Nanomaterial-Based Approaches for Neural Regeneration
Abstract
1. Introduction
2. Nervous System Regenerative Therapy
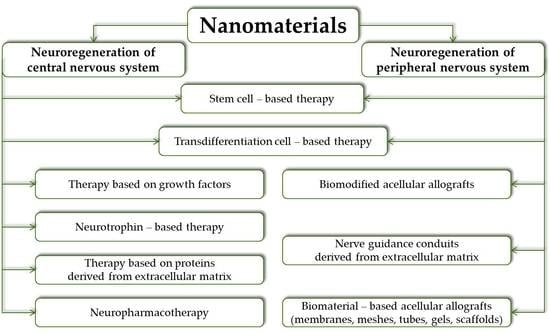
3. Nanomaterials for Central Nervous System Regenerative Applications
4. Nanomaterials for Peripheral Nervous System Regenerative Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADSCs | adipose-derived stem cells |
| BBB | blood-brain barrier |
| BDNF | brain-derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| BMSCs | bone mesenchymal stromal cells |
| BSA | bovine serum albumin |
| ChABC | chondroitinase ABC |
| CNS | central nervous system |
| cRGD | cyclic arginine-glycine-aspartate |
| CS | chitosan |
| db-cAMP | dibutyryl cyclic adenosine monophosphate |
| DNA | deoxyribonucleic acid |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EPO | erythropoietin |
| GDNF | glial derived neurotrophic factor |
| Gel | gelatin |
| GFs | growth factors |
| GO | graphene oxide |
| GS | gelatin sponge |
| HA | hyaluronic acid |
| HA-Lm | hyaluronic acid-laminin |
| HAMC | hyaluronic acid-methylcellulose |
| IMPs | immune-modifying nanoparticles |
| LL | ladder-like |
| MMP | matrix metalloproteinase |
| mNSCs | mouse-derived neural stem cells |
| MP | macroporous |
| MP | methylprednisolone |
| MSCs | mesenchymal stem cells |
| NECL1 | nectin-like molecule 1 |
| NF | nanofibrous |
| NGF | nerve growth factor |
| NPSCs | neural progenitor stem cells |
| NSCs | neural stem cells |
| NT-3 | neurotrophin-3 |
| PCL | polycaprolactone |
| PCN | polyelectrolyte complex nanoparticles |
| PCU | polycarbonate urethane |
| pDNA | plasmid DNA |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| PEG | polyethylene glycol |
| PEI | polyethyleneimine |
| PLCL | poly(l-lactide-co-ε-caprolactone) |
| PLGA | poly(lactide-co-glycolide) |
| PLL | poly-l-Lysine |
| PLLA | poly(l-lactic) acid |
| PLO | poly-l-Ornithine |
| PNS | peripheral nervous system |
| PPC | poly(propylene carbonate) |
| Ppy | polypyrrole |
| PSA | polysialic acid |
| PVA | polyvinyl alcohol |
| rGO | reduced graphene oxide |
| SA | sodium alginate |
| SDF-1 | stromal cell-derived factor 1 |
| SF | silk fibroin |
| SS | silk sericin |
| TAT | twin-arginine translocation |
| TMCSH-HC | thiolated trimethyl chitosan nanoparticles grafted with tetanus neurotoxin |
| XMC | cross-linked methylcellulose |
References
- Baars, B.J.; Edeman, D.B. Consciousness, biology and quantum hypotheses. Phys. Life Rev. 2012, 9, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Keppler, J. The Role of the Brain in Conscious Processes: A New Way of Looking at the Neural Correlates of Consciousness. Front. Psychol. 2018, 9, 1346. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Morrison, B.M. Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Exp. Neurol. 2018, 309, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.S.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.O.; Göritz, C. Fibrotic scarring following lesions to the central nervous system. Matrix Biol. 2018, 68–69, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, V.; Oñate, M.; Hetz, C.; Court, F.A. Injury to the nervous system: A look into the ER. Brain Res. 2016, 1648, 617–625. [Google Scholar] [CrossRef]
- Grotta, M.J.; Helgason, C. Ischemic stroke pathophysiology. J. Stroke Cerebrovasc. Dis. 1999, 8, 114–116. [Google Scholar] [CrossRef]
- Majid, A.; Kassab, M. Pathophysiology of ischemic stroke. UpToDate. 2018. Available online: https://www.uptodate.com/contents/pathophysiology-of-ischemic-stroke#references (accessed on 31 May 2019).
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Conforti, L.; Gilley, J.; Coleman, M.P. Wallerian degeneration: An emerging axon death pathway linking injury and disease. Nat. Rev. Neurosci. 2014, 15, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef] [PubMed]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.Y.; Fatemi, A.; Johnston, M.V. Cerebral plasticity: Windows of opportunity in the developing brain. Eur. J. Paediatr. Neurol. 2017, 21, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Serradj, N.; Agger, S.F.; Hollis, E.R., II. Corticospinal circuit plasticity in motor rehabilitation from spinal cord injury. Neurosci. Lett. 2017, 652, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; Carmichael, S.T. Promoting axonal rewiring to improve outcome after stroke. Neurobiol. Dis. 2010, 37, 259–266. [Google Scholar] [CrossRef]
- Voss, P.; Thomas, M.E.; Cisneros-Franco, M.; de Villers-Sidani, E. Dynamic Brains and the Changing Rules of Neuroplasticity: Implications for Learning and Recovery. Front. Psychol. 2017, 8, 1657. [Google Scholar] [CrossRef]
- Fawcett, J.W. The Paper that Restarted Modern Central Nervous System Axon Regeneration Research. Trends Neurosci. 2018, 41, 239–242. [Google Scholar] [CrossRef]
- Gaceb, A.; Barbariga, M.; Özen, I.; Paul, G. The pericyte secretome: Potential impact on regeneration. Biochimie 2018, 155, 16–25. [Google Scholar] [CrossRef]
- Muramatsu, R.; Yamashita, T. Concept and molecular basis of axonal regeneration after central nervous system injury. Neurosci. Res. 2014, 78, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Schwab, M.E.; Popovich, P.G. Central Nervous System Regenerative Failure: Role of Oligodendrocytes, Astrocytes, and Microglia. Cold Spring Harb. Perspect. Biol. 2014, 7, a020602. [Google Scholar] [CrossRef] [PubMed]
- Weil, Z.M.; Norman, G.J.; DeVries, A.C.; Nelson, R.J. The injured nervous system: A Darwinian perspective. Prog. Neurobiol. 2008, 86, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Temple, S. Neural Stem Cells: Generating and Regenerating the Brain. Neuron 2013, 80, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Lemke, K.A.; Aghayee, A.; Ashton, R.S. Deriving, regenerating, and engineering CNS tissues using human pluripotent stem cells. Curr. Opin. Biotechnol. 2017, 47, 36–42. [Google Scholar] [CrossRef]
- Yao, S.; Liu, X.; Wang, X.; Merolli, A.; Chen, X.; Cui, F. Directing neural stem cell fate with biomaterial parameters for injured brain regeneration. Prog. Nat. Sci. 2013, 23, 103–112. [Google Scholar] [CrossRef]
- All, A.H.; Gharibani, O.; Gupta, P.; Bazley, F.A.; Pashai, N.; Chou, B.; Shah, S.; Resar, L.M.; Cheng, L.; Gearhart, J.D.; et al. Early Intervention for Spinal Cord Injury with Human Induced Pluripotent Stem Cells Oligodendrocyte Progenitors. PLoS ONE 2015, 10, e0116933. [Google Scholar] [CrossRef]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-Distance Axonal Growth from Human Induced Pluripotent Stem Cells after Spinal Cord Injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef]
- Salewski, R.P.; Mitchell, R.A.; Li, L.; Shen, C.; Milekovskaia, M.; Nagy, A.; Fehlings, M.G. Transplantation of Induced Pluripotent Stem Cell-Derived Neural Stem Cells Mediate Functional Recovery Following Thoracic Spinal Cord Injury Through Remyelination of Axons. Stem Cells Transl. Med. 2015, 4, 743–754. [Google Scholar] [CrossRef]
- Zoldan, J.; Lytton-Jean, A.K.L.; Karagiannis, E.D.; Deiorio-Haggar, K.; Bellan, L.M.; Langer, R.; Anderson, D.G. Directing human embryonic stem cell differentiation by non-viral delivery of siRNA in 3D culture. Biomaterials 2011, 32, 7793–7800. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 253. [Google Scholar] [CrossRef]
- Liu, J.; Han, D.; Wang, Z.; Xue, M.; Zhu, L.; Yan, H.; Zheng, X.; Guo, Z.; Wang, H. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy 2013, 15, 185–191. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous Bone Marrow-Derived Cell Therapy Combined with Physical Therapy Induces Functional Improvement in Chronic Spinal Cord Injury Patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Jarocha, D.; Milczarek, O.; Wedrychowicz, A.; Kwiatkowski, S.; Majka, M. Continuous Improvement after Multiple Mesenchymal Stem Cell Transplantations in a Patient with Complete Spinal Cord Injury. Cell Transplant. 2015, 24, 661–672. [Google Scholar] [CrossRef]
- Jiang, P.C.; Xiong, W.P.; Wang, G.; Ma, C.; Yao, W.Q.; Kendell, S.F.; Mehling, B.M.; Yuan, X.H.; Wu, D.C. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp. Ther. Med. 2013, 6, 140–146. [Google Scholar] [CrossRef]
- Ang, C.; Wernig, M. Induced neuronal reprogramming. J. Comp. Neurol. 2014, 522, 2877–2884. [Google Scholar] [CrossRef]
- Kim, J.; Ambasudhan, R.; Ding, S. Direct lineage reprogramming to neural cells. Curr. Opin. Neurobiol. 2012, 22, 778–784. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Chen, G. In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron 2016, 91, 728–738. [Google Scholar] [CrossRef]
- Elliott Donaghue, I.; Tam, R.; Sefton, M.V.; Shoichet, M.S. Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system. J. Control. Release 2014, 190, 219–227. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Zadegan, S.A.; Vaccaro, A.R.; Rahimi-Movaghar, V.; Sabzevari, O. Biofunctionalized peptide-based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury 2019, 50, 278–285. [Google Scholar] [CrossRef]
- Villate-Beitia, I.; Puras, G.; Soto-Sánchez, C.; Agirre, M.; Ojeda, E.; Zarate, J.; Fernández, E.; Pedraz, J.L. Non-viral vectors based on magnetoplexes, lipoplexes and polyplexes for VEGF gene delivery into central nervous system cells. Int. J. Pharm. 2017, 521, 130–140. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Z.; Li, Y.; Zhu, S.; Ma, J.; Li, W.; Gao, G. Development and characterization of cores–shell poly(lactide-co-glycolide)-chitosan microparticles for sustained release of GDNF. Colloids Surf. B Biointerfaces 2017, 159, 791–799. [Google Scholar] [CrossRef]
- Zhao, H.; Zuo, X.; Ren, L.; Li, Y.; Tai, H.; Du, J.; Xie, X.; Zhang, X.; Han, Y.; Wu, Y.; et al. Combined use of bFGF/EGF and all-trans-retinoic acid cooperatively promotes neuronal differentiation and neurite outgrowth in neural stem cells. Neurosci. Lett. 2019, 690, 61–68. [Google Scholar] [CrossRef]
- Akyol, O.; Sherchan, P.; Yilmaz, G.; Reis, C.; Ho, W.M.; Wang, Y.; Huang, L.; Solaroglu, I.; Zhang, J.H. Neurotrophin-3 provides neuroprotection via TrkC receptor dependent pErk5 activation in a rat surgical brain injury model. Exp. Neurol. 2018, 307, 82–89. [Google Scholar] [CrossRef]
- Chung, C.Y.; Yang, J.T.; Kuo, Y.C. Polybutylcyanoacrylate nanoparticle-mediated neurotrophin-3 gene delivery for differentiating iPS cells into neurons. Biomaterials 2013, 34, 5562–5570. [Google Scholar] [CrossRef]
- Aronson, J.P.; Katnani, H.A.; Pomerantseva, I.; Shapir, N.; Tse, H.; Miari, R.; Goltsman, H.; Mwizerwa, O.; Neville, C.M.; Neil, G.A.; et al. Sustained intrathecal therapeutic protein delivery using genetically transduced tissue implants in a freely moving rat model. Int. J. Pharm. 2017, 534, 42–49. [Google Scholar] [CrossRef]
- Arulmoli, J.; Wright, H.J.; Phan, D.T.T.; Sheth, U.; Que, R.A.; Botten, G.A.; Keating, M.; Botvinick, E.L.; Pathak, M.M.; Zarembinski, T.I.; et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016, 43, 122–138. [Google Scholar] [CrossRef]
- Hayashi, H.; Yamada, M.; Kumai, J.; Takagi, N.; Nomizu, M. Biological activities of laminin-111-derived peptide-chitosan matrices in a primary culture of rat cortical neurons. Arch. Biochem. Biophys. 2018, 648, 53–59. [Google Scholar] [CrossRef]
- Rauck, B.M.; Novosat, T.L.; Oudega, M.; Wang, Y. Biocompatibility of a coacervate-based controlled release system for protein delivery to the injured spinal cord. Acta Biomater. 2015, 11, 204–211. [Google Scholar] [CrossRef]
- Das, S.; Carnicer-Lombarte, A.; Fawcett, J.W.; Bora, U. Bio-inspired nano tools for neuroscience. Prog. Neurobiol. 2016, 142, 1–22. [Google Scholar] [CrossRef]
- Huang, L.; Hu, J.; Huang, S.; Wang, B.; Siaw-Debrah, F.; Nyanzu, M.; Zhang, Y.; Zhuge, Q. Nanomaterial applications for neurological diseases and central nervous system injury. Prog. Neurobiol. 2017, 157, 29–48. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Loscher, W.; Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005, 76, 22–76. [Google Scholar] [CrossRef]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood-brain barrier. Semin. Cell. Dev. Biol. 2015, 38, 2–6. [Google Scholar] [CrossRef]
- Moura, R.P.; Almeida, A.; Sarmento, B. The role of non-endothelial cells on the penetration of nanoparticles through the blood brain barrier. Progr. Neurobiol. 2017, 159, 39–49. [Google Scholar] [CrossRef]
- Karanth, H.; Rayasa, M. Nanotechnology in Brain Targeting. Int. J. Pharm. Sci. Nanotech. 2018, 1, 9–24. [Google Scholar]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Parodi, A.; Rudzińska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A., Jr. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics 2019, 11, 245. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Lee, S.S.; Bhattacharya, M.; Nam, J.S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef]
- Chiono, V.; Tonda-Turo, C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 2015, 131, 87–104. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Yang, Y.; Liu, J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 2011, 93, 204–230. [Google Scholar] [CrossRef]
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, 171, 125–150. [Google Scholar] [CrossRef]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A. Advances in peripheral nervous system regenerative therapeutic strategies: A biomaterials approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 425–432. [Google Scholar] [CrossRef]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Jooste, E.A.J.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Rbia, N.; Shin, A.Y. The Role of Nerve Graft Substitutes in Motor and Mixed Motor/Sensory Peripheral Nerve Injuries. J. Hand. Surg. Am. 2017, 42, 367–377. [Google Scholar] [CrossRef]
- Kato, Y.; Chavez, J.; Yamada, S.; Hattori, S.; Takazawa, S.; Ohuchi, H. A large knee osteochondral lesion treated using a combination of osteochondral autograft transfer and second-generation autologous chondrocyte implantation: A case report. Regen. Ther. 2018, 10, 10–16. [Google Scholar] [CrossRef]
- Roballo, K.C.S.; Bushman, J. Evaluation of the host immune response and functional recovery in peripheral nerve autografts and allografts. Transpl. Immunol. 2019, 53, 61–71. [Google Scholar] [CrossRef]
- Boriani, F.; Fazio, N.; Bolognesi, F.; Pedrini, F.A.; Marchetti, C.; Baldini, N. Noncellular Modification of Acellular Nerve Allografts for Peripheral Nerve Reconstruction: A Systematic Critical Review of the Animal Literature. World Neurosurg. 2019, 122, 692–703.e2. [Google Scholar] [CrossRef]
- Safa, B.; Buncke, G. Autograft Substitutes: Conduits and Processed Nerve Allografts. Hand Clin. 2016, 32, 127–140. [Google Scholar] [CrossRef]
- Yampolsky, S.; Ziccardi, V.; Chuang, S.K. Efficacy of Acellular Nerve Allografts in Trigeminal Nerve Reconstruction. J. Oral Maxillofac. Surg. 2017, 75, 2230–2234. [Google Scholar] [CrossRef]
- Dixon, A.R.; Jariwala, S.H.; Bilis, Z.; Loverde, J.R.; Pasquina, P.F.; Alvarez, L.M. Bridging the gap in peripheral nerve repair with 3D printed and bioprinted conduits. Biomaterials 2018, 186, 44–63. [Google Scholar] [CrossRef]
- Kuffler, D.P. An assessment of current techniques for inducing axon regeneration and neurological recovery following peripheral nerve trauma. Prog. Neurobiol. 2014, 116, 1–12. [Google Scholar] [CrossRef]
- Narayan, S.K.; Arumugam, M.; Chittoria, R. Outcome of human peripheral nerve repair interventions using conduits: A systematic review. J. Neurol. Sci. 2019, 396, 18–24. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Yao, S.; Mao, S.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y.; Xiao, B.; Wang, Y.; et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017, 55, 296–309. [Google Scholar] [CrossRef]
- Haggerty, A.E.; Bening, M.R.; Pherribo, G.; Dauer, E.A.; Oudega, M. Laminin polymer treatment accelerates repair of the crushed peripheral nerve in adult rats. Acta Biomater. 2019, 86, 185–193. [Google Scholar] [CrossRef]
- Harris, G.M.; Madigan, N.N.; Lancaster, K.Z.; Enquist, L.W.; Windebank, A.J.; Schwartz, J.; Schwarzbauer, J.E. Nerve Guidance by a Decellularized Fibroblast Extracellular Matrix. Matrix Biol. 2017, 60–61, 176–189. [Google Scholar] [CrossRef]
- Lin, T.; Liu, S.; Chen, S.; Qiu, S.; Rao, Z.; Liu, J.; Zhu, S.; Yan, L.; Mao, H.; Zhu, Q.; et al. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater. 2018, 73, 326–338. [Google Scholar] [CrossRef]
- Saeki, M.; Tanaka, K.; Imatani, J.; Okamoto, H.; Watanabe, K.; Nakamura, T.; Gotani, H.; Ohi, H.; Nakamura, R.; Hirata, H. Efficacy and safety of novel collagen conduits filled with collagen filaments to treat patients with peripheral nerve injury: A multicenter, controlled, open-label clinical trial. Injury 2018, 49, 766–774. [Google Scholar] [CrossRef]
- Chang, W.; Shah, M.B.; Lee, P.; Yu, X. Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater. 2018, 73, 302–311. [Google Scholar] [CrossRef]
- Li, G.; Xiao, Z.Q.; Zhang, L.; Zhao, Y.; Yang, Y. Nerve growth factor loaded heparin/chitosan scaffolds for accelerating peripheral nerve regeneration. Carbohydr. Polym. 2017, 171, 39–49. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J.; et al. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, Y.; Qiao, J.; Yang, Y.; Zhang, W.; Liu, W.; Han, B. Rat sciatic nerve regeneration across a 10-mm defect bridged by a chitin/CM-chitosan artificial nerve graft. Int. J. Biol. Macromol. 2019, 129, 997–1005. [Google Scholar] [CrossRef]
- Peng, Y.; Li, K.Y.; Chen, Y.F.; Li, X.J.; Zhu, S.; Zhang, Z.Y.; Wang, X.; Duan, L.N.; Luo, Z.J.; Du, J.J.; et al. Beagle sciatic nerve regeneration across a 30 mm defect bridged by chitosan/PGA artificial nerve grafts. Injury 2018, 49, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.M.; McCarthy, D.J.; Torres-Fuentes, C.; Beltránd, C.J.; McCarthy, J.; Quinlan, A.; Ogier, J.R.; Darcy, R.; O’Driscoll, C.M.; Cryan, J.F. Differential nanotoxicological and neuroinflammatory liabilities of non-viral vectors for RNA interference in the central nervous system. Biomaterials 2014, 35, 489–499. [Google Scholar] [CrossRef]
- Khan, H.A.; Alamery, S.; Ibrahim, K.E.; El-Nagar, D.M.; Al-Harbi, N.; Rusop, M.; Alrokayan, S.H. Size and time-dependent induction of proinflammatory cytokines expression in brains of mice treated with gold nanoparticles. Saudi J. Biol. Sci. 2019, 26, 625–631. [Google Scholar] [CrossRef]
- Zhou, W.; Stukel, J.M.; Cebull, H.L.; Willits, R.K. Tuning the Mechanical Properties of Poly(Ethylene Glycol) Microgel-Based Scaffolds to Increase 3D Schwann Cell Proliferation. Macromol. Biosci. 2016, 16, 535–544. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, M.; Hao, Y.; Cheng, G. Precisely controllable hybrid graphene scaffold reveals size effects on differentiation of neural progenitor cells in mimicking neural network. Carbon 2019, 145, 90–99. [Google Scholar] [CrossRef]
- Shao, H.; Li, T.; Zhu, R.; Xu, X.; Yu, J.; Chen, S.; Song, L.; Ramakrishna, S.; Lei, Z.; Ruan, Y.; et al. Carbon nanotube multilayered nanocomposites as multifunctional substrates for actuating neuronal differentiation and functions of neural stem cells. Biomaterials 2018, 175, 93–109. [Google Scholar] [CrossRef]
- Wang, S.; Guan, S.; Li, W.; Ge, D.; Xu, J.; Sun, C.; Liu, T.; Ma, X. 3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 890–901. [Google Scholar] [CrossRef]
- Fornaguera, C.; Dols-Perez, A.; Calderó, G.; García-Celma, M.J.; Camarasa, J.; Solans, C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier. J. Control. Release 2015, 211, 134–143. [Google Scholar] [CrossRef]
- Huey, R.; O’Hagan, B.; McCarron, P.; Hawthorne, S. Targeted drug delivery system to neural cells utilizes the nicotinic acetylcholine receptor. Int. J. Pharm. 2017, 525, 12–20. [Google Scholar] [CrossRef]
- Niza, E.; Castro-Osma, J.C.; Posadas, I.; Alonso-Moreno, C.; Bravo, I.; Garzón, A.; Canales-Vázquez, J.; Ceña, V.; Lara-Sánchez, A.; Albaladejo, J.; et al. Assessment of doxorubicin delivery devices based on tailored bare polycaprolactone against glioblastoma. Int. J. Pharm. 2019, 558, 110–119. [Google Scholar] [CrossRef]
- Li, W.; Luo, R.; Lin, X.; Jadhav, A.D.; Zhang, Z.; Yan, L.; Chan, C.Y.; Chen, X.; He, J.; Chen, C.H.; et al. Remote modulation of neural activities via near-infrared triggered release of biomolecules. Biomaterials 2015, 65, 76–85. [Google Scholar] [CrossRef]
- Shyam, R.; Ren, Y.; Lee, J.; Braunstein, K.E.; Mao, H.Q.; Wong, P.C. Intraventricular Delivery of siRNA Nanoparticles to the Central Nervous System. Mol. Ther. Nucl. Acids 2015, 44, e242. [Google Scholar] [CrossRef]
- Wollenberg, A.L.; O’Shea, T.M.; Kim, J.H.; Czechanski, A.; Reinholdt, L.G.; Sofroniew, M.V.; Demingm, T.J. Injectable polypeptide hydrogels via methionine modification for neural stem cell delivery. Biomaterials 2018, 178, 527–545. [Google Scholar] [CrossRef]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef]
- Faustino, C.; Rijo, P.; Reis, C.P. Nanotechnological strategies for nerve growth factor delivery: Therapeutic implications in Alzheimer’s disease. Pharmacol. Res. 2017, 120, 68–87. [Google Scholar] [CrossRef]
- Ferguson, E.L.; Naseer, S.; Powell, L.C.; Hardwicke, J.; Young, F.I.; Zhu, B.; Liu, Q.; Song, B.; Thomas, D.W. Controlled release of dextrin-conjugated growth factors to support growth and differentiation of neural stem cells. Stem Cell Res. 2018, 33, 69–78. [Google Scholar] [CrossRef]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J.K. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef]
- Getts, D. Immune-Modifying Nanoparticles for the Treatment of Inflammatory Diseases. U.S. Patent US9,913,883B2, 20 March 2018. [Google Scholar]
- Jeong, S.J.; Cooper, J.G.; Ifergan, I.; McGuire, T.L.; Xu, D.; Hunter, Z.; Sharma, S.; McCarthy, D.; Miller, S.D.; Kessler, J.A. Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice. Neurobiol. Dis. 2017, 108, 73–82. [Google Scholar] [CrossRef]
- Lewellis, S.W.; Knaut, H. Attractive guidance: How the chemokine SDF1/CXCL12 guides different cells to different locations. Semin. Cell Dev. Biol. 2012, 23, 333–340. [Google Scholar] [CrossRef]
- Pino, A.; Fumagalli, G.; Bifari, F.; Decimo, I. New neurons in adult brain: Distribution, molecular mechanisms and therapies. Biochem. Pharmacol. 2017, 141, 4–22. [Google Scholar] [CrossRef]
- Zamproni, L.N.; Mundim, M.V.; Porcionatto, M.A.; Rieux, A. Injection of SDF-1 loaded nanoparticles following traumatic brain injury stimulates neural stem cell recruitment. Int. J. Pharm. 2017, 519, 323–331. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Donaghue, I.E.; Obermeyer, J.M.; Tuladhar, A.; McLaughlin, C.K.; Shendruk, T.N.; Shoichet, M.S. Encapsulation-free controlled release: Electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci. Adv. 2016, 2, e160519. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Carter, L.M. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 2011, 84, 306–316. [Google Scholar] [CrossRef]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving peripheral nerve regeneration: From molecular mechanisms to potential therapeutic targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Tator, C.H.; Shoichet, M.S. Local delivery of chondroitinase ABC with or without stromal cell-derived factor 1α promotes functional repair in the injured rat spinal cord. Biomaterials 2017, 134, 13–21. [Google Scholar] [CrossRef]
- Chalazonitis, A. Neurotrophin-3 in the development of the enteric nervous system. Prog. Brain Res. 2004, 146, 243–263. [Google Scholar] [CrossRef]
- Nayak, M.S.; Kim, Y.S.; Goldman, M.; Keirstead, H.S.; Kerr, D.A. Cellular therapies in motor neuron diseases. Biochim. Biophys. Acta-Mol. Basis Dis. 2006, 1762, 1128–1138. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Xu, X.; Guo, T.; Xie, D.; Zhu, R.; Chen, S.; Ramakrishna, S.; He, L. RGD/TAT-functionalized chitosan-graft-PEI-PEG gene nanovector for sustained delivery of NT-3 for potential application in neural regeneration. Acta Biomater. 2018, 72, 266–277. [Google Scholar] [CrossRef]
- Agarwala, S.; Tamplin, O.J. Neural Crossroads in the Hematopoietic Stem Cell Niche. Trends Cell Biol. 2018, 28, 987–998. [Google Scholar] [CrossRef]
- Andreas, K.; Sittinger, M.; Ringe, J. Toward in situ tissue engineering: Chemokine-guided stem cell recruitment. Trends Biotechnol. 2014, 32, 483–492. [Google Scholar] [CrossRef]
- Solis, M.A.; Chen, Y.H.; Yue, W.T.; Bittencourt, V.Z.; Lin, Y.C.; Huang, L.L.H. Hyaluronan Regulates Cell Behavior: A Potential Niche Matrix for Stem Cells. Biochem. Res. Int. 2011, 2012, 346972. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Addington, C.P.; Heffernan, J.M.; Millar-Haskell, C.S.; Tucker, E.W.; Sirianni, R.W.; Stabenfeldt, S.E. Enhancing neural stem cell response to SDF-1a gradients through hyaluronic acid-laminin hydrogels. Biomaterials 2015, 72, 11–19. [Google Scholar] [CrossRef]
- Addington, C.P.; Dharmawaj, S.; Heffernan, J.M.; Sirianni, R.W.; Stabenfeldt, S.E. Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol. 2017, 60–61, 206–216. [Google Scholar] [CrossRef]
- Cieplak, P.; Strongin, A.Y. Matrix metalloproteinases—From the cleavage data to the prediction tools and beyond. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1952–1963. [Google Scholar] [CrossRef]
- Kirchhain, A.; Poma, N.; Salvo, P.; Tedeschi, L.; Melai, B.; Vivaldi, F.; Bonini, A.; Franzini, M.; Caponi, L.; Tavanti, A.; et al. Biosensors for measuring matrix metalloproteinases: An emerging research field. Trac. Trends Anal. Chem. 2018, 110, 35–50. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef]
- Jian, W.H.; Wang, H.C.; Kuan, C.H.; Chen, M.H.; Wu, H.C.; Sun, J.S.; Wang, T.W. Glycosaminoglycan-based hybrid hydrogel encapsulated with polyelectrolyte complex nanoparticles for endogenous stem cell regulation in central nervous system regeneration. Biomaterials 2018, 174, 17–30. [Google Scholar] [CrossRef]
- Dumont, C.M.; Carlson, M.A.; Munsell, M.K.; Ciciriello, A.J.; Strnadova, K.; Park, J.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019, 86, 312–322. [Google Scholar] [CrossRef]
- Nune, M.; Manchineella, S.; Govindaraju, T.; Narayan, K.S. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 17–25. [Google Scholar] [CrossRef]
- Zeng, X.; Qiu, X.C.; Ma, Y.H.; Duan, J.J.; Chen, Y.F.; Gu, H.Y.; Wang, J.M.; Ling, E.A.; Wu, J.L.; Wu, W.; et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials 2015, 53, 184–201. [Google Scholar] [CrossRef]
- Wu, G.H.; Shi, H.J.; Che, M.T.; Huang, M.Y.; Wei, Q.S.; Feng, B.; Ma, Y.H.; Wang, L.J.; Jiang, B.; Wang, Y.Q.; et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials 2018, 181, 15–34. [Google Scholar] [CrossRef]
- Xia, T.; Huang, B.; Ni, S.; Gao, L.; Wang, J.; Wang, J.; Chen, A.; Zhu, S.; Wang, B.; Li, G.; et al. The combination of db-cAMP and ChABC with poly(propylene carbonate) microfibers promote axonal regenerative sprouting and functional recovery after spinal cord hemisection injury. Biomed. Pharmacother. 2017, 86, 354–362. [Google Scholar] [CrossRef]
- Gascon, E.; Vutskits, L.; Kiss, J.Z. Polysialic acid–neural cell adhesion molecule in brain plasticity: From synapses to integration of new neurons. Brain Res. Rev. 2007, 56, 101–118. [Google Scholar] [CrossRef]
- Li, X.; Katsanevakis, E.; Liu, X.; Zhang, N.; Wen, X. Engineering neural stem cell fates with hydrogel design for central nervous system regeneration. Prog. Polym. Sci. 2012, 37, 1105–1129. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.J.; Li, W.S.; Xu, X.L.; Hu, J.B.; Kang, X.Q.; Qi, J.; Ying, X.Y.; You, J.; Du, Y.Z. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018, 77, 15–27. [Google Scholar] [CrossRef]
- Gajendiran, M.; Choi, J.; Kim, S.J.; Kim, K.; Shin, H.; Koo, H.J.; Kim, K. Conductive biomaterials for tissue engineering applications. J. Ind. Eng. Chem. 2017, 51, 12–26. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef]
- Domínguez-Bajo, A.; González-Mayorga, A.; Guerrero, C.R.; Palomares, F.J.; García, R.; López-Dolado, E.; Serrano, M.C. Myelinated axons and functional blood vessels populate mechanically compliant rGO foams in chronic cervical hemisected rats. Biomaterials 2019, 192, 461–474. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P. Temperature and pH stimuli-responsive polymers and their applications in controlled and self-regulated drug delivery. J. Appl. Pharm. Sci. 2012, 2, 1–10. [Google Scholar]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Hunter, A.C.; Moghimi, S.M. Smart polymers in drug delivery: A biological perspective. Polym. Chem. 2017, 8, 41–51. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Manourasa, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic- and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Ninan, I. Synaptic regulation of affective behaviors; role of BDNF. Neuropharmacology 2014, 76, 684–695. [Google Scholar] [CrossRef]
- Pluchino, N.; Russo, M.; Santoro, A.N.; Litta, P.; Cela, V.; Genazzani, A.R. Steroid hormones and BDNF. Neuroscience 2013, 239, 271–279. [Google Scholar] [CrossRef]
- Lopes, C.D.F.; Gonçalves, N.P.; Gomes, C.P.; Saraiva, M.J.; Pêgo, A.P. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 2017, 121, 83–96. [Google Scholar] [CrossRef]
- Li, G.; Xue, C.; Wang, H.; Yang, X.; Zhao, Y.; Zhang, L.; Yang, Y. Spatially featured porous chitosan conduits with micropatterned inner wall and seamless sidewall for bridging peripheral nerve regeneration. Carbohydr. Polym. 2018, 194, 225–235. [Google Scholar] [CrossRef]
- Ahmadi, N.; Kharaziha, M.; Labbaf, S. (BaCa)TiO3 nanopowder: Synthesis and their electrical and biological characteristics. Mater. Chem. Phys. 2019, 226, 263–281. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Ehterami, A.; Kazemi, M.; Nazari, B.; Saraeian, P.; Azami, M. Fabrication and characterization of highly porous barium titanate based scaffold coated by Gel/HA nanocomposite with high piezoelectric coefficient for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2018, 79, 195–202. [Google Scholar] [CrossRef]
- Shokrollahi, H.; Salimi, F.; Doostmohammadi, A. The fabrication and characterization of barium titanate/akermanite nano-bio-ceramic with a suitable piezoelectric coefficient for bone defect recovery. J. Mech. Behav. Biomed. Mater. 2017, 74, 365–370. [Google Scholar] [CrossRef]
- Li, G.; Xiao, Q.; McNaughton, R.; Han, L.; Zhang, L.; Wang, Y.; Yang, Y. Nanoengineered porous chitosan/CaTiO3 hybrid scaffolds for accelerating Schwann cells growth in peripheral nerve regeneration. Colloid Surf. B-Biointerfaces 2017, 158, 57–67. [Google Scholar] [CrossRef]
- Ayanlaja, A.A.; Zhang, B.; Ji, G.Q.; Gao, Y.; Wang, J.; Kanwore, K.; Gao, D.S. The reversible effects of glial cell line–derived neurotrophic factor (GDNF) in the human brain. Semin. Cancer Biol. 2018, 53, 212–222. [Google Scholar] [CrossRef]
- Höke, A.; Ho, T.; Crawford, T.O.; LeBel, C.; Hilt, D.; Griffin, J.W. Glial Cell Line-Derived Neurotrophic Factor Alters Axon Schwann Cell Units and Promotes Myelination in Unmyelinated Nerve Fibers. J. Neurosci. 2003, 23, 561–567. [Google Scholar] [CrossRef]
- Painter, M.W. Aging Schwann cells: Mechanisms, implications, future directions. Curr. Opin. Neurobiol. 2017, 47, 203–208. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Walzer, M.A.; Snider, W.D. Turning On the Machine: Genetic Control of Axon Regeneration by c-Jun. Neuron 2004, 43, 1–2. [Google Scholar] [CrossRef]
- Lackington, W.A.; Raftery, R.M.; O’Brien, F.J. In vitro efficacy of a gene-activated nerve guidance conduit incorporating non-viral PEI-pDNA nanoparticles carrying genes encoding for NGF, GDNF and c-Jun. Acta Biomater. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, G.; Gao, Y.; Zhou, Y.; Liu, J.; Zhang, L.; Long, A.; Zhang, L.; Tang, P. A sequential delivery system employing the synergism of EPO and NGF promotes sciatic nerve repair. Colloids Surf. B Biointerfaces 2017, 159, 327–336. [Google Scholar] [CrossRef]
- Almeida, R.G.; Lyons, D.A. On the resemblance of synapse formation and CNS myelination. Neuroscience 2014, 276, 98–108. [Google Scholar] [CrossRef]
- Mori, M.; Rikitake, Y.; Mandai, K.; Takai, Y. Roles of Nectins and Nectin-Like Molecules in the Nervous System. In Advances in Neurobiology 8—Cell Adhesion Molecules: Implications in Neurological Diseases; Berezin, V., Walmod, P.S., Eds.; Springer: New York, NY, USA, 2014; pp. 91–116. [Google Scholar]
- Sakisaka, T.; Takai, Y. Biology and pathology of nectins and nectin-like molecules. Curr. Opin. Cell Biol. 2004, 16, 513–521. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Lv, P.; Lu, R.; Zheng, L.; Zhao, J. NECL1 coated PLGA as favorable conduits for repair of injured peripheral nerve. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1132–1140. [Google Scholar] [CrossRef]
- Rao, J.; Cheng, Y.; Liu, Y.; Ye, Z.; Zhan, B.; Quan, D.; Xu, Y. A multi-walled silk fibroin/silk sericin nerve conduit coated with poly(lactic-co-glycolic acid) sheath for peripheral nerve regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 319–332. [Google Scholar] [CrossRef]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef]
- Barroca, N.; Marote, A.; Vieira, S.I.; Almeida, A.; Fernandes, M.H.V.; Vilarinho, P.M.; da Cruz e Silva, O.A.B. Electrically polarized PLLA nanofibers as neural tissue engineeringscaffolds with improved neuritogenesis. Colloids Surf. B Biointerfaces 2018, 167, 93–103. [Google Scholar] [CrossRef]
- Jing, W.; Zuo, D.; Cai, Q.; Chen, G.; Wang, L.; Yang, X.; Zhong, W. Promoting neural transdifferentiation of BMSCs via applying synergetic multiple factors for nerve regeneration. Exp. Cell Res. 2019, 375, 80–91. [Google Scholar] [CrossRef]
- Jing, W.; Ao, Q.; Wang, L.; Huang, Z.; Cai, Q.; Chen, G.; Yang, X.; Zhong, W. Constructing conductive conduit with conductive fibrous infilling for peripheral nerve regeneration. Chem. Eng. J. 2018, 345, 566–577. [Google Scholar] [CrossRef]
- Sun, B.; Wu, T.; Wang, J.; Li, D.; Wang, J.; Gao, Q.; Bhutto, M.A.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous membranes promoting neural cell proliferation and differentiation with electrical stimulation. J. Mater. Chem. B 2016, 4, 6670–6679. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, Z.; Li, D.; Wu, T.; Zheng, H.; Liu, J.; Wang, G.; Yu, Y.; Mo, X. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous nerve guidance conduit induced nerve regeneration in rat. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 190–199. [Google Scholar] [CrossRef]
- Uz, M.; Büyüköz, M.; Sharma, A.D.; Sakaguchid, D.S.; Altinkaya, S.A.; Mallapragada, S.K. Gelatin-based 3D conduits for transdifferentiation of mesenchymal stem cells into Schwann cell-like phenotypes. Acta Biomater. 2017, 53, 293–306. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, M.; Lee, D.; Heo, D.N.; Lim, H.N.; Kwon, I.K. Fabrication and design of bioactive agent coated, highly-alignedelectrospun matrices for nerve tissue engineering: Preparation, characterization and application. Appl. Surf. Sci. 2017, 424, 359–367. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Kuzum, D. Graphene-based neurotechnologies for advanced neural interfaces. Curr. Opin. Biomed. Eng. 2018, 6, 138–147. [Google Scholar] [CrossRef]
- Reddy, S.; Xu, X.; Guo, T.; Zhu, R.; He, L.; Ramakrishana, S. Allotropic carbon (graphene oxide and reduced graphene oxide) based biomaterials for neural regeneration. Curr. Opin. Biomed. Eng. 2018, 6, 120–129. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Yan, K.; Shi, C.; Xu, X.; Wang, T.; Li, R.; Dong, W.; Zheng, J. Neurogenic differentiation of adipose derived stem cells on graphene-based mat. Mater. Sci. Eng. C 2018, 90, 685–692. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Niu, Q.; Fu, X.; Sun, X.; Tong, X. The application of graphene oxidized combining with decellularized scaffold to repair of sciatic nerve injury in rats. Saudi Pharm. J. 2017, 25, 469–476. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleanu, R.I.; Gherasim, O.; Gherasim, T.G.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, D.M. Nanomaterial-Based Approaches for Neural Regeneration. Pharmaceutics 2019, 11, 266. https://doi.org/10.3390/pharmaceutics11060266
Teleanu RI, Gherasim O, Gherasim TG, Grumezescu V, Grumezescu AM, Teleanu DM. Nanomaterial-Based Approaches for Neural Regeneration. Pharmaceutics. 2019; 11(6):266. https://doi.org/10.3390/pharmaceutics11060266
Chicago/Turabian StyleTeleanu, Raluca Ioana, Oana Gherasim, Tudor George Gherasim, Valentina Grumezescu, Alexandru Mihai Grumezescu, and Daniel Mihai Teleanu. 2019. "Nanomaterial-Based Approaches for Neural Regeneration" Pharmaceutics 11, no. 6: 266. https://doi.org/10.3390/pharmaceutics11060266
APA StyleTeleanu, R. I., Gherasim, O., Gherasim, T. G., Grumezescu, V., Grumezescu, A. M., & Teleanu, D. M. (2019). Nanomaterial-Based Approaches for Neural Regeneration. Pharmaceutics, 11(6), 266. https://doi.org/10.3390/pharmaceutics11060266