Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery
Abstract
:1. Introduction
2. Principles of Film-Forming Systems
3. The Generation of Film-Forming Systems
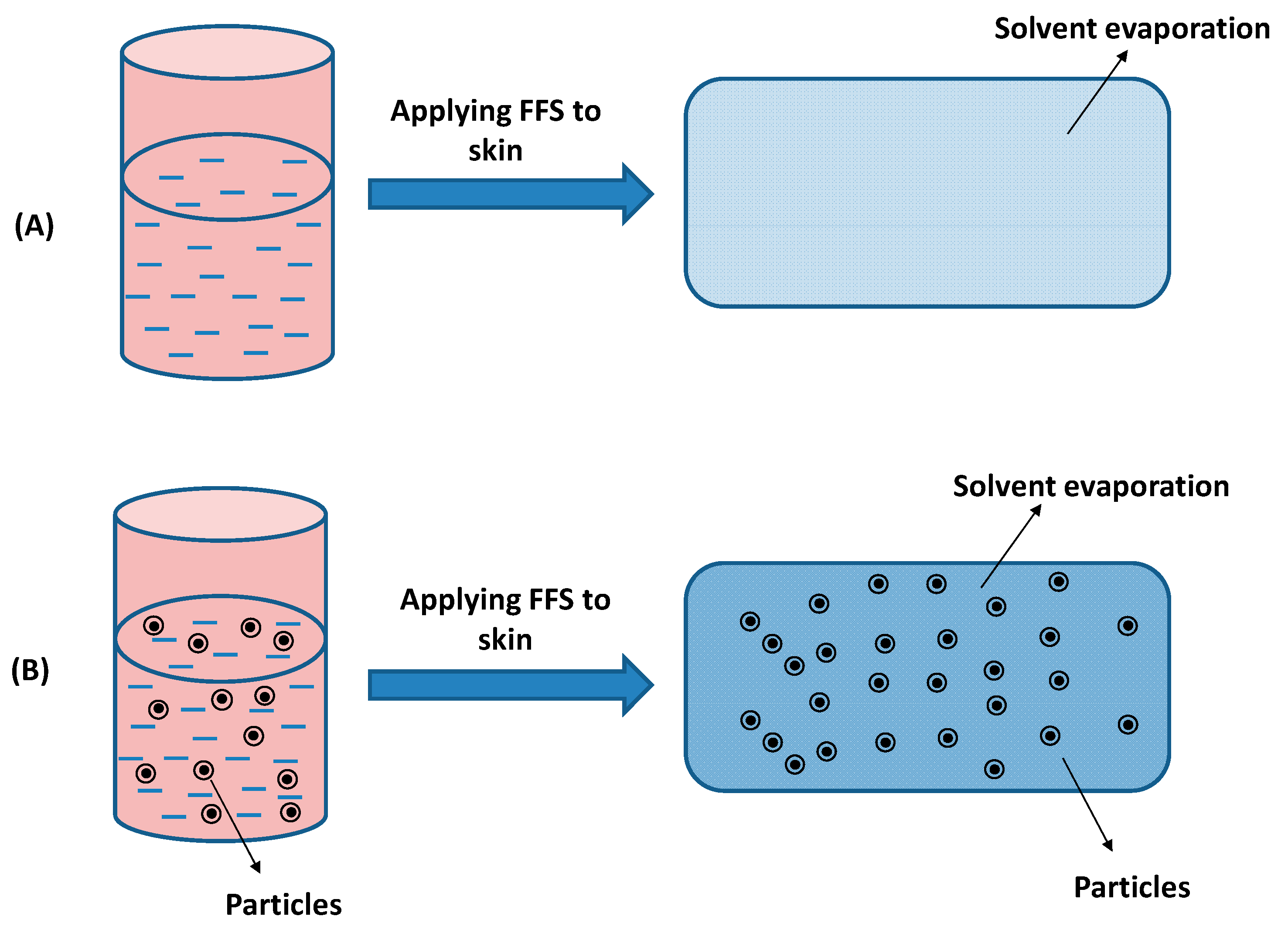
3.1. Film-Forming Solutions
3.2. Film-Forming Creams and Gels
3.3. Controlled Drug Delivery Film Forming Systems
4. Formulation Design of Controlled Drug Release Film-Forming Systems
4.1. Model Drugs
4.2. Polymers
4.3. Plasticizers
4.4. Solvents
5. Evaluation of the Physico-Chemical Properties of Controlled Drug Release Film-Forming Systems
5.1. Physicochemical Characterizations
5.2. Drug Release and Drug Penetration Evaluation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.H.; Kim, J.E.; Kim, Y.S.; Ryu, C.H.; Lee, H.J.; Kim, H.M.; Jeon, H.; Won, H.-J.; Lee, J.-Y.; et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J. Ginseng Res. 2018, 42, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.T.; Barua, S.; Yoo, S.-Y.; Hong, S.-C.; Lee, K.B.; Lee, J. Seeking better topical delivery technologies of moisturizing agents for enhanced skin moisturization. Expert Opin. Drug Deliv. 2018, 15, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Lee, D.I.; Kim, H.; Jo, K.; Yeo, S.; Yoo, S.-Y.; Jeon, H.; Lee, J.-Y.; Lee, J. Solid Lipid Nanoparticles of Serine Designed by Evaluating Affinity of Solid Lipids to Stratum Corneum for Enhanced Skin Hydration in Combination with Reed Root Extract. Bull. Korean Chem. Soc. 2018, 39, 220–226. [Google Scholar] [CrossRef]
- Padula, C.; Colombo, G.; Nicoli, S.; Catellani, P.L.; Massimo, G.; Santi, P. Bioadhesive film for the transdermal delivery of lidocaine: In vitro and in vivo behavior. J. Control. Release 2003, 88, 277–285. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Pescina, S.; Santi, P. Thin polymeric films for the topical delivery of propranolol. Colloids Surf. B Biointerfaces 2019, 174, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Engelke, L.; Winter, G.; Engert, J. Application of water-soluble polyvinyl alcohol-based film patches on laser microporated skin facilitates intradermal macromolecule and nanoparticle delivery. Eur. J. Pharm. Biopharm. 2018, 128, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mendes, I.T.; Ruela, A.L.M.; Carvalho, F.C.; Freitas, J.T.J.; Bonfilio, R.; Pereira, G.R. Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf. B Biointerfaces 2019, 177, 274–281. [Google Scholar] [CrossRef]
- Rostamkalaei, S.S.; Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Nokhodchi, A. Topical gel of Metformin solid lipid nanoparticles: A hopeful promise as a dermal delivery system. Colloids Surf. B Biointerfaces 2019, 175, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Michniak-Kohn, B.B. Investigation of microemulsion and microemulsion gel formulations for dermal delivery of clotrimazole. Int. J. Pharm. 2018, 536, 345–352. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M. Transdermal film-loaded finasteride microplates to enhance drug skin permeation: Two-step optimization study. Eur. J. Pharm. Sci. 2016, 88, 246–256. [Google Scholar] [CrossRef]
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldoni, L.; Bertorelli, R.; Athanassiou, A.; Bayer, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Navarro-García, F.; Heras, A.; Acosta, N. Chitosan based films as supports for dual antimicrobial release. Carbohydr. Polym. 2016, 146, 402–410. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-Y.; Liu, K.-L.; Yeh, H.-H.; Lin, H.-R.; Wu, H.-L.; Tsai, J.-C. Sustained release of recombinant thrombomodulin from cross-linked gelatin/hyaluronic acid hydrogels potentiate wound healing in diabetic mice. Eur. J. Pharm. Biopharm. 2019, 135, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Frederiksen, K.; Guy, R.H.; Petersson, K. The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs. Expert Opin. Drug Deliv. 2016, 13, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Schuren, J.; Becker, A.; Gary Sibbald, R. A liquid film-forming acrylate for peri-wound protection: A systematic review and meta-analysis (3M™ Cavilon™ no-sting barrier film). Int. Wound J. 2005, 2, 230–238. [Google Scholar] [CrossRef]
- Altmann, K.; Schulze, R.D.; Friedrich, J. Polymer deposition morphology by electrospray deposition—Modifications through distance variation. Thin Solid Film. 2014, 564, 269–276. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Choe, J.S.; Tran, T.T.D.; Park, Y.M.; Lee, B.J. Design and mechanism of on-off pulsed drug release using nonenteric polymeric systems via pH modulation. AAPS PharmSciTech 2011, 12, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Misra, A.; Raghuvanshi, R.S.; Ganga, S.; Diwan, M.; Talwar, G.P.; Singh, O. Formulation of a transdermal system for biphasic delivery of testosterone. J. Control. Release 1996, 39, 1–7. [Google Scholar] [CrossRef]
- Misra, A.; Pal, R.; Majumdar, S.S.; Talwar, G.P.; Singh, O. Biphasic Testosterone Delivery Profile Observed with Two Different Transdermal Formulations. Pharm. Res. 1997, 14, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Zurdo Schroeder, I.; Franke, P.; Schaefer, U.F.; Lehr, C.-M. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, I.Z.; Franke, P.; Schaefer, U.F.; Lehr, C.-M. Delivery of ethinylestradiol from film forming polymeric solutions across human epidermis in vitro and in vivo in pigs. J. Control. Release 2007, 118, 196–203. [Google Scholar] [CrossRef]
- Gohel, M.C.; Nagori, S.A. Fabrication of Modified Transport Fluconazole Transdermal Spray Containing Ethyl Cellulose and Eudragit® RS100 as Film Formers. AAPS PharmSciTech 2009, 10, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.M.; Patel, P.; Sheth, N.R.; Rathod, L.V.; Ashara, K.C. Fabrication and characterization of film-forming voriconazole transdermal spray for the treatment of fungal infection. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 41–51. [Google Scholar] [CrossRef]
- Acrux History. Available online: http://www.acrux.com.au/about/history/ (accessed on 25 March 2019).
- Wang, C.; Ilani, N.; Arver, S.; McLachlan, R.I.; Soulis, T.; Watkinson, A. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen-deficient men. Clin. Endocrinol. 2011, 75, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Axiron® (Testosterone) Pump Applicator, Axiron® (Testosterone) Twist Applicator. Available online: https://www.lilly.com/products/historic-products (accessed on 25 March 2019).
- Lamisil Once® 4g. Single Dose Treatment For Athlete’s Foot. Available online: http://lamisil.com.au/product-oncefilm.html (accessed on 25 March 2019).
- Kienzler, J.L.; Queille-Roussel, C.; Mugglestone, C.; Ortonne, J.P.; Larnier, C. Stratum corneum pharmaco-kinetics of the anti-fungal drug, terbinafine, in a novel topical formulation, for single-dose application in dermatophytoses. Curr. Med. Res. Opin. 2007, 23, 1293–1302. [Google Scholar] [CrossRef]
- MedSpray®. Available online: https://www.medpharm.com/en/medspray/ (accessed on 25 March 2019).
- Jones, S.A.; Reid, M.L.; Brown, M.B. Determining Degree of Saturation after Application of Transiently Supersaturated Metered Dose Aerosols for Topical Delivery of Corticosteroids. J. Pharm. Sci. 2009, 98, 543–554. [Google Scholar] [CrossRef]
- Platform Technology. Available online: https://epinamics.com/the-liqui-patch (accessed on 25 March 2019).
- Kim, D.W.; Kim, K.S.; Seo, Y.G.; Lee, B.-J.; Park, Y.J.; Youn, Y.S.; Kim, J.O.; Yong, C.S.; Jin, S.G.; Choi, H.-G. Novel sodium fusidate-loaded film-forming hydrogel with easy application and excellent wound healing. Int. J. Pharm. 2015, 495, 67–74. [Google Scholar] [CrossRef]
- Bryan, H.A.; Alster, T.S. The S-Caine Peel: A Novel Topical Anesthetic for Cutaneous Laser Surgery. Dermatol. Surg. 2002, 28, 999–1003. [Google Scholar] [CrossRef]
- Chen, J.Z.S.; Alexiades-Armenakas, M.R.; Bernstein, L.J.; Jacobson, L.G.; Friedman, P.M.; Geronemus, R.G. Two Randomized, Double-Blind, Placebo-Controlled Studies Evaluating the S-Caine Peel for Induction of Local Anesthesia Before Long-Pulsed Nd:YAG Laser Therapy for Leg Veins. Dermatol. Surg. 2003, 29, 1012–1018. [Google Scholar] [CrossRef]
- Doshi, S.N.; Friedman, P.M.; Marquez, D.K.; Goldberg, L.H. Thirty-Minute Application of the S-Caine Peel Prior to Nonablative Laser Treatment. Dermatol. Surg. 2003, 29, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.S.; Jacobson, L.G.; Bakus, A.D.; Garden, J.M.; Yaghmai, D.; Bernstein, L.J.; Geronemus, R.G. Evaluation of the S-Caine Peel for Induction of Local Anesthesia for Laser-Assisted Tattoo Removal: Randomized, Double-Blind, Placebo-Controlled, Multicenter Study. Dermatol. Surg. 2006, 31, 281–286. [Google Scholar] [CrossRef]
- Alster, T. Review of Lidocaine/Tetracaine Cream as a Topical Anesthetic for Dermatologic Laser Procedures. Pain Ther. 2013, 2, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, N.M.; Kim, D.D.; Lee, C.H.; Shin, Y.H. Development of a novel soft hydrogel for the transdermal delivery of testosterone. Drug Dev. Ind. Pharm. 2003, 29, 99–105. [Google Scholar] [CrossRef]
- Guo, J.-H. Investigating the Surface Properties and Bioadhesion of Buccal Patches. J. Pharm. Pharmacol. 1994, 46, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Du, X.; Zhang, R.; Deng, L.; Dong, A.; Zhang, J. Bioadhesive film formed from a novel organic–inorganic hybrid gel for transdermal drug delivery system. Eur. J. Pharm. Biopharm. 2011, 79, 574–583. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; Cui, S.; Feng, Z.; Du, Y.; Song, Z.; Tong, Y.; Yang, L.; Wang, Z.; Zeng, H.; et al. Chitosan-polyvinyl alcohol nanoscale liquid film-forming system facilitates MRSA-infected wound healing by enhancing antibacterial and antibiofilm properties. Int. J. Nanomed. 2018, 13, 4987–5002. [Google Scholar] [CrossRef]
- DuraPeel. Available online: http://www.crescitatherapeutics.com/technology/durapeel/ (accessed on 25 March 2019).
- Lunter, D.J.; Daniels, R. New film forming emulsions containing Eudragit® NE and/or RS 30D for sustained dermal delivery of nonivamide. Eur. J. Pharm. Biopharm. 2012, 82, 291–298. [Google Scholar] [CrossRef]
- Lunter, D.; Daniels, R. In vitro Skin Permeation and Penetration of Nonivamide from Novel Film-Forming Emulsions. Skin Pharmacol. Physiol. 2013, 26, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.; Hermann, S.; Lunter, D.J.; Daniels, R. Film-forming formulations containing porous silica for the sustained delivery of actives to the skin. Eur. J. Pharm. Biopharm. 2016, 108, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, K.; Guy, R.H.; Petersson, K. Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin. Eur. J. Pharm. Biopharm. 2015, 91, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, R.O.; Guy, R.H. Predicting Skin Permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yoshimura, M.; Tanaka, T.; Takada, K. Effect of Lipophilicity on the Bioavailability of Drugs After Percutaneous Administration by Dissolving Microneedles. J. Pharm. Sci. 2012, 101, 1145–1156. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Colombo, P.; Santi, P. Single-layer transdermal film containing lidocaine: Modulation of drug release. Eur. J. Pharm. Biopharm. 2007, 66, 422–428. [Google Scholar] [CrossRef]
- Benita, S.; Dor, P.; Aronhime, M.; Marom, G. Permeability and mechanical properties of a new polymer: Cellulose hydrogen phthalate. Int. J. Pharm. 1986, 33, 71–80. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Polymer blends used for the aqueous coating of solid dosage forms: Importance of the type of plasticizer. J. Control. Release 2004, 99, 1–13. [Google Scholar] [CrossRef]
- Iervolino, M.; Cappello, B.; Raghavan, S.L.; Hadgraft, J. Penetration enhancement of ibuprofen from supersaturated solutions through human skin. Int. J. Pharm. 2001, 212, 131–141. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Davis, A.F.; Hadgraft, J. Effect of supersaturation on membrane transport: 1. Hydrocortisone acetate. Int. J. Pharm. 1991, 76, 1–8. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Froehlich, C.; Kalia, Y.N.; Guy, R.H. Supersaturation: Enhancement of Skin Penetration and Permeation of a Lipophilic Drug. Pharm. Res. 2001, 18, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Qi, S.; Liu, F.; Brown, M.B.; McAuley, W.J. Rationalising polymer selection for supersaturated film forming systems produced by an aerosol spray for the transdermal delivery of methylphenidate. Eur. J. Pharm. Biopharm. 2017, 114, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Belton, P.; Nollenberger, K.; Clayden, N.; Reading, M.; Craig, D.Q.M. Characterisation and Prediction of Phase Separation in Hot-Melt Extruded Solid Dispersions: A Thermal, Microscopic and NMR Relaxometry Study. Pharm. Res. 2010, 27, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.; Lukić, M.Ž.; Savić, S.D.; Daniels, R.; Lunter, D.J. Ex vivo skin permeation and penetration of nonivamide from and in vivo skin tolerability of film-forming formulations containing porous silica. Eur. J. Pharm. Sci. 2017, 106, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-W.; Kang, J.-H.; Lee, H.-J.; Han, S.-D.; Kang, M.-H.; Kwon, Y.-H.; Jun, J.-H.; Kim, D.-W.; Rhee, Y.-S.; Kim, J.-Y.; et al. Formulation and in vitro/in vivo evaluation of chitosan-based film forming gel containing ketoprofen. Drug Deliv. 2017, 24, 1056–1066. [Google Scholar] [CrossRef] [Green Version]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S.N. Biophysical elucidation of the mechanism of enhanced drug release and topical delivery from polymeric film-forming systems. J. Control. Release 2015, 212, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trey, S.M.; Wicks, D.A.; Mididoddi, P.K.; Repka, M.A. Delivery of itraconazole from extruded HPC films. Drug Dev. Ind. Pharm. 2007, 33, 727–735. [Google Scholar] [CrossRef]
- Marucci, M.; Andersson, H.; Hjärtstam, J.; Stevenson, G.; Baderstedt, J.; Stading, M.; Larsson, A.; von Corswant, C. New insights on how to adjust the release profile from coated pellets by varying the molecular weight of ethyl cellulose in the coating film. Int. J. Pharm. 2013, 458, 218–223. [Google Scholar] [CrossRef]
- Thao, T.D.T.; Phuong, H.L.T. Perspectives on Strategies Using Swellable Polymers in Solid Dispersions for Controlled Drug Release. Curr. Pharm. Des. 2017, 23, 1639–1648. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J. Solvent exchange-induced in situ forming gel comprising ethyl cellulose-antimicrobial drugs. Int. J. Pharm. 2015, 494, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Setty, C.M.; Mistry, G.N.; Patel, S.L.; Patel, T.J.; Mistry, P.C.; Rana, A.K.; Patel, P.K.; Mishra, R.S. Development and Evaluation of Ethyl Cellulose-Based Transdermal Films of Furosemide for Improved In Vitro Skin Permeation. AAPS PharmSciTech 2009, 10, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Shin, T.H.; Ho, M.J.; Kim, S.R.; Im, S.H.; Kim, C.H.; Lee, S.; Kang, M.J.; Choi, Y.W. Formulation and in vivo pharmacokinetic evaluation of ethyl cellulose-coated sustained release multiple-unit system of tacrolimus. Int. J. Biol. Macromol. 2018, 109, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Polymer blends for controlled release coatings. J. Control. Release 2008, 125, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C. The effect of the molecular weight of ethyl cellulose on the drug release properties of mixed films of ethyl cellulose and hydroxypropylmethylcellulose. Int. J. Pharm. 1986, 29, 37–41. [Google Scholar] [CrossRef]
- Larsson, M.; Hjärtstam, J.; Berndtsson, J.; Stading, M.; Larsson, A. Effect of ethanol on the water permeability of controlled release films composed of ethyl cellulose and hydroxypropyl cellulose. Eur. J. Pharm. Biopharm. 2010, 76, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marucci, M.; Hjärtstam, J.; Ragnarsson, G.; Iselau, F.; Axelsson, A. Coated formulations: New insights into the release mechanism and changes in the film properties with a novel release cell. J. Control. Release 2009, 136, 206–212. [Google Scholar] [CrossRef]
- Marucci, M.; Ragnarsson, G.; von Corswant, C.; Welinder, A.; Jarke, A.; Iselau, F.; Axelsson, A. Polymer leaching from film coating: Effects on the coating transport properties. Int. J. Pharm. 2011, 411, 43–48. [Google Scholar] [CrossRef]
- Andersson, H.; Hjärtstam, J.; Stading, M.; von Corswant, C.; Larsson, A. Effects of molecular weight on permeability and microstructure of mixed ethyl-hydroxypropyl-cellulose films. Eur. J. Pharm. Sci. 2013, 48, 240–248. [Google Scholar] [CrossRef]
- Thombre, A.G.; DeNoto, A.R.; Falkner, F.C.; Lazar, J.D. In vitro/in vivo correlations of sustained-release coated multiparticulate formulations of doxazosin. Int. J. Pharm. 1994, 111, 181–189. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Liang, R.; Liu, W.; Wang, C.; Su, Z.; Sun, F.; Li, Y. Preparation and characterization of sustained-release rotigotine film-forming gel. Int. J. Pharm. 2014, 460, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Phuong, H.L.T.; Wei, D.; Beom-Jin, L.; Thao, T.D.T. Current Designs of Polymer Blends in Solid Dispersions for Improving Drug Bioavailability. Curr. Drug Metab. 2018, 19, 1111–1118. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives: I—Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, F.K.; Abdel Rahman, A.A.; Mahrous, G.M.; Alsarra, I.A. Formulation and physicochemical characterisation of buccoadhesive films containing ketorolac. J. Drug Deliv. Sci. Technol. 2007, 17, 183–192. [Google Scholar] [CrossRef]
- El-Setouhy, D.A.; El-Malak, N.S.A. Formulation of a Novel Tianeptine Sodium Orodispersible Film. AAPS PharmSciTech 2010, 11, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.D.; Tran, P.H.L.; Choi, H.G.; Han, H.K.; Lee, B.J. The roles of acidifiers in solid dispersions and physical mixtures. Int. J. Pharm. 2010, 384, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Rosa, R.M.; Silva, J.C.; Sanches, I.S.; Henriques, C. Simultaneous photo-induced cross-linking and silver nanoparticle formation in a PVP electrospun wound dressing. Mater. Lett. 2017, 207, 145–148. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Hu, H.; Zhai, M.; Peng, J.; Nho, Y.; Li, J.; Wei, G. Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 385–389. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.; Mahmoud, A.A.; Makram, T.S.; Ghoneim, A.M. Rapid pain relief using transdermal film forming polymeric solution of ketorolac. Pharm. Dev. Technol. 2013, 18, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Taylor, L.S.; Edgar, K.J. The role of polymers in oral bioavailability enhancement; a review. Polymer 2015, 77, 399–415. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Hameed, N.; Glattauer, V.; Ramshaw, J.A.M. Evaluation of polyvinyl alcohol composite membranes containing collagen and bone particles. J. Mech. Behav. Biomed. Mater. 2015, 48, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jeong Kim, S.; Jun Park, S.; Young Kim, I.; Hee Lee, Y.; Kim, S.I. Thermal characteristics of poly(vinyl alcohol) and poly(vinylpyrrolidone) IPNs. J. Appl. Polym. Sci. 2002, 86, 1844–1847. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M.; Teodorescu, M.; Avadanei, M. Tailoring the properties of poly(vinyl alcohol)/poly(vinylpyrrolidone) hydrogels for biomedical applications. Eur. Polym. J. 2016, 84, 313–325. [Google Scholar] [CrossRef]
- Thomas, J.; Gomes, K.; Lowman, A.; Marcolongo, M. The effect of dehydration history on PVA/PVP hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69B, 135–140. [Google Scholar] [CrossRef]
- Abou-Aiad, T.H.M.; Abd-El-Nour, K.N.; Hakim, I.K.; Elsabee, M.Z. Dielectric and interaction behavior of chitosan/polyvinyl alcohol and chitosan/polyvinyl pyrrolidone blends with some antimicrobial activities. Polymer 2006, 47, 379–389. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Furuike, T.; Komoto, D.; Hashimoto, H.; Tamura, H. Preparation of chitosan hydrogel and its solubility in organic acids. Int. J. Biol. Macromol. 2017, 104, 1620–1625. [Google Scholar] [CrossRef]
- Monti, D.; Tampucci, S.; Chetoni, P.; Burgalassi, S.; Mailland, F. Ciclopirox vs. Amorolfine: In Vitro Penetration Into and Permeation Through Human Healthy Nails of Commercial Nail Lacquers. J. Drugs Dermatol. 2014, 13, 143–147. [Google Scholar]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Cilurzo, F.; Selmin, F.; Gennari, C.G.M.; Montanari, L.; Minghetti, P. Application of methyl methacrylate copolymers to the development of transdermal or loco-regional drug delivery systems. Expert Opin. Drug Deliv. 2014, 11, 1033–1045. [Google Scholar] [CrossRef]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S. Characterization of Topical Film-Forming Systems Using Atomic Force Microscopy and Raman Microspectroscopy. Mol. Pharm. 2015, 12, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nollenberger, K.; Albers, J. Poly(meth)acrylate-based coatings. Int. J. Pharm. 2013, 457, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kucera, S.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Influence of an Acrylic Polymer Blend on the Physical Stability of Film-Coated Theophylline Pellets. AAPS PharmSciTech 2009, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.G.; Maus, M.; Kornherr, A.; Zifferer, G. Glass transition temperature of a cationic polymethacrylate dependent on the plasticizer content—Simulation vs. experiment. Chem. Phys. Lett. 2005, 406, 90–94. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.; El-Nahhas, S.A.; Kamel, R. Polymeric Matrix System for Prolonged Delivery of Tramadol Hydrochloride, Part I: Physicochemical Evaluation. AAPS PharmSciTech 2009, 10, 7–20. [Google Scholar] [CrossRef]
- Mahnaj, T.; Ahmed, S.U.; Plakogiannis, F.M. Evaluating the efficacy of a group of nontraditional plasticizers on the glass transition temperature of ethyl cellulose polymer. Drug Dev. Ind. Pharm. 2011, 37, 342–350. [Google Scholar] [CrossRef]
- Gennari, C.G.M.; Selmin, F.; Franzè, S.; Musazzi, U.M.; Quaroni, G.M.G.; Casiraghi, A.; Cilurzo, F. A glimpse in critical attributes to design cutaneous film forming systems based on ammonium methacrylate. J. Drug Deliv. Sci. Technol. 2017, 41, 157–163. [Google Scholar] [CrossRef]
- Kandile, N.G.; Mohamed, H.M. Chitosan nanoparticle hydrogel based sebacoyl moiety with remarkable capability for metal ion removal from aqueous systems. Int. J. Biol. Macromol. 2019, 122, 578–586. [Google Scholar] [CrossRef]
- Ashrafi, H.; Azadi, A. Chitosan-based hydrogel nanoparticle amazing behaviors during transmission electron microscopy. Int. J. Biol. Macromol. 2016, 84, 31–34. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Kamar, S.S.; Abdel-Kader, D.H.; Rashed, L.A. Beneficial effect of Curcumin Nanoparticles-Hydrogel on excisional skin wound healing in type-I diabetic rat: Histological and immunohistochemical studies. Ann. Anat. Anat. Anz. 2019, 222, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Dias, F.V.; Querobino, S.M.; Alberto-Silva, C.; Fraceto, L.F.; de Paula, E.; de Araujo, D.R. Influence of hybrid polymeric nanoparticle/thermosensitive hydrogels systems on formulation tracking and in vitro artificial membrane permeation: A promising system for skin drug-delivery. Colloids Surf. B Biointerfaces 2019, 174, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Choipang, C.; Chuysinuan, P.; Suwantong, O.; Ekabutr, P.; Supaphol, P. Hydrogel wound dressings loaded with PLGA/ciprofloxacin hydrochloride nanoparticles for use on pressure ulcers. J. Drug Deliv. Sci. Technol. 2018, 47, 106–114. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, O.; Ozay, H. A novel hydrogel containing thioether group as selective support material for preparation of gold nanoparticles: Synthesis and catalytic applications. Appl. Catal. B Environ. 2019, 241, 415–423. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Hernández, R.; Rescignano, N.; Boff, L.; Reginatto, F.H.; Simões, C.M.O.; de Campos, A.M.; Mijangos, C. Nanocomposite chitosan hydrogels based on PLGA nanoparticles as potential biomedical materials. Eur. Polym. J. 2018, 99, 456–463. [Google Scholar] [CrossRef]
- De Matos Fonseca, J.; de Fátima Medeiros, S.; Alves, G.M.; dos Santos, D.M.; Campana-Filho, S.P.; dos Santos, A.M. Chitosan microparticles embedded with multi-responsive poly(N-vinylcaprolactam-co-itaconic acid-co-ethylene-glycol dimethacrylate)-based hydrogel nanoparticles as a new carrier for delivery of hydrophobic drugs. Colloids Surf. B Biointerfaces 2019, 175, 73–83. [Google Scholar] [CrossRef]
- Tuong, N.G.N.; Van-Thanh, T.; Wei, D.; Phuong, H.L.T.; Thao, T.D.T. Nanoprecipitation for Poorly Water-Soluble Drugs. Curr. Drug Metab. 2017, 18, 1000–1015. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Pham, M.N.; Vo, T.V.; Duan, W.; Tran, P.H.; Tran, T.T. Strategies of Engineering Nanoparticles for Treating Neurodegenerative Disorders. Curr. Drug Metab. 2017, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dinh, H.T.T.; Tran, P.H.L.; Duan, W.; Lee, B.-J.; Tran, T.T.D. Nano-sized solid dispersions based on hydrophobic-hydrophilic conjugates for dissolution enhancement of poorly water-soluble drugs. Int. J. Pharm. 2017, 533, 93–98. [Google Scholar] [CrossRef]
- Tran, T.T.D.; Tran, P.H.L.; Nguyen, K.T.; Tran, V.T. Nano-precipitation: Preparation and application in the field of pharmacy. Curr. Pharm. Des. 2016, 22, 2997–3006. [Google Scholar] [CrossRef]
- Hurler, J.; Škalko-Basnet, N. Potentials of Chitosan-Based Delivery Systems in Wound Therapy: Bioadhesion Study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asasutjarit, R.; Larpmahawong, P.; Fuongfuchat, A.; Sareedenchai, V.; Veeranondha, S. Physicochemical properties and anti-propionibacterium acnes activity of film-forming solutions containing alpha-mangostin-rich extract. AAPS PharmSciTech 2014, 15, 306–316. [Google Scholar] [CrossRef]
- Veikauskait, I.; Briedis, V. Effect of Film-Forming Polymers on Release of Naftifine Hydrochloride from Nail Lacquers. Int. J. Polym. Sci. 2017, 2017, 7. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, T.T.D.; Park, J.B.; Lee, B.J. Controlled release systems containing solid dispersions: Strategies and mechanisms. Pharm. Res. 2011, 28, 2353–2378. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.D.; Tran, P.H.L.; Khanh, T.N.; Van, T.V.; Lee, B.J. Solubilization of poorly water-soluble drugs using solid dispersions. Recent Pat. Drug Deliv. Formul. 2013, 7, 122–133. [Google Scholar] [CrossRef]
- Nguyen, M.N.U.; Tran, P.H.L.; Tran, T.T.D. A single-layer film coating for colon-targeted oral delivery. Int. J. Pharm. 2019, 559, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Treffel, P.; Muret, P.; Muret-D’Aniello, P.; Coumes-Marquet, S.; Agache, P. Effect of Occlusion on in vitro Percutaneous Absorption of Two Compounds with Different Physicochemical Properties. Skin Pharmacol. Physiol. 1992, 5, 108–113. [Google Scholar] [CrossRef]
- Qiao, G.L.; Chang, S.K.; Riviere, J.E. Effects of Anatomical Site and Occlusion on the Percutaneous Absorption and Residue Pattern of 2,6-(ring-14C)Parathion in vivo in Pigs. Toxicol. Appl. Pharmacol. 1993, 122, 131–138. [Google Scholar] [CrossRef]
- Hotchkiss, S.A.M.; Miller, J.M.; Caldwell, J. Percutaneous absorption of benzyl acetate through rat skin in vitro. 2. Effect of vehicle and occlusion. Food Chem. Toxicol. 1992, 30, 145–153. [Google Scholar] [CrossRef]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig Ear Skin ex Vivo as a Model for in Vivo Dermatopharmacokinetic Studies in Man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Dermatopharmacokinetics of betamethasone 17-valerate: Influence of formulation viscosity and skin surface cleaning procedure. Eur. J. Pharm. Biopharm. 2009, 71, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Song, Y.; Page, S.W.; Garg, S. Evaluation of Transdermal Drug Permeation as Modulated by Lipoderm and Pluronic Lecithin Organogel. J. Pharm. Sci. 2018, 107, 587–594. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.D.; Tran, P.H.L. Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics 2019, 11, 290. https://doi.org/10.3390/pharmaceutics11060290
Tran TTD, Tran PHL. Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics. 2019; 11(6):290. https://doi.org/10.3390/pharmaceutics11060290
Chicago/Turabian StyleTran, Thao T. D., and Phuong H. L. Tran. 2019. "Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery" Pharmaceutics 11, no. 6: 290. https://doi.org/10.3390/pharmaceutics11060290
APA StyleTran, T. T. D., & Tran, P. H. L. (2019). Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics, 11(6), 290. https://doi.org/10.3390/pharmaceutics11060290




