171, 137–143. [Google Scholar] [CrossRef]
- Silva, C.G.; Monteiro, J.; Marques, R.R.N.; Silva, A.M.T.; Martinez, C.; Canle L., M.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, J.; Paseiro-Losada, P.; Sanches-Silva, A.T.; Lage-Yusty, M.A. Study of the changes of trans-resveratrol caused by ultraviolet light and determination of trans-and cis-resveratrol in Spanish white wines. Eur. Food Res. Technol. 2007, 225, 789–796. [Google Scholar] [CrossRef]
- Bernard, E.; Britz-McKibbin, P.; Gernigon, N. Resveratrol Photoisomerization: An integrative guided-inquiry experiment. J. Chem. Educ. 2007, 84, 1159. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Ha, J.-H.; Kim, S.-H.; Han, S.-Y.; Sung, Y.-K.; Lee, Y.-M.; Kang, I.-K.; Cho, C.-S. Albumin release from bioerodible hydrogels based on semi-interpenetrating polymer networks composed of poly(ε-caprolactone) and poly(ethylene glycol) macromer. J. Control. Release 1997, 49, 253–262. [Google Scholar] [CrossRef]
- Jeong, J.-C.; Lee, J.; Cho, K. Effects of crystalline microstructure on drug release behavior of poly(ε-caprolactone) microspheres. J. Control. Release 2003, 92, 249–258. [Google Scholar] [CrossRef]
- Billack, B.; Radkar, V.; Adiabouah, C. In vitro evaluation of the cytotoxic and anti-proliferative properties of resveratrol and several of its analogs. Cell. Mol. Biol. Lett. 2008, 13, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M.E.; Xiao, D.; Weinstein, I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer. Res. 2002, 8, 893–903. [Google Scholar] [PubMed]
- Wu, M.-L.; Li, H.; Yu, L.-J.; Chen, X.-Y.; Kong, Q.-Y.; Song, X.; Shu, X.-H.; Liu, J. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer cells. PLoS ONE 2014, 9, e89806. [Google Scholar] [CrossRef]
- Sevov, M.; Elfineh, L.; Cavelier, L.B. Resveratrol regulates the expression of LXR-α in human macrophages. Biochem. Biophys. Res. Commun. 2006, 348, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949. [Google Scholar] [CrossRef]
- Bourassa, P.; Kanakis, C.D.; Tarantilis, P.; Pollissiou, M.G.; Tajmir-Riahi, H.A. Resveratrol, genistein, and curcumin bind bovine serum albumin. J. Phys. Chem. B 2010, 114, 3348–3354. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Cho, D.-I.; Koo, N.-Y.; Chung, W.J.; Kim, T.-S.; Ryu, S.Y.; Im, S.Y.; Kim, K.-M. Effects of resveratrol-related hydroxystilbenes on the nitric oxide production in macrophage cells: Structural requirements and mechanism of action. Life Sci. 2002, 71, 2071–2082. [Google Scholar] [CrossRef]
- Pervaiz, S. Resveratrol: from grapevines to mammalian biology. Faseb J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.A.; Guan, X.Q.; Reis, J.C.; Papasian, C.J.; Jabre, S.; Morrison, D.C.; Qureshi, N. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids Health Dis. 2012, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.; Álvarez, E.; García, D.; Orallo, F. Resveratrol modulates rat macrophage functions. Int. Immunopharmacol. 2002, 2, 767–774. [Google Scholar] [CrossRef]
- Adiabouah Achy-Brou, C.A.; Billack, B. A comparative assessment of the cytotoxicity and nitric oxide reducing ability of resveratrol, pterostilbene and piceatannol in transformed and normal mouse macrophages. Drug Chem. Toxicol. 2017, 40, 2071. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Lee, W.-H.; Loo, C.-Y.; Young, P.M.; Traini, D.; Scalia, S. Co-spray dried resveratrol and budesonide inhalation formulation for reducing inflammation and oxidative stress in rat alveolar macrophages. Eur. J. Pharm. Sci. 2016, 86, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.-Y.; Mattiacci, J.A.; Hwang, H.S.; Shah, A.; Fong, D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem. Pharmacol. 2000, 60, 1539–1548. [Google Scholar] [CrossRef]
- Pandey, R.P.; Lee, J.; Park, Y.; Sohng, J.K. Glucosylation of resveratrol improves its immunomodulating activity and the viability of murine macrophage RAW 264.7 cells. Microbiol. Biotechnol. Lett. 2017, 45, 19–26. [Google Scholar] [CrossRef]
- Jaswal, S.; Mehta, H.C.; Sood, A.K.; Kaur, J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin. Chim. Acta 2003, 338, 123–129. [Google Scholar] [CrossRef]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Del Río Nájera, D.; Pacheco-Tena, C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: A systematic review. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Zhou, W.-L.; Wu, Q.-L.; Li, X.-Y.; Zhao, W.-M.; Zou, J.-P. Low dose of resveratrol enhanced immune response of mice. Acta Pharmacol. Sin. 2002, 23, 893–897. [Google Scholar] [PubMed]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Falchetti, R.; Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Ravagnan, G. Effects of resveratrol on human immune cell function. Life Sci. 2001, 70, 81–96. [Google Scholar] [CrossRef]
- Shanmugam, N.; Figarola, J.L.; Li, Y.; Swiderski, P.M.; Rahbar, S.; Natarajan, R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes 2008, 57, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Weismann, D.; Binder, C.J. The innate immune response to products of phospholipid peroxidation. Biochim. Biophys. Acta 2012, 1818, 2465–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, J.G.; De Moura, E.G.; Koury, J.C.; Trotta, P.A.; Cordeiro, A.; Souza, L.L.; Almeida, N.A.D.S.; Lima, N.D.S.; Pazos-Moura, C.C.; Lisboa, P.C.; et al. Resveratrol reduces lipid peroxidation and increases sirtuin 1 expression in adult animals programed by neonatal protein restriction. J. Endocrinol. 2010, 207, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Chen, R.; Fu, R.; Xiong, J.; Hu, Y. Cytotoxicity and inhibition of lipid peroxidation activity of resveratrol/cyclodextrin inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 313–320. [Google Scholar] [CrossRef]










| Formulation | [PCL] | Power | [PVA] | Process Yield | Encapsulation Efficiency (E.E) |
|---|---|---|---|---|---|
| F1 | 0.08% | 5 W | 3.5% | 85.83% | 39.66% |
| F2 | 0.5% | 4 W | 3.5% | 85.45% | 63.80% |
| F3 | 0.5% | 6 W | 3.5% | 80.43% | 50.20% |
| F4 | 1.5% | 3.58 W | 3.5% | 85.30% | 83.97% |
| F5 | 1.5% | 5 W | 3.5% | 92.07% | 96.00% |
| F5 | 1.5% | 5 W | 3.5% | 92.07% | 96.00% |
| F5 | 1.5% | 5 W | 3.5% | 92.07% | 96.00% |
| F5 | 1.5% | 5 W | 3.5% | 92.07% | 96.00% |
| F5 | 1.5% | 5 W | 3.5% | 92.07% | 96.00% |
| F6 | 1.5% | 6.41 W | 3.5% | 80.22% | 66.16% |
| F7 | 2.5% | 4 W | 3.5% | 86.12% | 77.30% |
| F8 | 2.5% | 6 W | 3.5% | 89.88% | 91.48% |
| F9 | 2.91% | 5 W | 3.5% | 91.88% | 92.76% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzardo-Álvarez, A.; Lamela-Gómez, I.; Otero-Espinar, F.; Blanco-Méndez, J. Development, Characterization, and In Vitro Evaluation of Resveratrol-Loaded Poly-(ε-caprolactone) Microcapsules Prepared by Ultrasonic Atomization for Intra-Articular Administration. Pharmaceutics 2019, 11, 249. https://doi.org/10.3390/pharmaceutics11060249
Luzardo-Álvarez A, Lamela-Gómez I, Otero-Espinar F, Blanco-Méndez J. Development, Characterization, and In Vitro Evaluation of Resveratrol-Loaded Poly-(ε-caprolactone) Microcapsules Prepared by Ultrasonic Atomization for Intra-Articular Administration. Pharmaceutics. 2019; 11(6):249. https://doi.org/10.3390/pharmaceutics11060249
Chicago/Turabian StyleLuzardo-Álvarez, Asteria, Iván Lamela-Gómez, Francisco Otero-Espinar, and José Blanco-Méndez. 2019. "Development, Characterization, and In Vitro Evaluation of Resveratrol-Loaded Poly-(ε-caprolactone) Microcapsules Prepared by Ultrasonic Atomization for Intra-Articular Administration" Pharmaceutics 11, no. 6: 249. https://doi.org/10.3390/pharmaceutics11060249
APA StyleLuzardo-Álvarez, A., Lamela-Gómez, I., Otero-Espinar, F., & Blanco-Méndez, J. (2019). Development, Characterization, and In Vitro Evaluation of Resveratrol-Loaded Poly-(ε-caprolactone) Microcapsules Prepared by Ultrasonic Atomization for Intra-Articular Administration. Pharmaceutics, 11(6), 249. https://doi.org/10.3390/pharmaceutics11060249