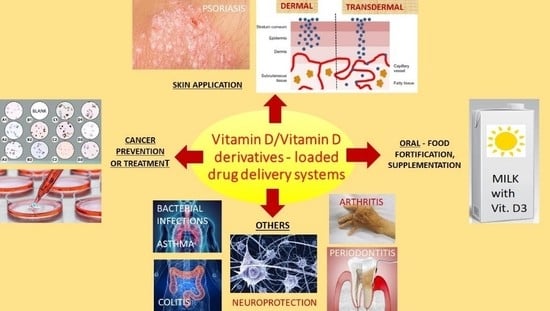
Drug Delivery Systems for Vitamin D Supplementation and Therapy
Abstract
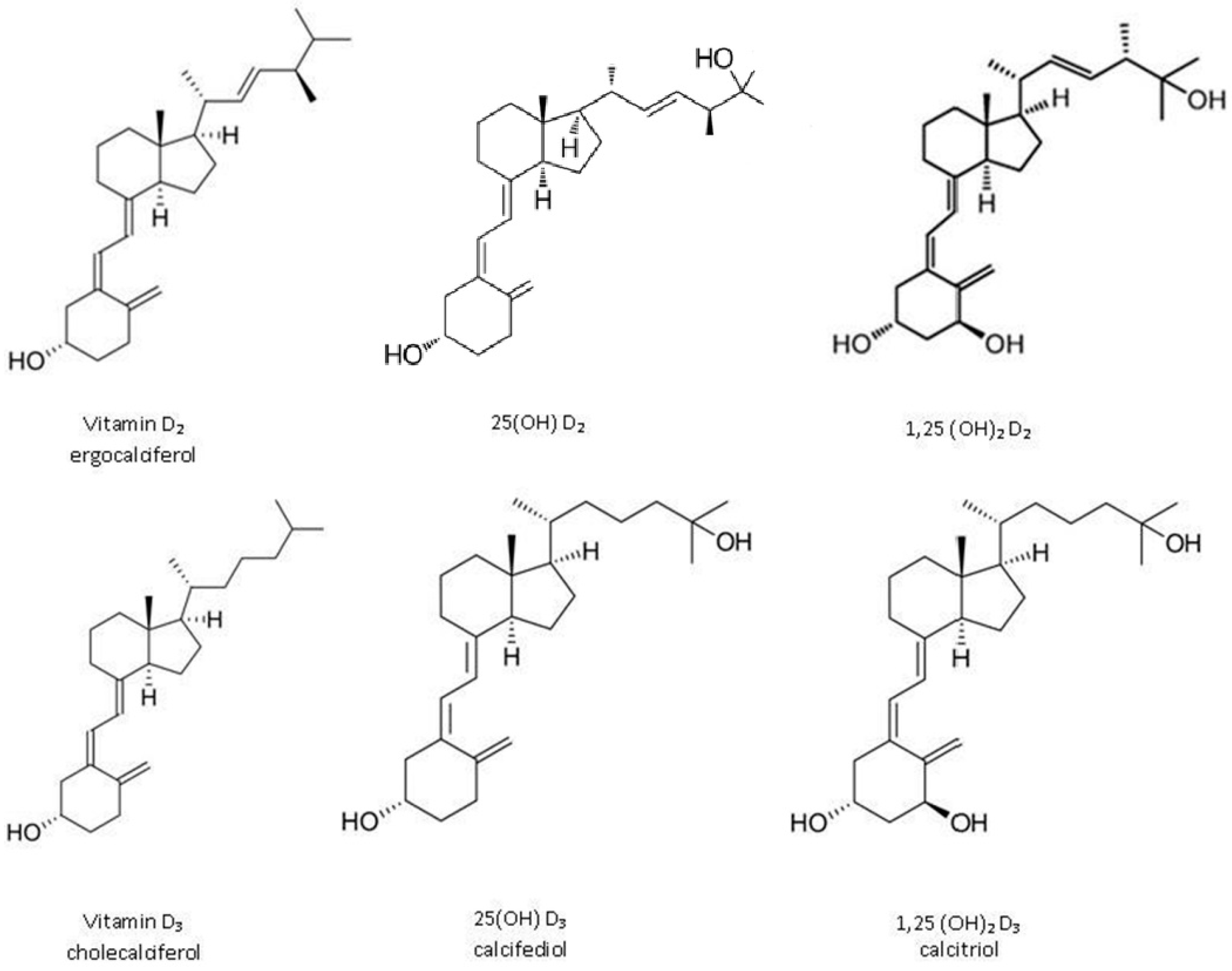
:1. Introduction
2. Drug Delivery Systems for Oral Administration (Food Fortification)
2.1. Delivery Systems from a Food Processing Point of View
2.2. Types of Vitamin D Delivery Systems for Food Fortification
3. Drug Delivery Systems for Application on the Skin
3.1. Transdermal Delivery
3.2. Topical Delivery
4. Drug Delivery Systems for the Prevention and Treatment of Cancer
4.1. Vitamin D as an Adjuvant in Cancer Therapy
4.2. Vitamin D and Its Metabolites as Anticancer Drugs
4.3. Active Targeting of Nanoparticles Loaded with Vitamin D to Cancer Cells
5. Drug Delivery Systems for Other Diseases or Routes of Administration
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wimalawansa, S.J. Vitamin D in the New Millennium. Curr. Osteoporos Rep. 2012, 10, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Cavalier, E.; Delanaye, P.; Chapelle, J.P.; Souberbielle, J.C. Vitamin D: Current status and perspectives. Clin. Chem. Lab. Med. 2009, 47, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. Available online: http://www.nature.com/nrc/journal/v14/n5/abs/nrc3691.html#supplementary-information (accessed on 23 March 2019). [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moukayed, M.; Grant, W.B. The roles of UVB and vitamin D in reducing risk of cancer incidence and mortality: A review of the epidemiology, clinical trials, and mechanisms. Rev. Endocr. Metab. Disord. 2017, 18, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Merchan, B.B.; Morcillo, S.; Martin-Nunez, G.; Tinahones, F.J.; Macias-Gonzalez, M. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J. Steroid Biochem. Mol. Biol. 2017, 167, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Franza, L.; Mandolini, C.; Conti, P. Immune Modulation by Vitamin D: Special Emphasis on Its Role in Prevention and Treatment of Cancer. Clin. Ther. 2017, 39, 884–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, M.J.; Murray, A.; Synnott, N.C.; O’Donovan, N.; Crown, J. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol. Hematol. 2017, 112, 190–197. [Google Scholar] [CrossRef]
- Corcoran, A.; Bermudez, M.A.; Seoane, S.; Perez-Fernandez, R.; Krupa, M.; Pietraszek, A.; Chodyński, M.; Kutner, A.; Brown, G.; Marcinkowska, E. Biological evaluation of new vitamin D2 analogues. J. Steroid Biochem. Mol. Biol. 2016, 164, 66–71. [Google Scholar] [CrossRef]
- Maestro, M.A.; Molnár, F.; Mouriño, A.; Carlberg, C. Vitamin D receptor 2016: Novel ligands and structural insights. Expert Opin. Ther. Pat. 2016, 26, 1291–1306. [Google Scholar] [CrossRef]
- Hansen, C.M.; Hamberg, K.J.; Binderup, E.; Binderup, L. Seocalcitol (EB 1089) A Vitamin D Analogue of Anti-cancer Potential. Background, Design, Synthesis, Pre-clinical and Clinical Evaluation. Cur. Pharm. Des. 2000, 6, 803–828. [Google Scholar] [CrossRef]
- Dobnig, H. A review of the health consequences of the vitamin D deficiency pandemic. J. Neurol. Sci. 2011, 311, 15–18. [Google Scholar] [CrossRef]
- Jain, K.K. Current status and future prospects of drug delivery systems. Methods Mol. Biol. 2014, 1141, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip Rev. Nanomed Nanobiotechnol 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.; Florence, A.T. Towards more effective advanced drug delivery systems. Int. J. Pharm. 2013, 454, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Mihov, G.; Welting, T.; Thies, J.; Emans, P. Drugs and Polymers for Delivery Systems in OA Joints: Clinical Needs and Opportunities. Polymers 2014, 6, 799–819. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Wang, Z.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Formulation of monodisperse oil-in-water emulsions loaded with ergocalciferol and cholecalciferol by microchannel emulsification: Insights of production characteristics and stability. Int. J. Food Sci. Technol. 2015, 50, 1807–1814. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D Bioavailability: State of the Art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.-G.; Huang, Z.-H.; Xue, F.-F. Preparation and Characterization of Nanoparticles Based on Hydrophobic Alginate Derivative as Carriers for Sustained Release of Vitamin D3. J. Agric. Food Chem. 2011, 59, 1962–1967. [Google Scholar] [CrossRef]
- Grossmann, R.E.; Tangpricha, V. Evaluation of vehicle substances on vitamin D bioavailability: A systematic review. Mol. Nutr. Food Res. 2010, 54, 1055–1061. [Google Scholar] [CrossRef]
- Coelho, I.M.G.; de Andrade, L.D.; Saldanha, L.; Diniz, E.T.; Griz, L.; Bandeira, F. Bioavailability of vitamin D3 in non-oily capsules: The role of formulated compounds and implications for intermittent replacement. Arq. Bras. Endocrinol. Metab. 2010, 54, 239–243. [Google Scholar] [CrossRef]
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro- and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Haham, M.; Ish-Shalom, S.; Nodelman, M.; Duek, I.; Segal, E.; Kustanovich, M.; Livney, Y.D. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct. 2012, 3, 737–744. [Google Scholar] [CrossRef]
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: Factors affecting particle size and stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.; Emam-Djomeh, Z.; Mousavi, M.A.E.; Davoodi, D. Stability of vitamin D3 encapsulated in nanoparticles of whey protein isolate. Food Chem. 2014, 143, 379–383. [Google Scholar] [CrossRef]
- Cohen, Y.; Levi, M.; Lesmes, U.; Margier, M.; Reboul, E.; Livney, Y.D. Re-assembled casein micelles improve in vitro bioavailability of vitamin D in a Caco-2 cell model. Food Funct. 2017, 8, 2133–2141. [Google Scholar] [CrossRef]
- Ziani, K.; Fang, Y.; McClements, D.J. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: Vitamin E, vitamin D, and lemon oil. Food Chem. 2012, 134, 1106–1112. [Google Scholar] [CrossRef]
- Kirilenko, V.; Gregoriadis, G. Fat Soluble Vitamins in Liposomes: Studies on Incorporation Efficiency and Bile Salt Induced Vesicle Disintegration. J. Drug Target. 1993, 1, 361–368. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Wang, Q. Development of Zein Nanoparticles Coated with Carboxymethyl Chitosan for Encapsulation and Controlled Release of Vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Fumiaki, B.; Park, Y.; McClements, D.J. The influence of lipid droplet size on the oral bioavailability of vitamin D2 encapsulated in emulsions: An in vitro and in vivo study. Food Funct. 2017, 8, 767–777. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Nanoemulsion delivery systems for oil-soluble vitamins: Influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. 2015, 187, 499–506. [Google Scholar] [CrossRef]
- Sun, F.; Ju, C.; Chen, J.; Liu, S.; Liu, N.; Wang, K.; Liu, C. Nanoparticles Based on Hydrophobic Alginate Derivative as Nutraceutical Delivery Vehicle: Vitamin D3 Loading. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 113–119. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Encapsulation of cholecalciferol and ergocalciferol in oil-in-water emulsions by different homogenization techniques. Eur. J. Lipid Sci. Technol. 2017, 119, 1600247. [Google Scholar] [CrossRef]
- Shu, G.F.; Khalid, N.; Zhao, Y.G.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Formulation and stability assessment of ergocalciferol loaded oil-in-water nanoemulsions: Insights of emulsifiers effect on stabilization mechanism. Food Res. Int. 2016, 90, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Walia, N.; Dasgupta, N.; Ranjan, S.; Chen, L.; Ramalingam, C. Fish oil based vitamin D nanoencapsulation by ultrasonication and bioaccessibility analysis in simulated gastro-intestinal tract. Ultrason. Sonochem 2017, 39, 623–635. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; van der Meeren, P. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Hassanvand, E.; Fathi, M.; Bassiri, A.; Javanmard, M.; Abbaszadeh, R. Novel starch based nanocarrier for Vitamin D fortification of milk: Production and characterization. Food Bioprod. Process. 2015, 96, 264–277. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Wang, Q. Carboxymethyl chitosan–soy protein complex nanoparticles for the encapsulation and controlled release of vitamin D3. Food Chem. 2013, 141, 524–532. [Google Scholar] [CrossRef]
- Patel, M.R.; Martin-Gonzalez, M.F.S. Characterization of Ergocalciferol Loaded Solid Lipid Nanoparticles. J. Food Sci. 2012, 77, N8–N13. [Google Scholar] [CrossRef]
- Mohammadi, M.; Pezeshki, A.; Mesgari Abbasi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Vitamin D (3)-Loaded Nanostructured Lipid Carriers as a Potential Approach for Fortifying Food Beverages; in Vitro and in Vivo Evaluation. Adv. Pharm. Bull. 2017, 7, 61–71. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- Li, W.; Peng, H.; Ning, F.; Yao, L.; Luo, M.; Zhao, Q.; Zhu, X.; Xiong, H. Amphiphilic chitosan derivative-based core–shell micelles: Synthesis, characterisation and properties for sustained release of Vitamin D3. Food Chem. 2014, 152, 307–315. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin d3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4, 569–575. [Google Scholar] [CrossRef]
- Barba, A.A.; Dalmoro, A.; d’Amore, M.; Lamberti, G. Liposoluble vitamin encapsulation in shell-core microparticles produced by ultrasonic atomization and microwave stabilization. LWT-Food Sci. Technol. 2015, 64, 149–156. [Google Scholar] [CrossRef]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Levinson, Y.; Ish-Shalom, S.; Segal, E.; Livney, Y.D. Bioavailability, rheology and sensory evaluation of fat-free yogurt enriched with VD3 encapsulated in re-assembled casein micelles. Food Funct. 2016, 7, 1477–1482. [Google Scholar] [CrossRef]
- Cohen, Y.; Ish-Shalom, S.; Segal, E.; Nudelman, O.; Shpigelman, A.; Livney, Y.D. The bioavailability of vitamin D3, a model hydrophobic nutraceutical, in casein micelles, as model protein nanoparticles: Human clinical trial results. J. Funct. Food 2017, 30, 321–325. [Google Scholar] [CrossRef]
- Diarrassouba, F.; Remondetto, G.; Garrait, G.; Alvarez, P.; Beyssac, E.; Subirade, M. Self-assembly of β-lactoglobulin and egg white lysozyme as a potential carrier for nutraceuticals. Food Chem. 2015, 173, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric nanospheres for topical delivery of vitamin D3. Int. J. Pharm. 2017, 516, 196–203. [Google Scholar] [CrossRef]
- D’Angelo Costa, G.M.; Sales de Oliveira Pinto, C.A.; Rodrigues Leite-Silva, V.; Rolim Baby, A.; Robles Velasco, M.V. Is Vitamin D3 Transdermal Formulation Feasible? An Ex Vivo Skin Retention and Permeation. AAPS PharmSciTech 2018, 19, 2418–2425. [Google Scholar] [CrossRef]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Bubshait, D.A.; Al-Dakheel, D.A.; Alanii, F.M. Topical vitamin D3: A randomized controlled trial (RCT). Clin. Nutr. ESPEN 2018, 27, 16–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadat-Ali, M.; Bubshait, D.A.; Al-Turki, H.A.; Al-Dakheel, D.A.; Al-Olayani, W.S. Topical delivery of vitamin d3: A randomized controlled pilot study. Int. J. Biomed. Sci. 2014, 10, 21–24. [Google Scholar] [PubMed]
- Kim, H.-G.; Gater, D.L.; Kim, Y.-C. Development of transdermal vitamin D3 (VD3) delivery system using combinations of PLGA nanoparticles and microneedles. Drug Deliv. Transl. Res. 2018, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, N.; Namioka, T.; Okura, N.; Sato, S.; Kim, D.; Furuhama, K.; Naito, Y. Application of a Reservoir-Type Calcitriol Transdermal Patch in Dairy Cattle. J. Vet. Med. Sci. 2009, 71, 845–848. [Google Scholar] [CrossRef] [Green Version]
- Prüfer, K.; Jirikowski, G.F. Liposomal incorporation changes the effect of 1.25-dihydroxyvitamin D3 on the phospholipase C signal transduction pathway and the eicosanoid cascade on keratinocytes in vitro. Biochem. Pharmacol. 1996, 51, 247–252. [Google Scholar] [CrossRef]
- Prüfer, K.; Merz, K.; Barth, A.; Wollina, U.; Sternberg, B. Interaction of Liposomal Incorporated Vitamin D3-Analogues and Human Keratinocytes. J. Drug Target. 1994, 2, 419–429. [Google Scholar] [CrossRef]
- Knudsen, N.O.; Ronholt, S.; Salte, R.D.; Jorgensen, L.; Thormann, T.; Basse, L.H.; Hansen, J.; Frokjaer, S.; Foged, C. Calcipotriol delivery into the skin with PEGylated liposomes. Eur. J. Pharm. Biopharm. 2012, 81, 532–539. [Google Scholar] [CrossRef]
- Merz, K.; Sternberg, B. Incorporation of Vitamin D3-Derivatives in Liposomes of Different Lipid Types. J. Drug Target. 1994, 2, 411–417. [Google Scholar] [CrossRef]
- Knudsen, N.Ø.; Jorgensen, L.; Hansen, J.; Vermehren, C.; Frokjaer, S.; Foged, C. Targeting of liposome-associated calcipotriol to the skin: Effect of liposomal membrane fluidity and skin barrier integrity. Int. J. Pharm. 2011, 416, 478–485. [Google Scholar] [CrossRef]
- Körbel, J.N.; Sebök, B.; Kerényi, M.; Mahrle, G. Enhancement of the Antiparakeratotic Potency of Calcitriol and Tacalcitol in Liposomal Preparations in the Mouse Tail Test. Skin Pharmacol. Physiol. 2001, 14, 291–295. [Google Scholar] [CrossRef]
- Sonawane, R.; Harde, H.; Katariya, M.; Agrawal, S.; Jain, S. Solid lipid nanoparticles-loaded topical gel containing combination drugs: An approach to offset psoriasis. Expert Opin. Drug Deliv. 2014, 11, 1833–1847. [Google Scholar] [CrossRef]
- Lalloz, A.; Bolzinger, M.-A.; Faivre, J.; Latreille, P.-L.; Garcia Ac, A.; Rakotovao, C.; Rabanel, J.-M.; Hildgen, P.; Banquy, X.; Briançon, S. Effect of surface chemistry of polymeric nanoparticles on cutaneous penetration of cholecalciferol. Int. J. Pharm. 2018, 553, 120–131. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhou, T.; Cao, N.; Ni, J.; Wang, X. Role of Vitamin D Metabolism and Activity on Carcinogenesis. Oncol. Res. 2015, 22, 129–137. [Google Scholar] [CrossRef]
- Mondul, A.M.; Weinstein, S.J.; Layne, T.M.; Albanes, D. Vitamin D and Cancer Risk and Mortality: State of the Science, Gaps, and Challenges. Epidemiol. Rev. 2017, 39, 28–48. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhang, Y.; Dang, S.; He, S. Astemizole promotes the anti-tumor effect of vitamin D through inhibiting miR-125a-5p-meidated regulation of VDR in HCC. Biomed. Pharm. 2018, 107, 1682–1691. [Google Scholar] [CrossRef]
- Sabzichi, M.; Mohammadian, J.; Mohammadi, M.; Jahanfar, F.; Movassagh Pour, A.; Hamishehkar, H.; Ostad-Rahimi, A. Vitamin D-Loaded Nanostructured Lipid Carrier (NLC): A New Strategy for Enhancing Efficacy of Doxorubicin in Breast Cancer Treatment. Nutr. Cancer 2017, 69, 840–848. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Zhang, T.; Forrest, M.L.; Alrushaid, S.; Doschak, M.R.; Davies, N.M.; El-Kadi, A.O.S. DOX-Vit D, a Novel Doxorubicin Delivery Approach, Inhibits Human Osteosarcoma Cell Proliferation by Inducing Apoptosis While Inhibiting Akt and mTOR Signaling Pathways. Pharmaceutics 2018, 10, 144. [Google Scholar] [CrossRef]
- Almouazen, E.; Bourgeois, S.; Jordheim, L.P.; Fessi, H.; Briancon, S. Nano-encapsulation of Vitamin D-3 Active Metabolites for Application in Chemotherapy: Formulation Study and in Vitro Evaluation. Pharm. Res. 2013, 30, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Gomes, B.; Frasco, M.F.; Coelho, M.A.N.; Pereira, M.C. PLGA nanoparticles as a platform for vitamin D-based cancer therapy. Beilstein J. Nanotechnol. 2015, 6, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.; Sita, V.G.; Vavia, P. Zero order controlled release delivery of cholecalciferol from injectable biodegradable microsphere: In-vitro characterization and in-vivo pharmacokinetic studies. Eur. J. Pharm. Sci. 2017, 107, 78–86. [Google Scholar] [CrossRef]
- Nguyen, T.L.U.; Tey, S.Y.; Pourgholami, M.H.; Morris, D.L.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Synthesis of semi-biodegradable crosslinked microspheres for the delivery of 1,25 dihydroxyvitamin D3 for the treatment of hepatocellular carcinoma. Eur. Polymer. J. 2007, 43, 1754–1767. [Google Scholar] [CrossRef]
- Liu, C.; Shaurova, T.; Shoemaker, S.; Petkovich, M.; Hershberger, P.A.; Wu, Y. Tumor-Targeted Nanoparticles Deliver a Vitamin D-Based Drug Payload for the Treatment of EGFR Tyrosine Kinase Inhibitor-Resistant Lung Cancer. Mol. Pharm. 2018, 15, 3216–3226. [Google Scholar] [CrossRef]
- Maleklou, N.; Allameh, A.; Kazemi, B. Targeted delivery of vitamin D3-loaded nanoparticles to C6 glioma cell line increased resistance to doxorubicin, epirubicin, and docetaxel in vitro. Vitro Cell. Dev. Biol. Anim. 2016, 52, 989–1000. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Da Silveira, K.L.; da Silveira, L.L.; Thorstenberg, M.L.P.; Cabral, F.L.; Castilhos, L.G.; Rezer, J.F.P.; de Andrade, D.F.; Beck, R.C.R.; Einloft Palma, H.; de Andrade, C.M.; et al. Free and nanoencapsulated vitamin D3: Effects on E-NTPDase and E-ADA activities in an animal model with induced arthritis. Cell Biochem. Function 2016, 34, 262–273. [Google Scholar] [CrossRef]
- Wei-hong, T.; Min-chang, G.; Zhen, X.; Jie, S. Pharmacological and Pharmacokinetic Studies with Vitamin D-loaded Nanoemulsions in Asthma Model. Inflammation 2014, 37, 723–728. [Google Scholar] [CrossRef]
- Goff, J.P.; Koszewski, N.J.; Haynes, J.S.; Horst, R.L. Targeted delivery of vitamin D to the colon using β-glucuronides of vitamin D: Therapeutic effects in a murine model of inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G460–G469. [Google Scholar] [CrossRef]
- Castoldi, A.; Herr, C.; Niederstraßer, J.; Labouta, H.I.; Melero, A.; Gordon, S.; Schneider-Daum, N.; Bals, R.; Lehr, C.-M. Calcifediol-loaded liposomes for local treatment of pulmonary bacterial infections. Eur. J. Pharm. Biopharm. 2017, 118, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Q.; Xiao, Y.; Bao, C.; Li, W. 25-Hydroxyvitamin D(3)-Loaded PLA Microspheres: In Vitro Characterization and Application in Diabetic Periodontitis Models. AAPS PharmSciTech 2013, 14, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.; Wang, Q.; Xiao, Y.; Chen, X.M.; Li, W. Attenuation of Inflammatory Response by 25- hydroxyvitamin D3-loaded Polylactic Acid Microspheres in Treatment of Periodontitis in Diabetic Rats. Chin. J. Dent. Res. 2014, 17, 91–98. [Google Scholar] [PubMed]
- Ślusarczyk, J.; Piotrowski, M.; Szczepanowicz, K.; Regulska, M.; Leśkiewicz, M.; Warszyński, P.; Budziszewska, B.; Lasoń, W.; Basta-Kaim, A. Nanocapsules with Polyelectrolyte Shell as a Platform for 1,25-dihydroxyvitamin D3 Neuroprotection: Study in Organotypic Hippocampal Slices. Neurotox. Res. 2016, 30, 581–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Delivery System | Active | Main Components | Technique/Method | Reference |
|---|---|---|---|---|
| O/W emulsions and microemulsions | VD2 VD3 | Oil phase: Soybean oil, olive oil, or MCT; water phase containing Tween 20 or sodium cholate | Microchannel emulsification | [19] |
| VD2 VD3 | Oil phase: Soybean oil, olive oil, or MCT; water phase containing Tween 20 | Rotor-stator and high-pressure homogenization | [36] | |
| VD3 | Oil phase: MCT; water phase containing Tween 20, 60, or 80 | High-speed blender and high-pressure homogenization | [30] | |
| VD2 | Oil phase: Soybean oil; water phase containing modified lecithin, sodium caseinate, or decaglycerol monooleate | Rotor-stator and high-pressure homogenization | [37] | |
| O/W nanoemulsions | VD3 | Oil phase: MCT, corn oil, fish oil, mineral oil, or orange oil; water phase containing a natural surfactant | High-speed blender and high-pressure homogenization | [34] |
| VD3 | Oil phase: Fish oil; water phase containing Tween 20 | Ultrasonication | [38] | |
| Biopolymer-based nanoparticles | VD3 | Zein nanoparticles coated with carboxymethyl chitosan | Phase separation method and coating by cross-linking with calcium | [32] |
| VD2 | Beta-lactoglobulin–sodium alginate complex | Electrostatic interactions | [39] | |
| VD3 | High amylose corn starch | Ultrasonication | [40] | |
| VD3 | Carboxymethyl chitosan–soy protein complex | Ionic gelation method | [41] | |
| Lipid-based nanoparticles | VD2 | Solid lipid nanoparticles (glyceryl tripalmitate) stabilized by Tween 20 | Hot homogenization technique using a high-pressure homogenizer | [42] |
| VD3 | Nanostructured lipid carriers (solid lipids: Precirol or Compritol, liquid lipid: Miglyol or octyloctanoat, surfactants: Tween 80 or 20 or Poloxamer 407) | Hot homogenization method | [43] | |
| VD3 | Nanostructured lipid carriers (glycerol monostearate as solid lipid, oleic acid as liquid lipid, and Tween 80) | Hot high-pressure homogenization | [44] | |
| Micelles | VD3 | Amphiphilic chitosan derivative of N,N-dimethylhexadecyl carboxymethyl chitosan | Synthesis | [45] |
| Liposomes | VD3 | Soybean phosphatidylcholine, cholesterol | Thin film hydration-sonication technique | [46] |
| Microparticles | VD2 | Medium molecular weight sodium alginate | Ultrasonic atomization and microwave stabilization | [47] |
| Delivery System | Active | Main Components | Technique/Method | Reference |
|---|---|---|---|---|
| Liposomes | Calcipotriol | Dipalmitoylphosphatidyl-choline (DPPC) and dilauroylphosphatidylcholine (DLPC) | Thin film method and extrusion | [63] |
| Calcipotriol | Distearoylphosphatidylcholine (DSPC), poly(ethylene glycol)-distearoylphosphoethanolamine (PEG2000-DSPE), sodium cholate | Thin film method and extrusion | [61] | |
| Calcitriol and tacalcitol | Phosphatidylcholine, phosphatidic acid, phospha-tidylethanolamine | Made from concentrate (commercial kit) | [64] | |
| Solid lipid nanoparticles | Bethamethasone and calcipotriol | Precirol® ATO 5 | Hot melt high shear homogenization technique and incorporation in Carbol gel matrix | [65] |
| Polymeric nanoparticles | VD3 | Poly(lactic acid) nanoparticles with non-ionic poly(ethylene glycol) or zwitterionic poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) coating | Flash nanoprecipitation | [66] |
| VD3 | ABA triblock copolymers composed of hydrophilic A blocks and hydrophobic B blocks that form TyroSpheres® | TyroSpheres® preparation | [52] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glowka, E.; Stasiak, J.; Lulek, J. Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics 2019, 11, 347. https://doi.org/10.3390/pharmaceutics11070347
Glowka E, Stasiak J, Lulek J. Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics. 2019; 11(7):347. https://doi.org/10.3390/pharmaceutics11070347
Chicago/Turabian StyleGlowka, Eliza, Joanna Stasiak, and Janina Lulek. 2019. "Drug Delivery Systems for Vitamin D Supplementation and Therapy" Pharmaceutics 11, no. 7: 347. https://doi.org/10.3390/pharmaceutics11070347
APA StyleGlowka, E., Stasiak, J., & Lulek, J. (2019). Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics, 11(7), 347. https://doi.org/10.3390/pharmaceutics11070347