Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics
Abstract
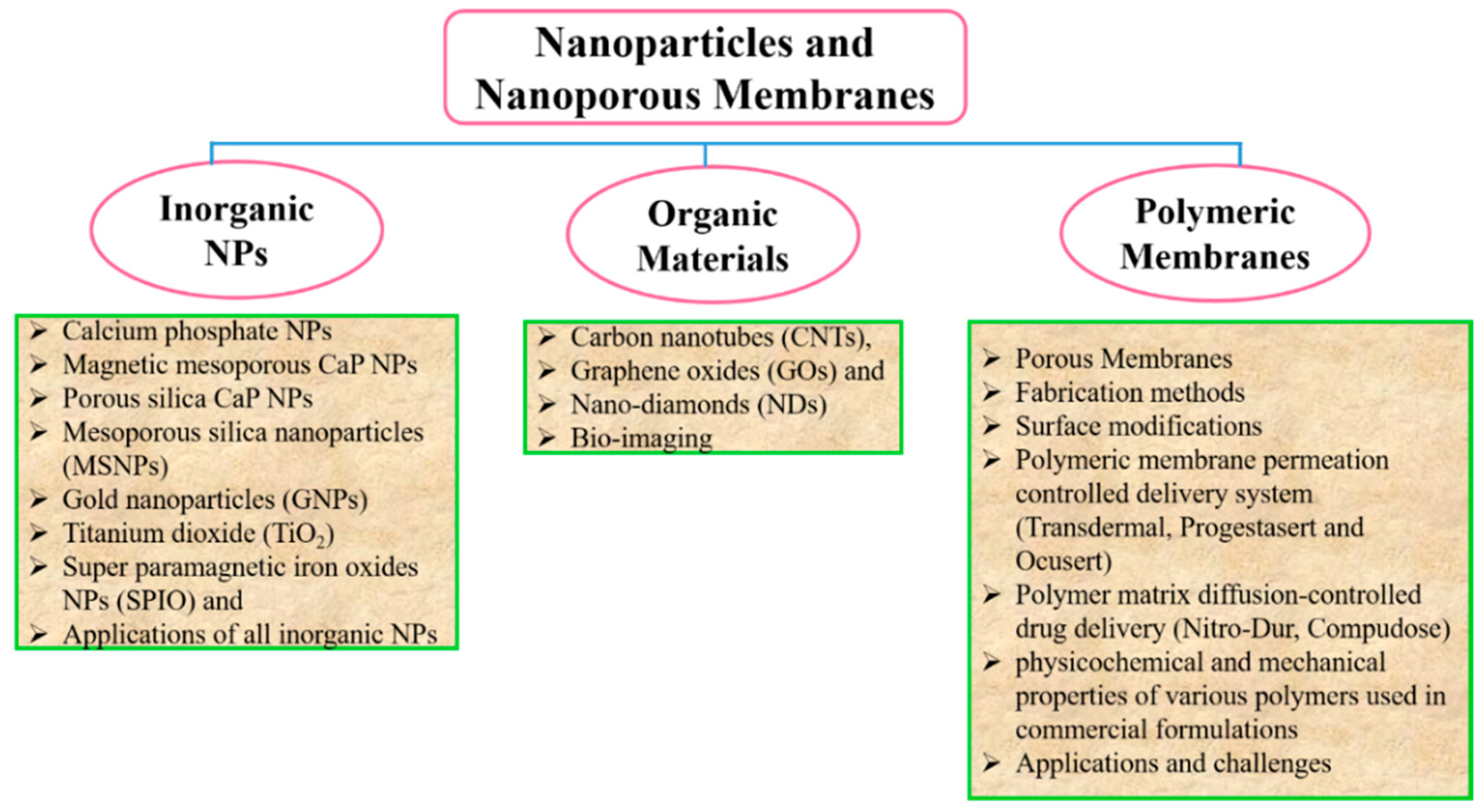
:1. Introduction
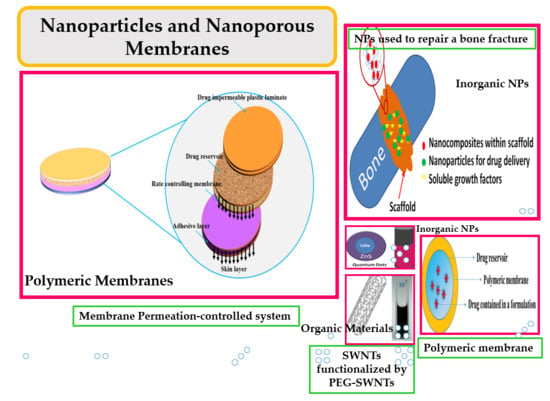
1.1. Why Nanoparticles?
1.2. Why a Nanoporous Membrane?
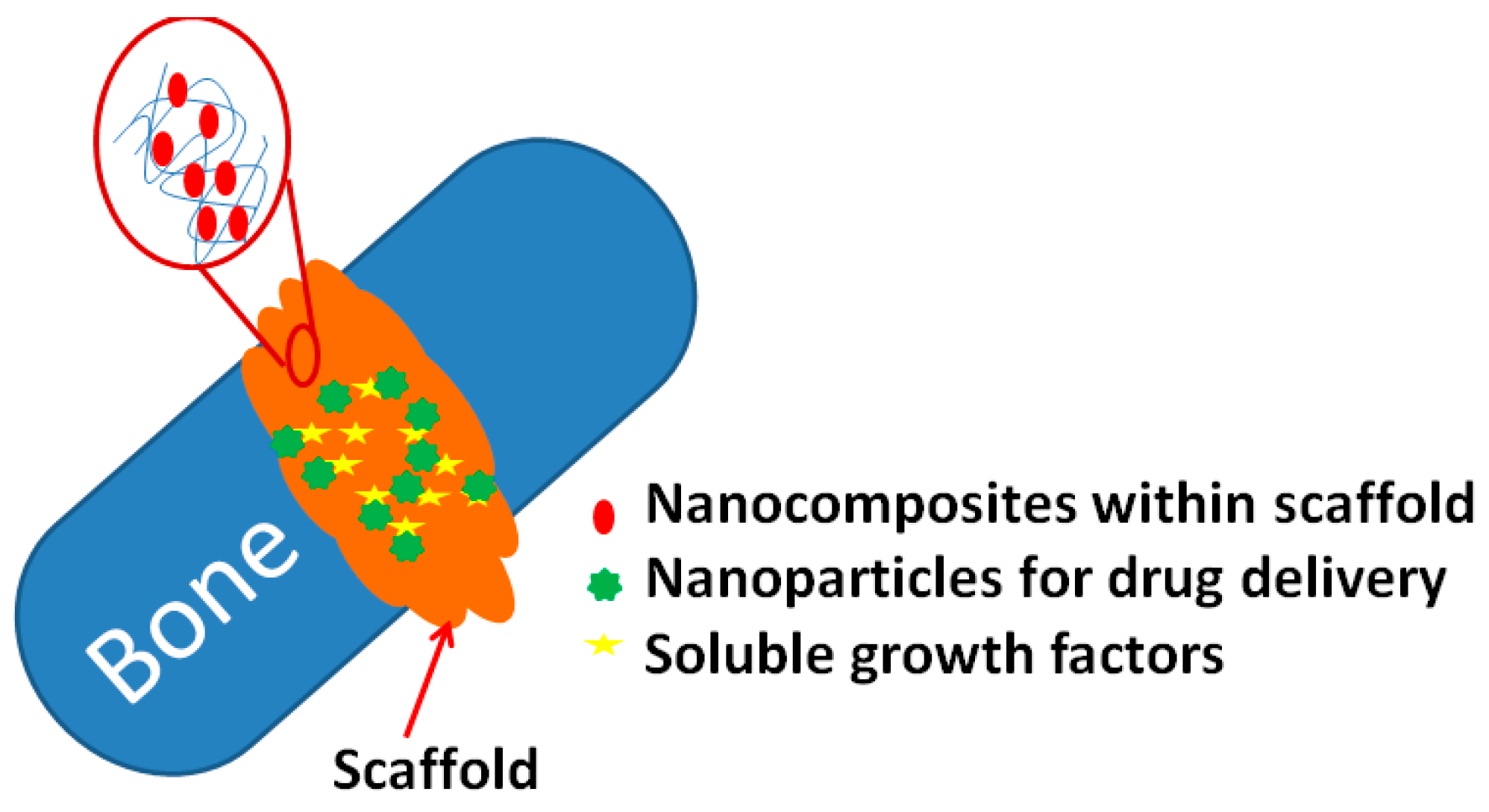
2. Drug Delivery System
2.1. Calcium Phosphate NPs
2.1.1. Magnetic Mesoporous CaP NPs
2.1.2. Porous Silica CaP NPs
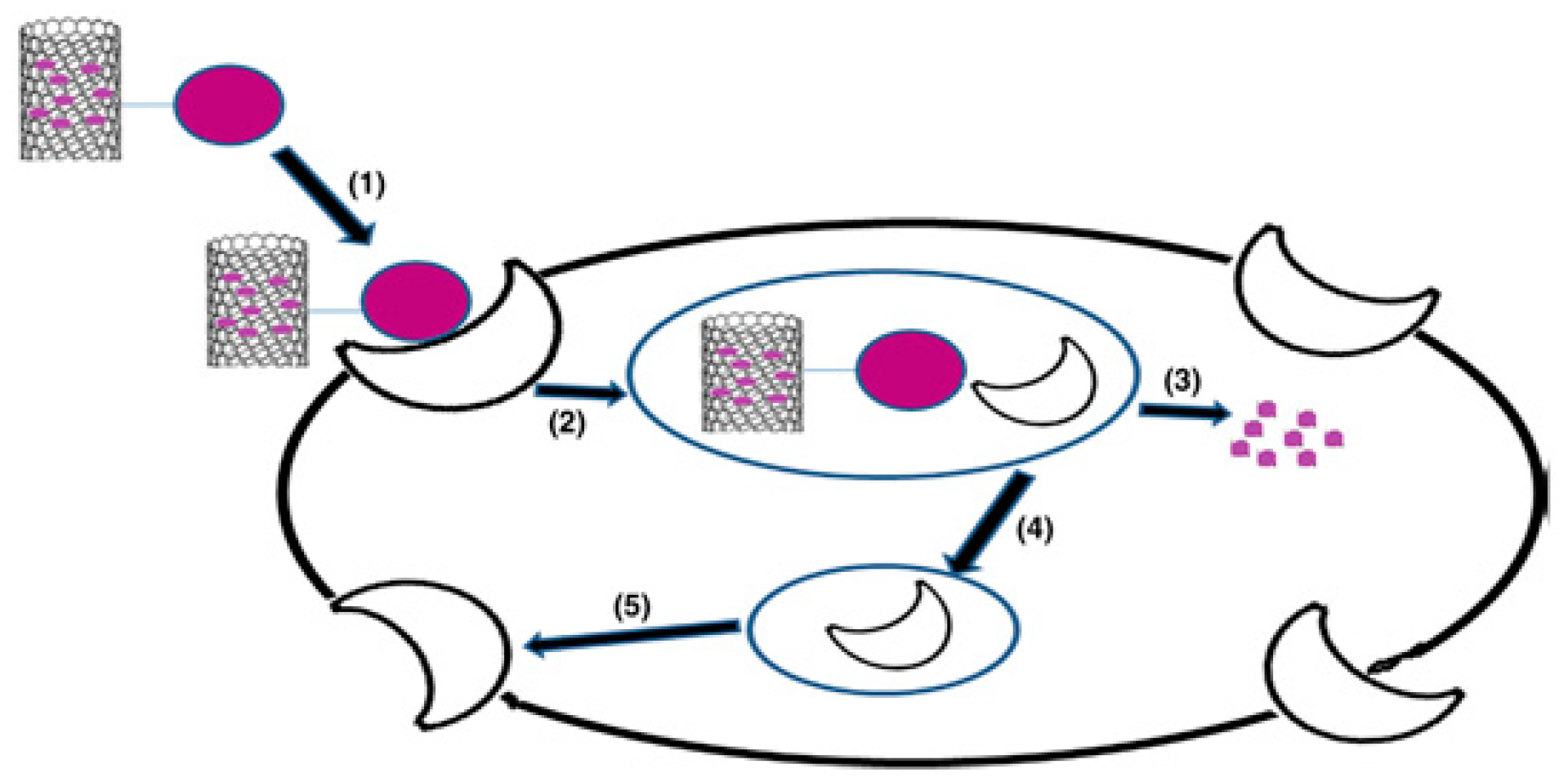
2.2. Carbon-Based Nanomaterials
2.2.1. Carbon Nanotubes
2.2.2. Graphene Oxide
2.2.3. Nano-Diamond
2.3. Mesoporous Silica Nanoparticles (MSNPs)
2.4. Gold Nanoparticles (GNPs)
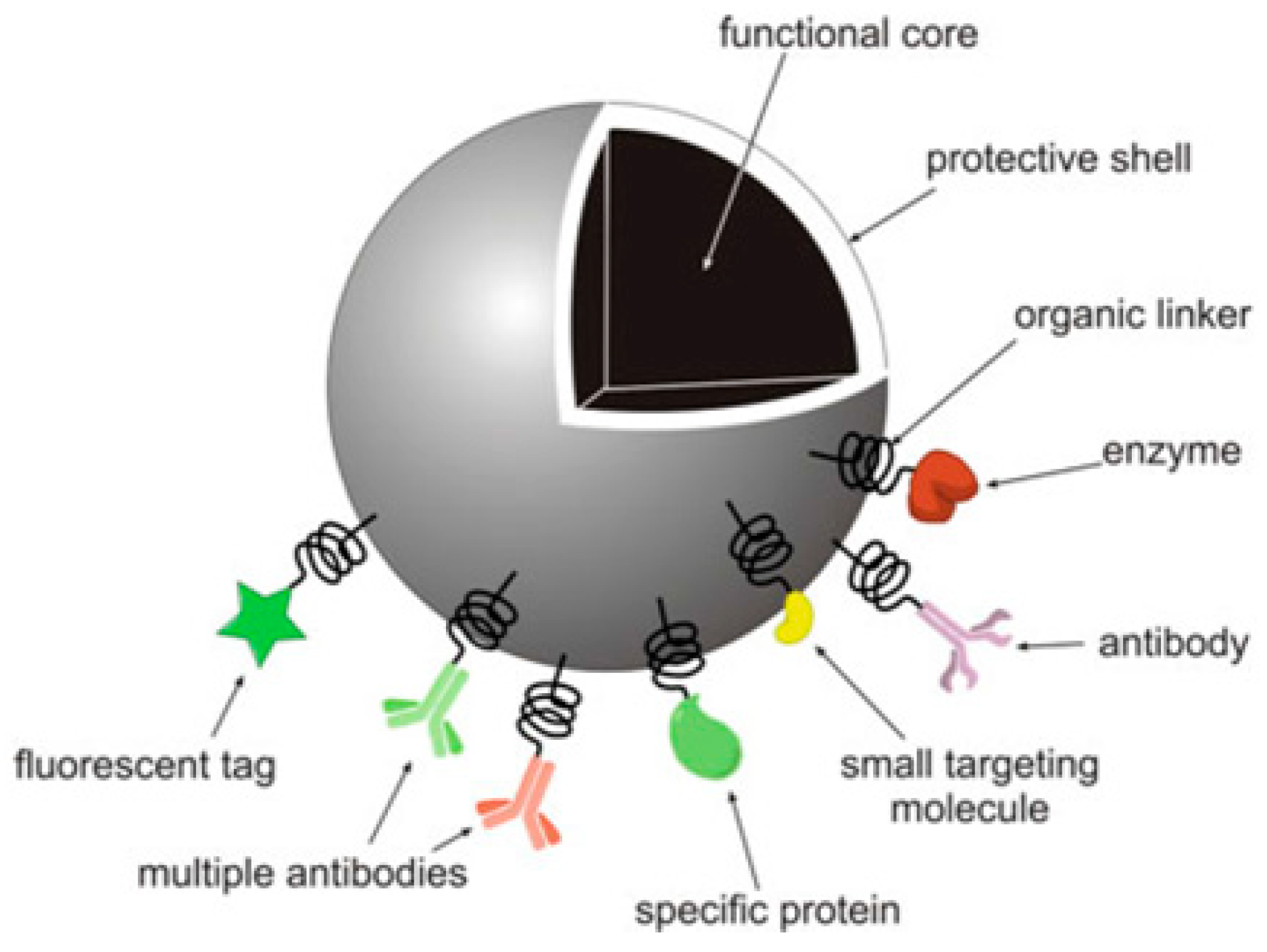
2.5. Other NPs Used as Drug Delivery Carriers
3. Inorganic NPs for Hard Tissue Regeneration
3.1. Carbon Nanotubes
3.2. Titanium Dioxide (TiO2)
4. Bio-Imaging
4.1. Quantum Dots
4.2. Carbon Nanotubes
4.3. Super Paramagnetic Iron Oxides NPs
4.4. Gold NPs
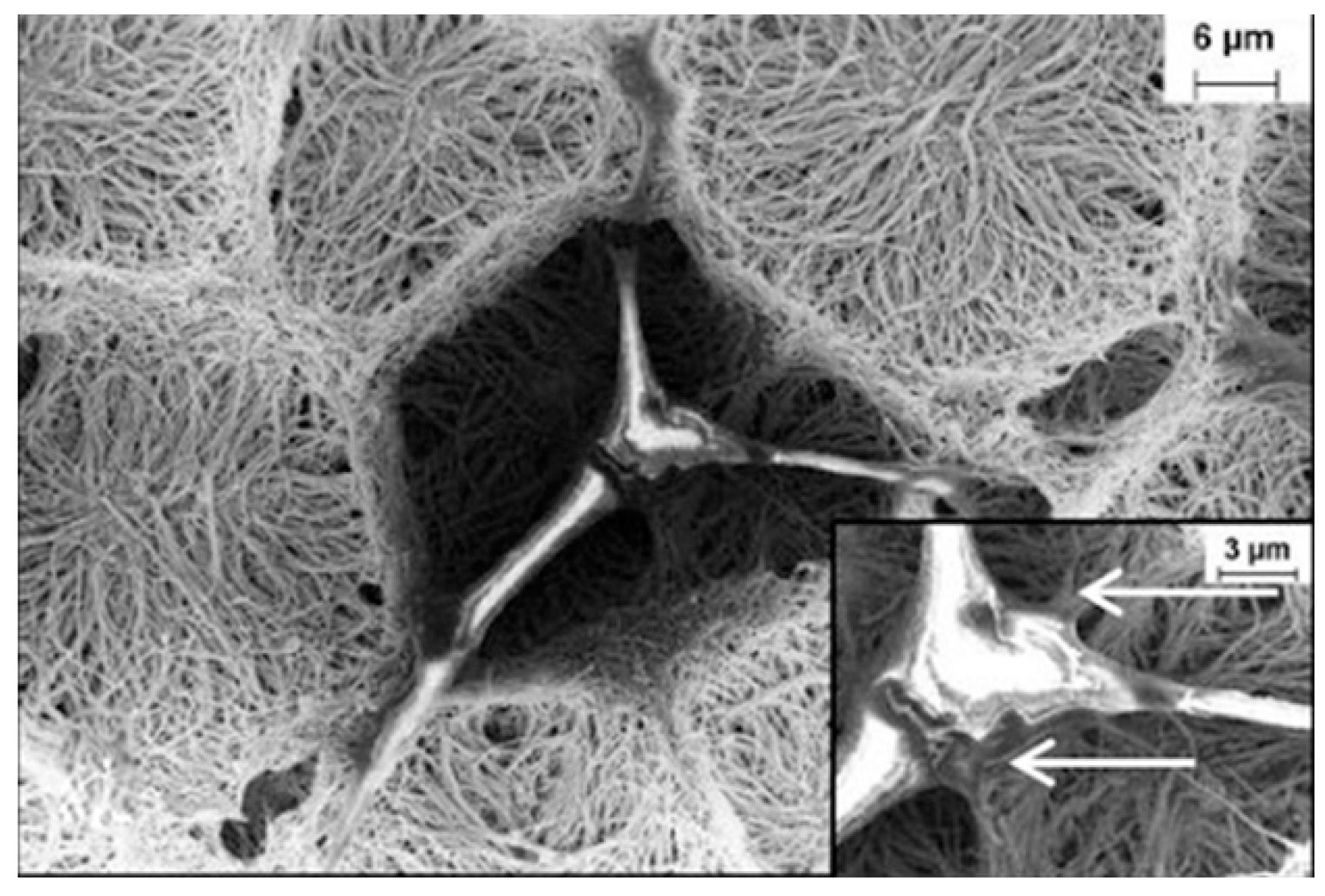
5. Porous Membranes
5.1. Membrane Classifications
5.1.1. Material
5.1.2. Size, Shape, and Order of Pores
- Optimum pore size with narrow distribution;
- Less flow resistance to allow high flux;
- Sufficient mechanical strength with adequate chemical and thermal stability, and;
- Biocompatibility.
5.1.3. Fabrication Method
Ion-Track Etching
Micromolding
Lithography
Focused Ion Beam Etching
Electrochemical Etching
5.1.4. Surface Modifications
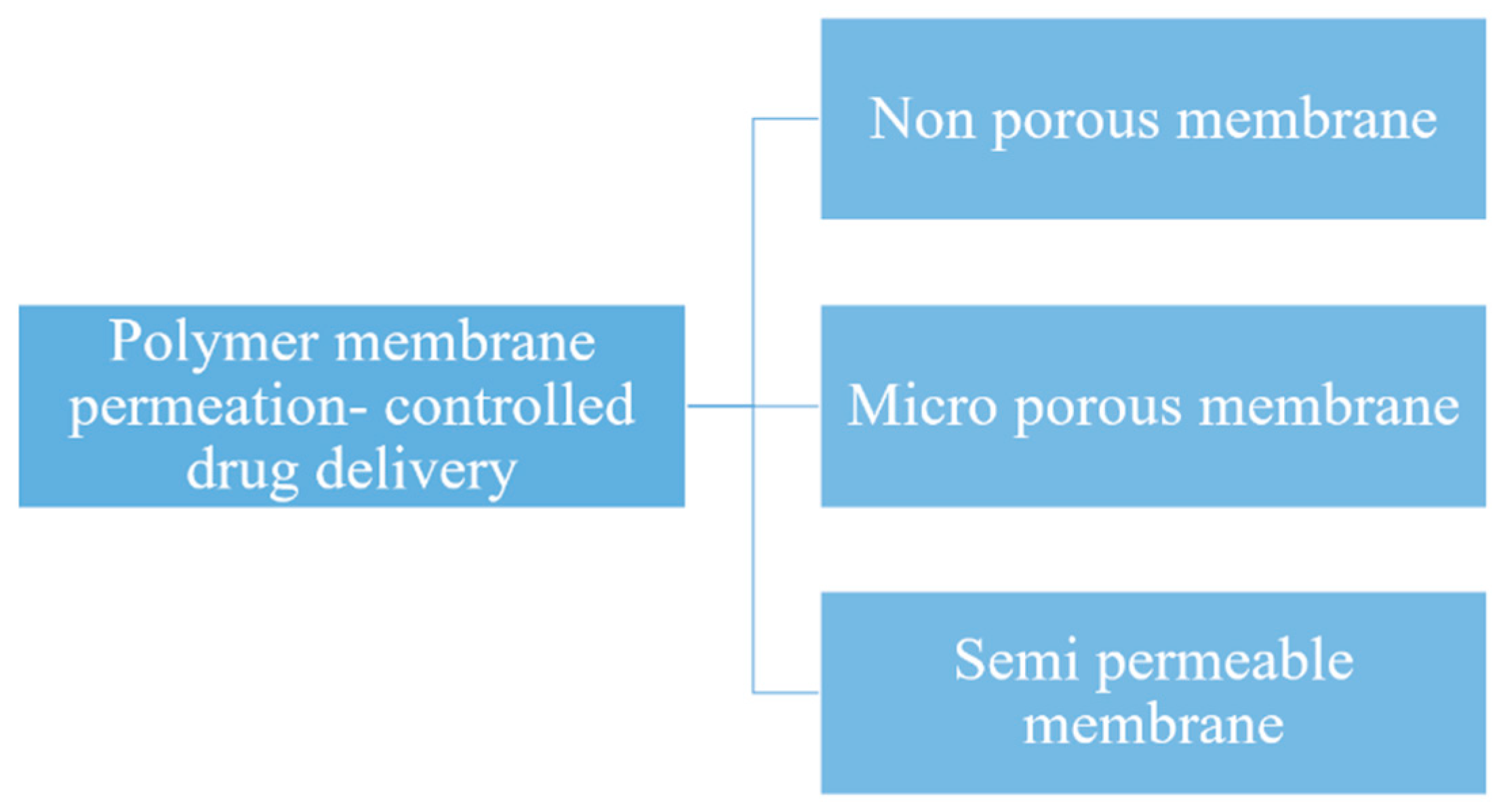
5.2. Drug Delivery by Membranes
5.2.1. Polymeric Membranes
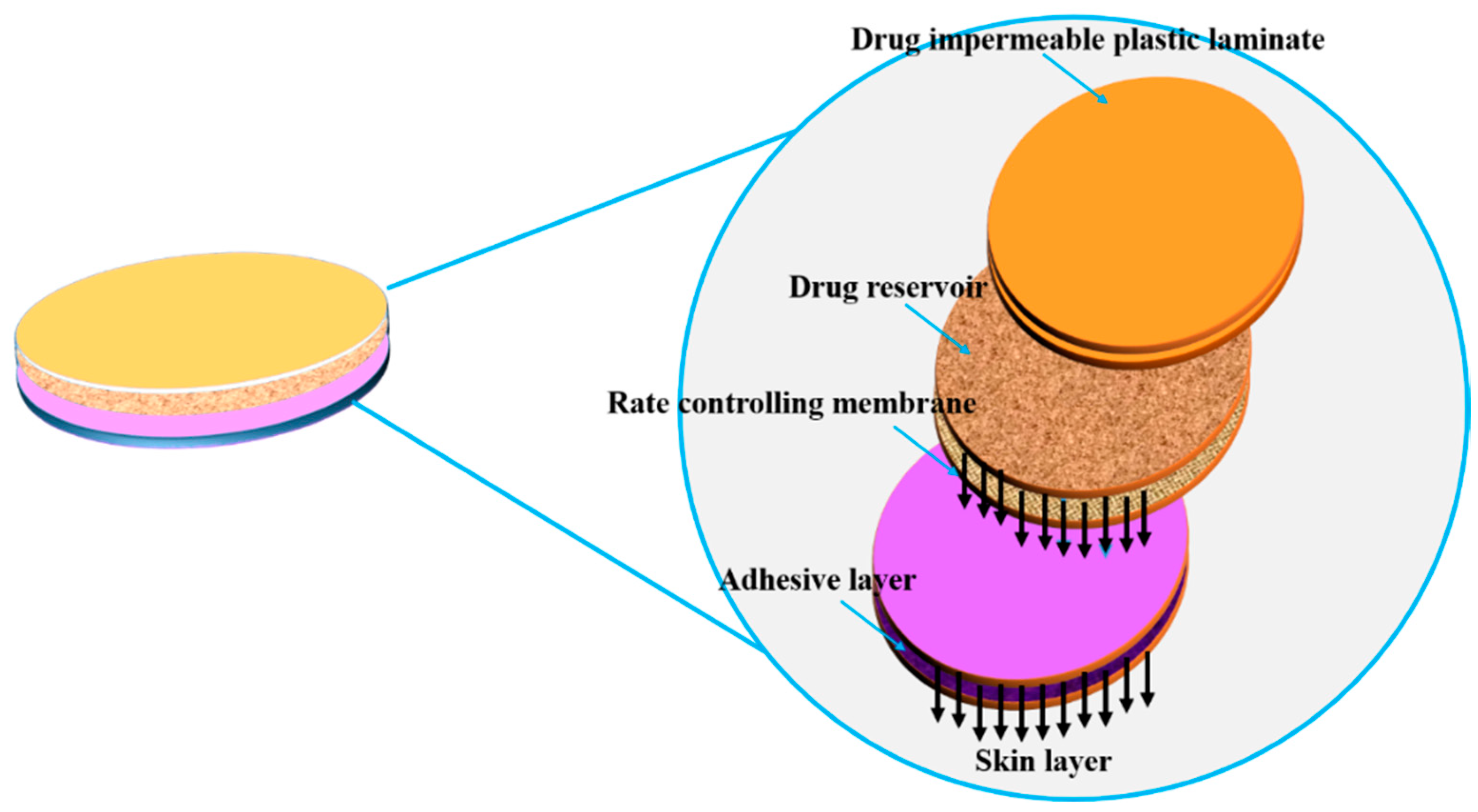
5.2.2. Transdermal Systems
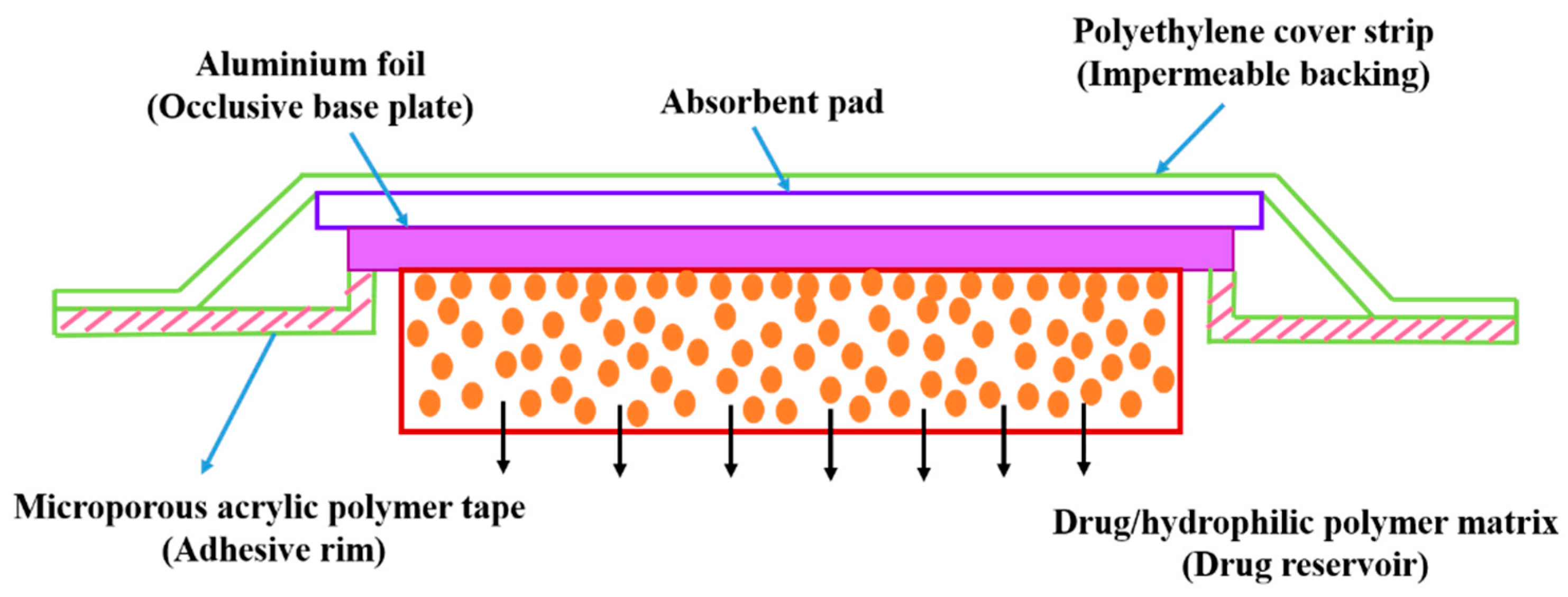
5.2.3. Ocusert
5.2.4. Progestasert
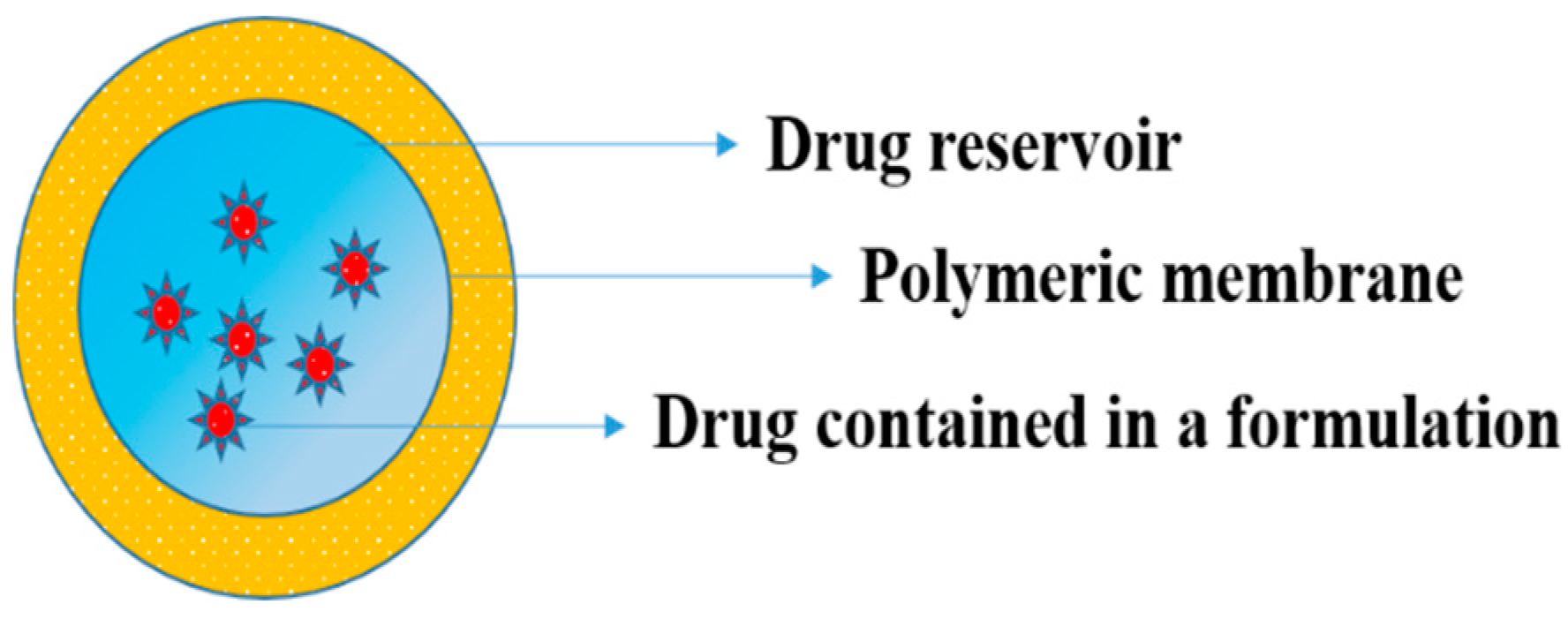
5.2.5. Polymer Matrix Diffusion-Controlled Drug Delivery
- Loading dose;
- Polymer solubility of the drug, and;
- Diffusivity in the polymer matrix.
5.2.6. Nitro-Dur System
5.2.7. Compudose
5.2.8. Polymeric Membrane-Based Drug Delivery Systems as Commercialized Formulations
5.3. Future Challenges
6. Conclusions
Funding
Conflicts of Interest
References
- Kim, K.; Fisher, J.P. Nanoparticle technology in bone tissue engineering. J. Drug Target. 2007, 15, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Uludag, H. Nanoparticulate Systems for Growth Factor Delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.P. Biointegration of Medical Implant. Materials; Science and Design, Inorganic Nanoparticles for Targeted Drug Delivery; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Zhang, R.; Olin, H. Carbon nanomaterials as drug carriers: Real time drug release investigation. Mater. Sci. Eng. C 2012, 32, 1247–1252. [Google Scholar] [CrossRef]
- Öchsner, A.; Shokuhfar, A. Novel Principles and Techniques, (Inorganic–Organic Hybrid Nanoparticles for Medical Applications. In New Frontiers of Nanoparticles and Nanocomposite Materials; Olariu, C.I., Yiu, H.P., Bouffier, L., Eds.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Javad, S.; Zohre, Z. Advanced drug delivery systems: Nanotechnology of health design A review. J. Saudi Chem. Soc. 2014, 18, 85–99. [Google Scholar]
- El Ghannam, A.; Ricci, K.; Malkawi, A.; Jahed, K.; Vedantham, K.; Wyan, H.; Allen, L.D.; Dréau, D. A ceramic-based anticancer drug delivery system to treat breast cancer. J. Mater. Sci. Mater. Med. 2010, 21, 2701–2710. [Google Scholar] [CrossRef]
- Uskoković, V.; Batarni, S.S.; Schweicher, J.; King, A.; Desai, T.A. The Effect of Calcium Phosphate Particle Shape and Size on their Antibacterial and Osteogenic Activity in the Delivery of Antibiotics in vitro. ACS Appl. Mater. Interfaces 2013, 5, 2422–2431. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, A.; Teja, B.V.; Pandey, G.; Mittapelly, N.; Trivedi, R.; Mishra, P. An insight into functionalized calcium based inorganic nanomaterials in biomedicine: Trends and transitions. Colloids Surf. B Biointerfaces 2015, 133, 120–139. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y.J.; Zhang, K.H.; Wu, J.; Wang, K.W.; Tang, Q.L.; Mo, X.M. Europium-doped amorphous calcium phosphate porous nanospheres: Preparation and application as luminescent drug carriers. Nanoscale Res. Lett. 2011, 6, 67–76. [Google Scholar] [CrossRef]
- Kester, M.; Heakal, Y.; Fox, T.; Sharma, A.; Robertson, G.P.; Morgan, T.T.; Altinoğlu, E.I.; Tabaković, A.; Parette, M.R.; Rouse, S.M.; et al. Calcium Phosphate Nanocomposite Particles for In Vitro Imaging and Encapsulated Chemotherapeutic Drug Delivery to Cancer Cells. Nano Lett. 2008, 8, 4116–4121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastakoti, B.P.; Hsu, Y.C.; Liao, S.H.; Wu, K.C.W.; Inoue, M.; Yusa, S.I.; Nakashima, K.; Yamauchi, Y. Inorganic-Organic Hybrid Nanoparticles with Biocompatible Calcium Phosphate Thin Shells for Fluorescence Enhancement. Chem. Asian J. 2013, 8, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Shinto, Y.; Uchida, A.; Korkusuz, F.; Araki, N.; Ono, K. Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. J. Bone Jt. Surg. Br. 1992, 74, 600–604. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhu, Y.J.; Chen, F.; Lu, B.Q.; Qi, C.; Zhao, J.; Wu, J. Calcium Phosphate Hybrid Nanoparticles: Self-Assembly Formation, Characterization, and Application as an Anticancer Drug Nanocarrier. Chem. Asian J. 2013, 8, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, U.; Kulkarni, V.; Tushar, R.; Murthy, R.S.R. Methotrexate Loaded Self Stabilized Calcium Phosphate Nanoparticles: A Novel Inorganic Carrier for Intracellular Drug Delivery. J. Biomed. Nanotechnol. 2009, 5, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zhao, D.; Wang, C.Q.; Zong, J.Y.; Zhuo, R.X.; Cheng, S.X. Facile preparation of heparin/CaCO3/CaP hybrid nano-carriers with controllable size for anticancer drug delivery. Colloids Surf. B Biointerfaces 2013, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.R.; Behera, B.; Maiti, T.K.; Mohapatra, S. Multifunctional magnetic calcium phosphate nanoparticles for targeted platin delivery. Dalton Trans. 2012, 41, 10777–10783. [Google Scholar] [CrossRef]
- Meihua, Y.; Siddharth, J.; Peter, T.; Jiezhong, C.; Wenyi, G.; Chengzhong, Y. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanomaterials 2013, 5, 178–183. [Google Scholar]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, Y.Y.; Zhang, Q.; Xie, H.H.; Qian, W.Z.; Wei, F. Growth of Half-Meter Long Carbon Nanotubes Based on Schulz–Flory Distribution. ACS Nano 2013, 7, 6156–6161. [Google Scholar] [CrossRef]
- Im, O.; Li, J.; Wang, M.; Zhang, L.G.; Keidar, M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int. J. Nanomed. 2012, 7, 2087–2099. [Google Scholar] [Green Version]
- Pastorin, G.; Wu, W.; Wieckowski, S.; Briand, J.P.; Kostarelos, K.; Prato, M.; Bianco, A. Double functionalisation of carbon nanotubes for multimodel drug delivery. Chem. Commun. 2006, 11, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Bafkary, R.; Fayyazi, F. DOX delivery based on chitosan-capped graphene oxide mesoporous silica nanohybride as pH-responsive nanocarriers. J. Sol. Gel. Sci. Technol. 2017, 81, 493–504. [Google Scholar] [CrossRef]
- Ren, J.; Shen, S.; Wang, D.; Xi, Z.; Guo, L.; Pang, Z.; Qian, Y.; Sun, X.; Jiang, X. The Targeted Delivery of Anticancer Drugs to Brain Glioma by PEGylated Oxidized Multi-Walled Carbon Nanotubes Modified with Angiopep-2. Biomaterials 2012, 33, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, X.; Hu, N.; Tam, U.C.; Blixt, O.; Zettl, A.; Bertozzi, C.R. Biocompatible Carbon Nanotubes Generated by Functionalization with Glycodendrimers. Angew. Chem. Int. Ed. Engl. 2008, 47, 5022–5025. [Google Scholar] [CrossRef]
- Bhatt, A.; Jain, A.; Gurnany, E.; Jain, R.; Modi, A.; Jain, A. Carbon Nanotubes: A Promising Carrier for Drug Delivery and Targeting. In Nanoarchitectonics for Smart Delivery and Drug Targeting, 1st ed.; William Andrew: Norwich, NY, USA, 2016; pp. 465–501. [Google Scholar]
- Chauhan, G.; Chopra, V.; Tyagi, A.; Rath, G.; Sharma, K.; Goyal, K. Gold nanoparticles composite-Folic Acid Conjugated Graphene Oxide Nanohybrids for Targeted Chemo-Thermal Cancer Ablation: In-vitro Screening and In-vivo Studies. Eur. J. Pharm. Sci. 2017, 1, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.S.; Lu, Y.J.; Chen, J.P. Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J. Magn. Magn. Mater. 2017, 427, 34–40. [Google Scholar] [CrossRef]
- Barahuie, F.; Saifullah, B.; Dorniani, D.; Fakurazi, S.; Karthivashan, G.; Hussein, M.Z.; Elfghi, F.M. Graphene oxide as a nanocarrier for controlled release and targeted delivery of an anticancer active agent, chlorogenic acid. Mater. Sci. Eng. C 2107, 74, 177–185. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Qin, X.; Guo, Z.; Liu, Z.; Zhang, W.; Wan, M.; Yang, B. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, Z.; Zhao, Z.; Miao, Y.; Qiu, Y.; Yang, W.; Jia, X.; Liu, Z.; Hou, H. Design and Development of Graphene Oxide Nanoparticle/Chitosan Hybrids Showing pH-Sensitive Surface Charge-Reversible Ability for Efficient Intracellular Doxorubicin Delivery. ACS Appl. Mater. Interfaces 2018, 10, 6608–6617. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nano-Graphene Oxide for Delivery of Water Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Masoudipour, E.; Kashanian, S.; Maleki, N. A targeted drug delivery system based on dopamine functionalized nano graphene oxide. Chem. Phys. Lett. 2017, 668, 56–63. [Google Scholar] [CrossRef]
- Khalid, M.E. Nanodiamond as a drug delivery system: Applications and prospective. J. Appl. Pharm. Sci. 2011, 1, 29–39. [Google Scholar]
- Mochalin, V.; Olga, S.; Dean, H.; Yury, G. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Kang, M.W.; Chang, H.C.; Chen, K.M.; Yu, Y.C. Bright fluorescent NDs: No photobleaching and low cytotoxicity. J. Am. Chem. Soc. 2005, 127, 17604–17605. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Pierstorff, E.D.; Lam, R.; Li, S.Y.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-Mediated Delivery of Water-Insoluble Therapeutics. ACS Nano 2009, 3, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Amanda, M.; Suzanne, A.; Ciftan, H.; Olga, A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar]
- Upal, R.; Vadym, D.; Andriy, D.; Jesse, R.; Paul, B.; Venkata, A.; Liu, X.; Thomas, G.; Surendra, S.; Madhavan, N. Characterization of Nanodiamond based anti-HIV drug Delivery to the Brain. Sci. Rep. 2018, 8, 1603–1615. [Google Scholar]
- Slowing, I.; Viveroescoto, J.; Wu, C.; Lin, V. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kaushik, A.; Jayant, R.; Nair, M. Nanomaterials for Drug Delivery. In Advances in Personalized Nanotherapeutics; Springer: Berlin, Germany, 2017; pp. 57–77. [Google Scholar]
- Slowing, I.; Trewyn, B.; Victor, S.Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silicaon the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Sabu, T.; Yves, G.; Neethu, N.; Parvathy, R.C.; Reny, T.T. Nanotechnology Applications for Tissue Engineering; gold nanoparticles in cancer drug delivery. Elsevier Inc. 2015, 13, 5637–5655. [Google Scholar]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Daniel, W.L.; Giljohann, D.A.; Mirkin, C.A.; Lippard, S.J. Polyvalent Oligonucleotide Gold Nanoparticle Conjugates as Delivery Vehicles for Platinum(IV) Warheads. J. Am. Chem. Soc. 2009, 131, 14652–14653. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tsai, C.Y.; Huang, P.Y.; Chang, M.Y.; Cheng, P.C.; Chou, C.H.; Chen, D.H.; Wang, C.R.; Shiau, A.L.; Wu, C.L. Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Oh, K.S.; Park, D.Y.; Lee, J.Y.; Lee, B.S.; Kim, I.S.; Kim, K.; Kwon, I.C.; Sang, Y.K.; Yuk, S.H. Doxorubicin/gold-loaded core/shell nanoparticles for combination therapy to treat cancer through the enhanced tumor targeting. J. Control. Release 2016, 228, 141–149. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.O.; Lim, I.G.; Oh, J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Biol. Macromol. 2016, 91, 578–588. [Google Scholar] [CrossRef]
- Dhamechaa, D.; Jalalpure, S.; Jadhav, K.; Jagwani, S.; Chavan, R. Doxorubicin loaded gold nanoparticles: Implication of passive targeting on anticancer efficacy. Pharmacol. Res. 2016, 113, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Perin, E.; Gabano, E.; Zanellato, I.; Panzarasa, G.; Sparnacci, K.; Laus, M.; Osella, D. Functional fluorescent nonporous silica nanoparticles as carriers for Pt(IV) anticancer prodrugs. J. Inorg. Biochem. 2015, 151, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajmi, M.F.; Hussain, A.; Ahmed, F. Novel synthesis of ZnO nanoparticles and their enhanced anticancer activity: Role of ZnO as a drug carrier. Ceram. Int. 2016, 42, 4462–4469. [Google Scholar] [CrossRef]
- Vajtai, R. Springer Handbook of Nanomaterials; Springer: Berlin, Germany, 2013. [Google Scholar]
- Sasaki, N.; Sudoh, Y. X-ray pole figure analysis of apatite crystals and collagen molecules in bone. Calcif. Tissue Int. 1997, 60, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Webster, T.J. Nanotechnology and biomaterials for orthopaedic medical applications. Nanomedicine 2006, 1, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ahn, E.S. Nanostructured biomaterials for tissue engineering bone. Adv. Biochem. Eng. Biotechnol. 2007, 103, 275–308. [Google Scholar]
- Zhang, Z.G.; Li, Z.H.; Mao, X.Z.; Wang, W.C. Advances in bone repair with nanobiomaterials: Mini-review. Cytotechnology 2011, 63, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Nanotechnology for bone materials. WIREs Nanomed. Nanobiotech. 2009, 1, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Marquis, M.E.; Lord, E.; Bergeron, E.; Drevelle, O.; Park, H.; Cabana, F.; Senta, H.; Faucheux, N. Bone cells-biomaterials interactions. Front. Biosci. 2009, 14, 1023–1067. [Google Scholar] [CrossRef]
- Scheller, E.L.; Krebsbach, P.H.; Kohn, D.H. Tissue engineering: State of the art in oral rehabilitation. J. Oral Rehabil. 2009, 36, 368–389. [Google Scholar] [CrossRef]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J. Biomed. Mater. Res. A 2008, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kwok, C.T. Hydroxyapatite-anatase-carbon nanotube nanocomposite coatings fabricated by electrophoretic codeposition for biomedical applications. J. Mater. Sci. Mater. Med. 2011, 22, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Kealley, C.; Elcombe, M.; Van Riessen, A.; Ben-Nissan, B. Development of carbon nanotube-reinforced hydroxyapatite bioceramics. Phys. B Condens. Matter 2006, 385, 496–498. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Yao, C.; Xiao, X.; Sheldon, B.W.; Webster, T.J. Greater osteoblast functions on multiwalled carbon nanotubes grown from anodized nanotubular titanium for orthopedic applications. Nanotechnology 2007, 18, 365102. [Google Scholar] [CrossRef]
- Abrishamchian, A.; Hooshmand, T.; Mohammadi, M.; Najafi, F. Preparation and characterization of multi-walled carbon nanotube/hydroxyapatite nanocomposite film dip coated on Ti–6Al–4V by sol–gel method for biomedical applications: An in vitro study. Mater. Sci. Eng. C 2013, 33, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Facca, S.; Lahiri, D.; Fioretti, F.; Messadeq, N.; Mainard, D.; Benkirane-Jessel, N.; Agarwal, A. In Vivo Osseointegration of Nano-Designed Composite Coatings on Titanium Implants. ACS Nano 2011, 5, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Balani, K.; Agarwal, A. Wetting of carbon nanotubes by aluminum oxide. Nanotechnology 2008, 19, 165701. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Wagner, N.; Rojas Chapana, J.; Morsczeck, C.; Thie, M.; Giersig, M. Fabrication and Biocompatibility of Carbon Nanotube-Based 3D Networks as Scaffolds for Cell Seeding and Growth. Nano Lett. 2004, 4, 2233–2236. [Google Scholar] [CrossRef]
- Abarrategi, A.; Gutierrez, M.C.; Moreno Vicente, C.; Hortiguela, M.J.; Ramos, V.; Lopez Lacomba, J.L.; Ferrer, M.L.; Del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef]
- Huisheng, P.; Qingwen, L.; Tao, C. Industrial Applications of Carbon Nanotubes; Xue, Y., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Keefer, E.W.; Botterman, B.R.; Romero, M.I.; Rossi, A.F.; Gross, G.W. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 2008, 3, 434–439. [Google Scholar] [CrossRef]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials. 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Li, H.; Khor, K.; Cheang, P.; Khor, K. Impact formation and microstructure characterization of thermal sprayed hydroxyapatite/titania composite coatings. Biomaterials 2003, 24, 949–957. [Google Scholar] [CrossRef]
- Siddharthan, A.; Sampath, K.S.; Seshadri, S.K. In situ composite coating of titania hydroxyapatite on commercially pure titanium by microwave processing. Surf. Coat. Technol. 2010, 204, 1755–1763. [Google Scholar] [CrossRef]
- Kuwabara, A.; Hori, N.; Sawada, T.; Hoshi, N.; Watazu, A.; Kimoto, K. Enhanced biological responses of a hydroxyapatite/TiO2 hybrid structure when surface electric charge is controlled using radiofrequency sputtering. Dent. Mater. J. 2012, 31, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.H.; Aglan, H.A.; Mabrouk, M.; Abd Rabou, A.A.; Beherei, H.H. Enhanced mesenchymal stem cell proliferation through complexation of selenium/titanium nanocomposites. J. Mater. Sci. Mater. Med. 2019, 30, 24. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, S.; Nikpour, M.R.; Amani, A.; Soltani, M.; Rabiee, S.M.; Rezayat, S.M.; Chen, P.; Jahanshahi, M. Bone regeneration based on nanohydroxyapatite and hydroxyapatite/chitosan nanocomposites: An in-vitro and in-vivo comparative study. J. Nanopart. Res. 2013, 15, 1373–1389. [Google Scholar] [CrossRef]
- Si, Y. Fluorescent Nanomaterials for Bioimaging and Biosensing: Application on E.coli Bacteria. PhD Thesis, École normale supérieure de Cachan—ENS Cachan, Paris, France, 2015. [Google Scholar]
- Cai, W.; Shin, D.W.; Chen, K.; Gheysens, O.; Cao, Q.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-Labeled Near-Infrared Quantum Dots for Imaging Tumor Vasculature in Living Subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef]
- Keren, S.; Zavaleta, C.; Cheng, Z.; De La Zerda, A.; Gheysens, O.; Gambhir, S.S. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 5844–5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulder, W.J.; Castermans, K.; Van Beijnum, J.R.; Oude Egbrink, M.G.; Chin, P.T.; Fayad, Z.A.; Löwik, C.W.; Kaijzel, E.L.; Que, I.; Storm, G.; et al. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis 2009, 12, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Z.B.; Wang, H.; Cai, W.; Chen, X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2235–2244. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.T.; Hong, D.; Indrajit, R.; Wing Cheung, L.; Earl, J.; Anirban, M.; Paras, N. Imaging Pancreatic Cancer Using BioconjugatedInP Quantum Dots. ACS Nano 2009, 3, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.B.; Chen, X.Y. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat. Protoc. 2008, 3, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cao, X.; Hou, W.; Zheng, L.; Peng, C.; Xiao, T.; Guo, R.; Shen, M.; Zhang, G.; Shi, X. Facile formation of dendrimer-stabilized gold nanoparticles modified with diatrizoic acid for enhanced computed tomography imaging applications. Nanoscale 2012, 4, 6768. [Google Scholar]
- Kravets, V.; Almemar, Z.; Jiang, K.; Culhane, K.; Machado, R.; Hagen, G.; Kotko, A.; Dmytruk, I.; Spendier, K.; Pinchuk, A. Imaging of Biological Cells Using Luminescent Silver Nanoparticles. Nanoscale Res. Lett. 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, M.; Douma, K.; Hackeng, T.M.; Dirksen, A.; Post, M.J.; Van Zandvoort, M.A.; Backes, W.H. Quantitative Molecular Magnetic Resonance Imaging of Tumor Angiogenesis Using cNGR-Labeled Paramagnetic Quantum Dots. Cancer Res. 2008, 68, 7676–7683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Jugold, M.; Woenne, E.C.; Lammers, T.; Morgenstern, B.; Mueller, M.M.; Zentgraf, H.; Bock, M.; Eisenhut, M.; Semmler, W.; et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultra small superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007, 15, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- CaSeo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2007, 5, 971–976. [Google Scholar]
- Liu, Z.; Li, X.; Tabakman, S.M.; Jiang, K.; Fan, S.; Dai, H. Multiplexed Multi-Color Raman Imaging of Live Cells with Isotopically Modified Single Walled Carbon Nanotubes. J. Am. Chem. Soc. 2008, 130, 13540–13541. [Google Scholar] [CrossRef]
- Smith, B.R.; Cheng, Z.; De, A.; Koh, A.L.; Sinclair, R.; Gambhir, S.S. Real-Time Intravital Imaging of RGD–Quantum Dot Binding to Luminal Endothelium in Mouse Tumor Neovasculature. Nano Lett. 2008, 8, 2599–2606. [Google Scholar] [CrossRef]
- Cai, W.; Chen, K.; Li, Z.B.; Gambhir, S.S.; Chen, X. Dual-Function Probe for PET and Near-Infrared Fluorescence Imaging of Tumor Vasculature. J. Nucl. Med. 2007, 48, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Rui, P. Inorganic nanomaterials for tumor angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 147–163. [Google Scholar]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug Delivery with Carbon Nanotubes for In vivo Cancer Treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Seyednejad, H.; Laurent, S.; Atyabi, F.; Saei, A.A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging. Contrast Media Mol. Imaging 2015, 10, 329–355. [Google Scholar] [CrossRef]
- Afsaneh, L.; Saeed, S.; Sophie, L.; Saeed, S. Dual nano-sized contrast agents in PET/MRI: A systematic review. Contrast Media Mol. Imaging 2016, 11, 428–447. [Google Scholar]
- Li, C.; Tao, C.; Ismail, O.; Guizhi, Z.; Emir, Y.; Mingxu, Y.; Cuichen, W.; Jing, Z.; Erqun, S.; Cheng, Z.; et al. Gold-Coated Fe3O4 Nanoroses with Five Unique Functions for Cancer Cell Targeting, Imaging, and Therapy. Adv. Funct. Mater. 2014, 26, 1772–1780. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, S.; Zhao, L.; Li, D.; Liu, C.; Jiang, W.; Gao, X.; Gu, W.; Ma, N.; Zhao, J.; et al. Ultra stable polyethyleneimine-stabilized gold nanoparticles modified with polyethylene glycol for blood pool, lymph node and tumor CT imaging. Nanoscale 2015, 8, 5567–5577. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.V.; Zhu, L.; Ifediba, M.A.; Zhang, Y.; Carr, K.; Moore, A.; Medarova, Z. Noninvasive MRI-SERS Imaging in Living Mice Using an Innately Bimodal Nanomaterial. ACS Nano 2011, 5, 1056–1066. [Google Scholar] [CrossRef]
- Wisniewski, N.; Reichert, M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B Biointerfaces 2000, 18, 197–219. [Google Scholar] [CrossRef]
- Desai, T.A.; Hansford, D.; Ferrari, M. Characterization of micromachined silicon membranes for immunoisolation and bioseparation applications. J. Memb. Sci. 1999, 159, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Max, L.G.Q.; Song, Z.X. Nanoporous Materials: Science and Engineering; World Scientific: Singapore, 2004; Volume 4, ISBN 178326179X. [Google Scholar]
- Langley, P.J.; Hulliger, J. Nanoporous and mesoporous organic structures: New openings for materials research. Chem. Soc. Rev. 1999, 28, 279–291. [Google Scholar] [CrossRef]
- Beherei, H.H.; Shaltout, A.A.; Mabrouk, M.; Abdelwahed, N.A.M.; Das, D.B. Influence of niobium pentoxide particulates on the properties of brushite/gelatin/alginate membranes. J. Pharm. Sci. 2018, 107, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, C.; Brüchle, W.; Spohr, R.; Vetter, J.; Angert, N. Pore geometry of etched ion tracks in polyimide. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 1996, 111, 70–74. [Google Scholar] [CrossRef]
- Tsuru, T. Inorganic porous membranes for liquid phase separation. Sep. Purif. Methods 2001, 30, 191–220. [Google Scholar] [CrossRef]
- Metz, S.; Trautmann, C.; Bertsch, A.; Renaud, P. Polyimide microfluidic devices with integrated nanoporous filtration areas manufactured by micromachining and ion track technology. J. Micro. Microeng. 2003, 14, 324. [Google Scholar] [CrossRef]
- Desai, T.A.; Chu, W.H.; Tu, J.K.; Beattie, G.M.; Hayek, A.; Ferrari, M. Microfabricated immunoisolatingbiocapsules. Biotechnol. Bioeng. 1998, 57, 118–120. [Google Scholar] [CrossRef]
- Desai, T.; Bhatia, S. Therapeutic Micro/Nano Technology; Springer: New York, NY, USA, 2006; Volume 1, ISBN 9780387255651. [Google Scholar]
- Tong, H.D.; Jansen, H.V.; Gadgil, V.J.; Bostan, C.G.; Berenschot, E.; van Rijn, C.J.M.; Elwenspoek, M. Silicon nitride nanosieve membrane. Nano Lett. 2004, 4, 283–287. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Hasegwa, F.; Ono, S. Self-ordering of cell arrangement of anodic porous alumina formed in sulfuric acid solution. J. Electrochem. Soc. 1997, 144, L127–L130. [Google Scholar] [CrossRef]
- Foll, H.; Christophersen, M.; Carstensen, J.; Hasse, G. Formation and application of porous silicon. Mater. Sci. Eng. R 2002, 39, 93–141. [Google Scholar] [CrossRef]
- Lewis, A.L. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf. B Biointerfaces 2000, 18, 261–275. [Google Scholar] [CrossRef]
- Harrison, D.J.; Turner, R.F.B.; Baltes, H.P. Characterization of Perfluorosulfonic Acid Polymer Coated Enzyme Electrodes and a Miniaturized Integrated Potentiostat for Glucose Analysis in Whole Blood. Anal. Chem. 1988, 60, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.A.; Hillmyer, M.A. Nanoporous membranes derived from block copolymers: From drug delivery to water filtration. ACS Nano 2010, 4, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.; Yang, S.Y.; Kim, J.K. Functional nanoporous membranes for drug delivery. J. Mater. Chem. 2012, 22, 14814–14834. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330. [Google Scholar] [CrossRef]
- Lee, J.H.; Kopecek, J.; Andrade, J.D. Protein-resistant surfaces prepared by PEO-containing block copolymer surfactants. J. Biomed. Mater. Res. 1989, 23, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Cosofret, V.V.; Ufer, S.; Buck, R.P.; Kao, W.J.; Neuman, M.R.; Anderson, J.M. Ion-selective membranes with low plasticizer content: Electroanalytical characterization and biocompatibility studies. J. Biomed. Mater. Res. 1994, 28, 591–601. [Google Scholar] [CrossRef]
- Morra, M. On the molecular basis of fouling resistance. J. Biomater. Sci. Polym. Ed. 2000, 11, 547–569. [Google Scholar] [CrossRef]
- McHugh, A.J. The role of polymer membrane formation in sustained release drug delivery systems. J. Control. Release 2005, 109, 211–221. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Papenburg, B.J.; Gironés, M.; Saiful, S.; Bettahalli, S.N.M.; Schmitmeier, S.; Wessling, M. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Memb. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.; Das, R.; Das, P.; Mukhopadhyay, A. Membrane Technology; Carbon Nanostructures, Wiley: Hoboken, NJ, USA, 2018; ISBN 9780080479385. [Google Scholar]
- Wick, S.M. Transdermal Nitroglycerin Delivery System. U.S. Patent 2017, 4,751,087, 14 June 1988. [Google Scholar]
- Merus Labs Luxco II S.à R.L. Estraderm MX 50 summary of product characteristics. 2019. Available online: https://www.medicines.org.uk/emc/product/5839/smpc (accessed on 27 January 2019).
- Pharmaceuticals, J. Duragesic (Fentanyl Transdermal System) for transdermal administration, summary of product charcteristics. 2016. Available online: https://www.fda.gov/…/fentanyl-transdermal-system-marketed-duragesic-information (accessed on 28 January 2019).
- Pharma, T. Androderm Transdermal Patch (New Zealand data sheet) 2018. Available online: https://www.nps.org.au/medicine-finder/androderm-transdermal-patch (accessed on 28 January 2019).
- Orosz, K.E.; Gupta, S.; Hassink, M.; Abdel-Rahman, M.; Moldovan, L.; Davidorf, F.H.; Moldovan, N.I. Delivery of antiangiogenic and antioxidant drugs of ophthalmic interest through a nanoporous inorganic filter. Mol. Vis. 2004, 10, 555–565. [Google Scholar] [PubMed]
- Pharriss, B.B.; Erickson, R.; Bashaw, J.; Hoff, S.; Place, V.A.; Zaffaroni, A. Progestasert: A Uterine Therapeutic System for Long-term Contraception: I. Philosophy and Clinical Efficacy. In Proceedings of the 29th Annual Meeting of The American Fertility Society, San Francisco, CA, USA, 5–7 April 1974. [Google Scholar]
- Pharmaceuticals, B.H. Levonorgestrel-releasing intrauterine system (Mirena). Data sheet 2008, Bayer Heal. Available online: https://www.mirena-us.com/about-miren (accessed on 27 January 2019).
- Chien, Y.W.; Lin, S. Drug Delivery: Controlled Release. In Encycl. Pharm. Technol, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1082–1103. [Google Scholar]
- Transdermal Infusion System summary of product characteristics. 2014. Available online: www.merck.com/product/patent/home.html%0ACopyright (accessed on 27 January 2019).
- Eiyo, T. Frandol tape Drug Information Sheet 2016. Available online: https://bciq.biocentury.com/products/frandol_tape_isosorbide_dinitrate (accessed on 27 January 2019).
- Gupta, S.K.; Okerholm, R.A.; Eller, M.; Wei, G.; Rolf, C.N.; Gorsline, J. Comparison of the pharmacokinetics of two nicotine transdermal systems: Nicoderm and habitrol. J. Clin. Pharmacol. 1995, 35, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Valeant MINITRAN- nitroglycerin patch summary of product characteristics, Dly. med 2019. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=15b3ea32-3422-4aa8-b4c2-41ee6d1aa566 (accessed on 28 January 2019).
- Place, V.A.; Nichols, K.C. Transdermal Delivery of Testosterone with TESTODERMTM to Provide a Normal Circadian Pattern of Testosterone. Ann. N. Y. Acad. Sci. 1991, 618, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ajithkumar, T.V.; Hatcher, H.M. Breast cancer. Spec. Train. Oncol. 2017, 5, 115–133. [Google Scholar]
- Preeti, K.; Jain, R.; Choukse, R.; Dubey, P.K.; Agrawal, S. Ocusert as A Novel Drug Delivery System. Int. J. Pharm. Biol. Arch. 2013, 4, 614–619. [Google Scholar]
- Ferguson, T.H.; Needham, G.F.; Wagner, J.F. Compudose: An implant system for growth promotion and feed efficiency in cattle. J. Control. Release 1988, 8, 45–54. [Google Scholar] [CrossRef]
- Labs, M.; Luxco, S. Deponit 5 summary of product characteristics. eMC 2019. Available online: http://www.datapharm.org.uk (accessed on 28 January 2019).
- Yang, W.W.; Pierstorff, E. Reservoir-based polymer drug delivery systems. J. Lab. Autom. 2012, 17, 50–58. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. BiosurfaceBiotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Macleod, A.M.; Campbell, M.; Cody, J.D.; Daly, C.; Donaldson, C.; Grant, A.; Khan, I.; Rabindranath, K.S.; Vale, L.; Wallace, S.; et al. Cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease. Cochrane Database Syst. Rev. 2005, 20, 20–42. [Google Scholar] [CrossRef]
- Pruitt, L.; Furmanski, J. Polymeric biomaterials for load-bearing medical devices. JOM 2009, 61, 1–24. [Google Scholar] [CrossRef]
- Jeon, G.; Yang, S.Y.; Byun, J.; Kim, J.K. Electrically actuatable smart nanoporous membrane for pulsatile drug release. Nano Lett. 2011, 11, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.N. Microbial Degradation of Polyurethane, Polyester Polyurethanes and Polyether Polyurethanes. Appl. Microbiol. Biotechnol. 1999, 51, 134–140. [Google Scholar] [CrossRef]
- Shenoy, D.; Little, S.; Langer, R.; Amiji, M. Poly (ethylene oxide)-modified poly (β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs In vitro evaluations. Mol. Pharm. 2005, 2, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Foll, H.; Christophersen, M.; Carstensen, J.; Hasse, G. Formation and application of porous silicon. Mater. Sci. Eng. R 2002, 280, 1–49. [Google Scholar] [CrossRef]
- Yang, Q.; Adrus, N.; Tomicki, F.; Ulbricht, M. Composites of functional polymeric hydrogels and porous membranes. J. Mater. Chem. 2011, 21, 2783–2811. [Google Scholar] [CrossRef]
- Groth, T.; Seifert, B.; Albrecht, W.; Malsch, G.; Gross, U.; Fey-Lamprecht, F.; Michanetzis, G.; Missirlis, Y.; Engbers, G. Development of polymer membranes with improved haemocompatibility for biohybrid organ technology. Clin. Hemorheol. Microcirc. 2005, 32, 129. [Google Scholar]
- Park, Y.J.; Nam, K.H.; Ha, S.J.; Pai, C.M.; Chung, C.P.; Lee, S.J. Porous poly(l-lactide) membranes for guided tissue regeneration and controlled drug delivery: Membrane fabrication and characterization. J.Control. Release 1997, 43, 151–160. [Google Scholar] [CrossRef]
- Causa, F.; Netti, P.A.; Ambrosio, L.; Ciapetti, G.; Baldini, N.; Pagani, S.; Martini, D.; Giunti, A. Poly-caprolactone/hydroxyapatite composites for bone regeneration: In vitro characterization and human osteoblast response. J. Biomed. Mater. Res. Part. A 2006, 76, 151–162. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kanamori, T.; Noumi, Y.; Wang, O.C.; Shinbo, T. Preparation of porous poly(d,l-lactide) and poly(d,l-lactide-co-glycolide) membranes by a phase inversion process and investigation of their morphological changes as cell culture scaffolds. J. Appl. Polym. Sci. 2004, 92, 2082–2092. [Google Scholar] [CrossRef]
- Vienken, J.; Diamantoglou, M.; Hahn, C.; Kamusewitz, H.; Paul, D. Considerations on developmental aspects of biocompatible dialysis membranes. Artif. Organs 1995, 19, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Uchiyama, S.; Kurita, K.; Morimoto, N.; Nakabayashi, N. A nonthrombogenic gas-permeable membrane composed of a phospholipid polymer skin film adhered to a polyethylene porous membrane. Biomaterials 2002, 23, 3421. [Google Scholar] [CrossRef]
- Queiroz, D.P.; Norberta de Pinho, M. Structural characteristics and gas permeation properties of polydimethylsiloxane/poly(propylene oxide) urethane/urea bi-soft segment membranes. Polymer 2005, 46, 2346. [Google Scholar] [CrossRef]









| Inorganic Carrier | Drug Loaded | Purpose | References |
|---|---|---|---|
| CaP | Docetaxel | Breast, lung, and ovarian cancer. | [8] |
| CaP, dopamine nanographene oxide (NGO) | Methotrexate | Anti-rheumatic drug, breast adenocarcinoma. | [38] |
| Heparin/CaCo/CaP | Doxorubicin hydrochloride | Breast, lung, bladder, stomach, and ovarian cancer, and leukaemia. | [18] |
| Porous silica CaP | 5-fluorouracil | Mammary tumors | [8,20] |
| NGO | SN38 | Colon cancer | [31,32,33,34,35,36] |
| Carboxylate NDs | Purvalanol and 4-hydroxytamoxitan | Liver and breast cancer | [40,41,42] |
| Single-walled carbon nanotubes (SWNTs)-polyethylene glycol (PEG), NGO-PEG and MSNPs-GO-chitosan (CHI) | Doxorubicin | Leukaemia, breast cancer, gastric cancer, head and neck cancer, Hodgkin’s lymphoma, liver cancer, kidney cancer, ovarian cancer, small cell lung cancer, soft tissue sarcoma, thyroid cancer, bladder cancer, uterine sarcoma. | [53,54,55] |
| NDs-NaCl | HT-29 colorectal cancer cells. | [43] | |
| Gold NPs | Paclitaxel | Breast, lung, and pancreatic cancer. | [49] |
| Polymers | Structure | Fabrication Method | Commercial Products/Literatures | Comments | Reference |
|---|---|---|---|---|---|
| Polycarbonate (PC) | 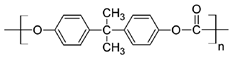 | Ion-track etching | Estrogen | Excellent stability against oxidation and biodegradation and improves antifouling properties | [151] |
| Polyethylene (PE) |  | Ion-track etching | Catapress (Clonidine), Boehringer IngelheimClimara (Estradiol), Berlex | Physico-chemical stability andordered pore formation with superior membrane performance | [152] |
| Polyethylene terephthalate (PET) | 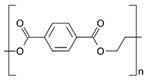 | Lithography | Ketoprofen | Biostable, antifouling, has better performance of membranes, in useful in preparing surgical meshes and ligaments | [153] |
| Polystyrene (PS) | 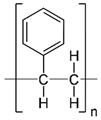 | Lithography | d-limonene, ibuprofen | Chemical resistance, easy processing, lower cost, exhibits enhancements in strength, stiffness, toughness, and ductility | [154] |
| PC, PE | - | Ion-track etching | Estraderm (Nitroglycerin), Rotta Research | Cost-effective and biocompatibility is fairly good | [155] |
| PC, PE, PET, PS | - | Phase separation | Deponit (Nitroglycerin), Pharma SchwarzHabitrol (Nicotine), Novartis | Cost-effective and biocompatibility is fairly good | [154,156] |
| Polyurethane (PU) |  | Sol-gel/solvent casting | Vivelle (Estradiol), Novartis | Good elasticity, biodegradable, suitable for hydrophilic drugs, biocompatibility is fairly good | [154] |
| Polysiloxane (silicone) | 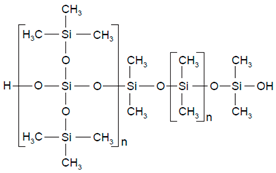 | Sol-gel/solvent casting | Prostep (Nicotine), Lederle, Transderm Nitro (Nitroglycerin), AlzaSyncro-Mate-C (Norgestomet) | Better insulation, excellent biocompatibility, and fabricated easily for hydrophilic drugs | [157] |
| Polyisobutylene (PIB) | 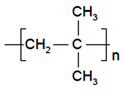 | Solvent casting | Aminopyrene, Mitsubishi Petrochem Co., Japan | Good adhesive drug impermeable layer and high degree of tack or self-adhesion | [158] |
| Polymethyl methacrylate (PMMA), poly (2-hydroxy ethyl methacrylate) |  | Layer by layer deposition | Androderm (Testosterone), SmithKline Beecham | Physical strength and transparency | [159] |
| Polyvinyl alcohol (PVA), Poly (ethylene-co-vinyl acetate) | 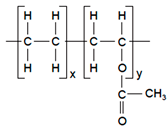 | Solvent casting | Nitro-Dur I (Nitroglycerin), Key PharmaTestoderm TTS (Testosterone), Alza | Rate-controlling membranes, high membrane permeability, hydrophilicity and strength, suitable for lipophilic drugs | [160] |
| Polyacrylic acid, polyacrylate, polyacrylamide |  | Layer by layer deposition | Epinitril (Nitroglycerin), Rotta ResearchMonsanto (Fentanyl), Dow Corning | Good adhesivity and spreadability and contains a drug impermeable layer | [160] |
| Polylactides (PLA), polylactic-co-glycolic acid (PLGA), polyglycolides (PGA) | 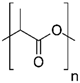 , , 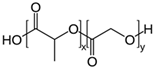 | Sol-gel/solvent casting | Propranalol, Exxon Chemical Co. | Good biocompatibility; lactic and glycolic acids are the degradation products and they are easily eliminated from the body | [161,162] |
| Polyvinyl pyrrolidone (PVP), poly (N-vinyl pyrrolidone) | 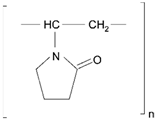 | Sol-gel/solvent casting | Cytarabine, ara-ADA, Polyscience | Superior biocompatibility, has suspension capabilities, antinucleating agent, and enhances release rate | [163] |
| Polyethylene glycol (PEG) | Sol-gel/solvent casting | Miconozale, Rohm, Germany | Chemically inert and free of leachable impurities | [164] | |
| Oxide plus polymer | - | Sol-gel/solvent casting | Superior biocompatibility and has narrow pore size | ||
| Polymer coating on support membrane | - | Layer by layer deposition | [165] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabrouk, M.; Rajendran, R.; Soliman, I.E.; Ashour, M.M.; Beherei, H.H.; Tohamy, K.M.; Thomas, S.; Kalarikkal, N.; Arthanareeswaran, G.; Das, D.B. Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics. Pharmaceutics 2019, 11, 294. https://doi.org/10.3390/pharmaceutics11060294
Mabrouk M, Rajendran R, Soliman IE, Ashour MM, Beherei HH, Tohamy KM, Thomas S, Kalarikkal N, Arthanareeswaran G, Das DB. Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics. Pharmaceutics. 2019; 11(6):294. https://doi.org/10.3390/pharmaceutics11060294
Chicago/Turabian StyleMabrouk, Mostafa, Rajakumari Rajendran, Islam E. Soliman, Mohamed M. Ashour, Hanan H. Beherei, Khairy M. Tohamy, Sabu Thomas, Nandakumar Kalarikkal, Gangasalam Arthanareeswaran, and Diganta B. Das. 2019. "Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics" Pharmaceutics 11, no. 6: 294. https://doi.org/10.3390/pharmaceutics11060294
APA StyleMabrouk, M., Rajendran, R., Soliman, I. E., Ashour, M. M., Beherei, H. H., Tohamy, K. M., Thomas, S., Kalarikkal, N., Arthanareeswaran, G., & Das, D. B. (2019). Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics. Pharmaceutics, 11(6), 294. https://doi.org/10.3390/pharmaceutics11060294