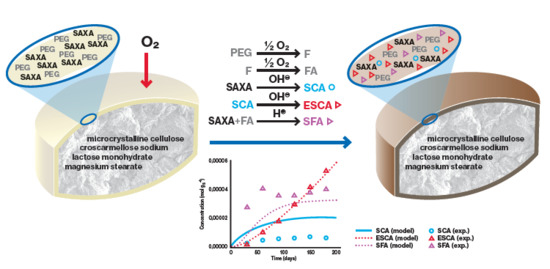
Understanding and Kinetic Modeling of Complex Degradation Pathways in the Solid Dosage Form: The Case of Saxagliptin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film-Coated Tablets
2.3. Stability Study Protocol
2.4. Saxagliptin Related Substances and Degradation Products Determination
2.5. Low Molecular Weight Organic Impurities Determination
2.6. Other Measurements (pH, Water Activity, Coloration)
2.7. Statistical Evaluation of Data
2.8. Kinetic Model Development
2.9. Computational Methods
3. Results and Discussion
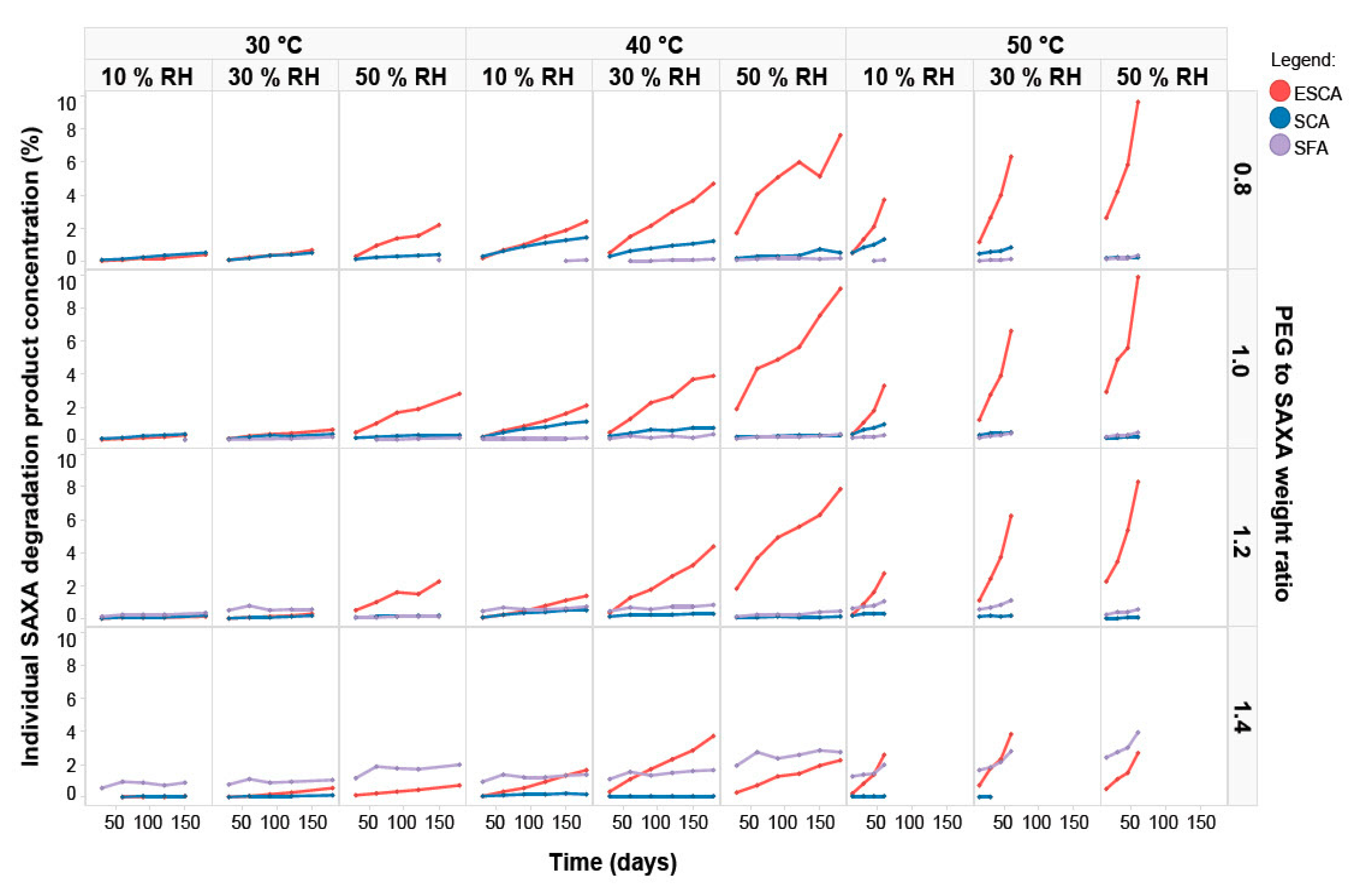
3.1. Film-Coated Tablet Stability Study
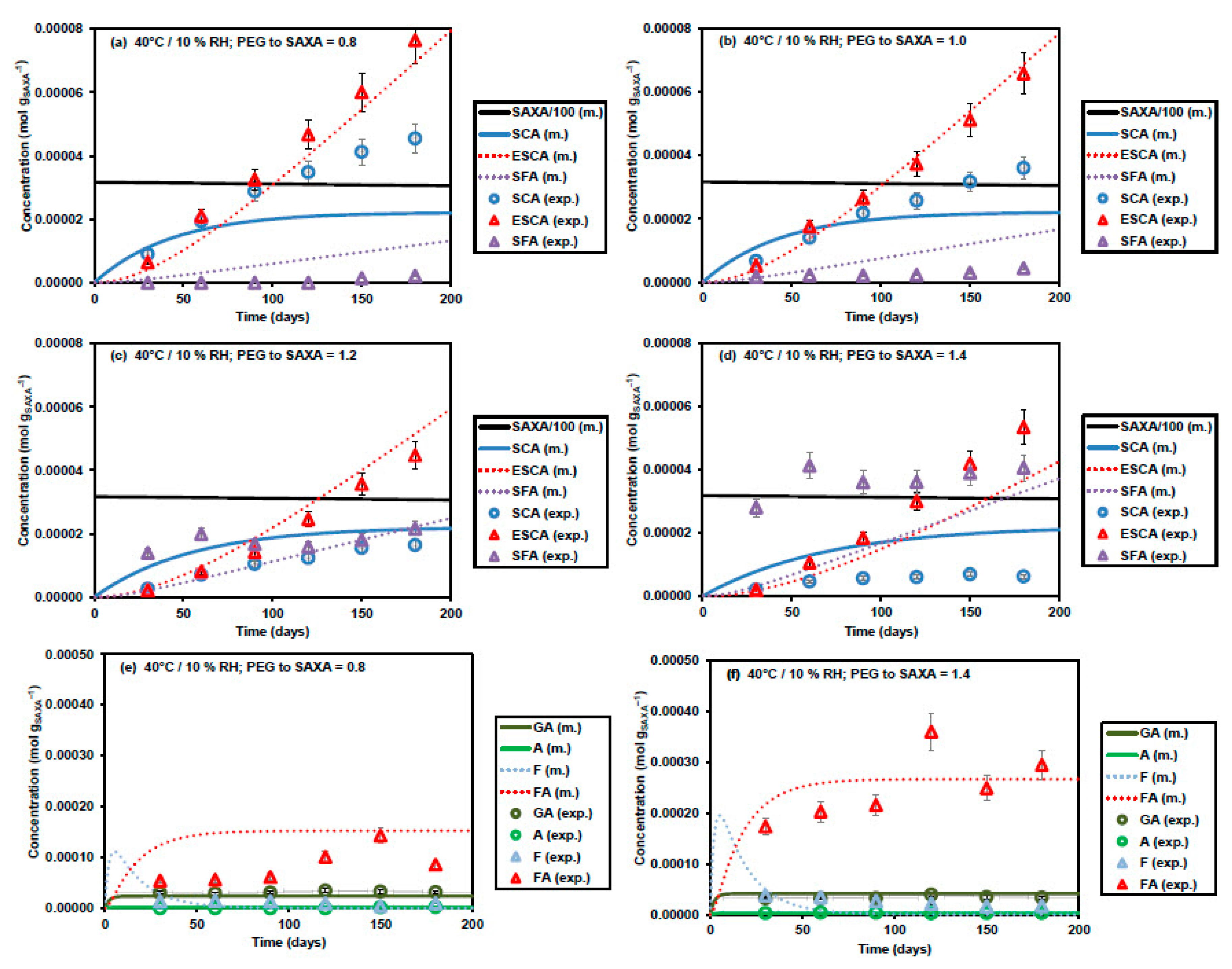
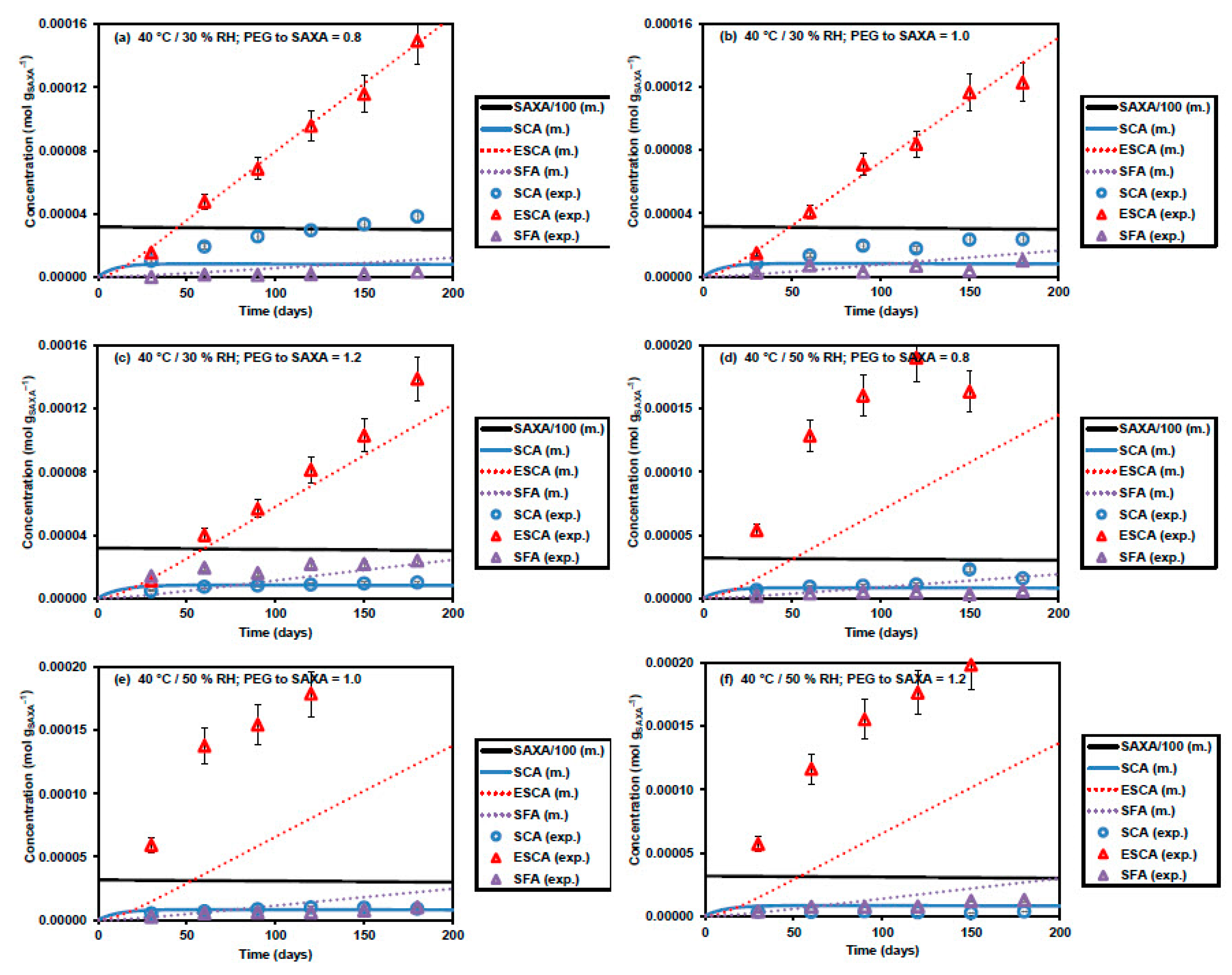
3.2. Kinetic Model Development
3.3. Additional Model Optimization
3.4. Ab Initio Calculation of Ea
3.5. Binary Component Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zong, Z.; Qiu, J.; Tinmanee, R.; Kirsch, L.E. Kinetic model for solid-state degradation of gabapentin. J. Pharm. Sci. 2012, 101, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Long, G.T.; Vyazovkin, S.; Gamble, N.; Wight, C.A. Hard to swallow dry: Kinetics and mechanism of the anhydrous thermal decomposition of acetylsalicylic acid. J. Pharm. Sci. 2002, 91, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; La Cruz, T.E.; Tábora, J.E. A photochemical kinetic model for solid dosage forms. Eur. J. Pharm. Biopharm. 2017, 120, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Stella, V.J. Stability of Drugs and Dosage Forms; Springer US: New York, NY, USA, 2000. [Google Scholar]
- Hemenway, J.N.; Carvalho, T.C.; Rao, V.M.; Wu, Y.; Levons, J.K.; Narang, A.S.; Paruchuri, S.R.; Stamato, H.J.; Varia, S.A. Formation of reactive impurities in aqueous and neat polyethylene glycol 400 and effects of antioxidants and oxidation inducers. J. Pharm. Sci. 2012, 101, 3305–3318. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, R.P.; Mazzitelli, C.L. Polyethylene glycols in oral and parenteral formulations--A critical review. Int. J. Pharm. 2015, 496, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pellett, J.D.; Narang, A.S.; Wang, Y.J.; Zhang, Y.T. Reactive impurities in large and small molecule pharmaceutical excipients – A review. Trends Anal. Chem. 2017, 101, 34–42. [Google Scholar] [CrossRef]
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive Impurities in Excipients: Profiling, Identification and Mitigation of Drug–Excipient Incompatibility. AAPS Pharm. Sci. Tech. 2011, 12, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fiske, J.D.; Jennings, S.P.; Tomasella, F.P.; Palaniswamy, V.A.; Ray, K.L. Identification and Control of a Degradation Product in Avapro™ Film-Coated Tablet: Low Dose Formulation. Pharm. Dev. Technol. 2008, 13, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Yamniuk, A.; Zhang, L.; Comezoglu, S.N.; Bindra, D.S.; Varia, S.A.; Doyle, M.; Badawy, S. Drug Excipient Interactions. In Excipient Applications in Formulation Design and Drug Delivery; Narang, A.S., Boddu, S.H.S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–35. [Google Scholar]
- Waterman, K.C.; Arikpo, W.B.; Fergione, M.B.; Graul, T.W.; Johnson, B.A.; Macdonald, B.C.; Roy, M.C.; Timpano, R.J. N-methylation and N-formylation of a secondary amine drug (varenicline) in an osmotic tablet. J. Pharm. Sci. 2008, 97, 1499–1507. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Dill, A.L.; Kramer, T.T.; Scrivens, G.; Suruzhon, M. Degradation Rate Observations as a Function of Drug Load in Solid-State Drug Products. J. Pharm. Sci. 2019, 108, 1746–1755. [Google Scholar] [CrossRef]
- AstraZeneca Pharmaceuticals LP. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022350s023lbl.pdf. (accessed on 8 August 2019).
- Bing, V.L.; Desai Divyakant, S. Coated Tablet Formulation and Method. U.S. Patent 7951400 B2, 31 May 2011. [Google Scholar]
- Chen, W.; Wang, J.; Desai, D.; Chang, S.-Y.; Kiang, S.; Lyngberg, O. A Strategy for Tablet Active Film Coating Formulation Development Using a Content Uniformity Model and Quality by Design Principles. In Comprehensive Quality by Design for Pharmaceutical Product Development and Manufacture; Reklaitis, G.V., Seymour, C., García-Munoz, S., Eds.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Jones, G.S.; Savage, S.A.; Ivy, S.; Benitez, P.L.; Ramirez, A. Kinetic and Mechanistic Insight into the Thermodynamic Degradation of Saxagliptin. J. Org. Chem. 2011, 76, 10332–10337. [Google Scholar] [CrossRef] [PubMed]
- Desai Divyakant, S.; Narang, A.; Rao Venkatramana, M. Pharmaceutical Formulations Including an Amine Compound. U.S. Patent Application 2014/0079778 A1, 20 March 2014. [Google Scholar]
- Rao Venkatramana, M.; Narang, A.; Desai Divyakant, S. Drug Formulations Using Water Soluble Antioxidants. U.S. Patent Application 2013/0224296 A1, 29 August 2013. [Google Scholar]
- Gerack, C.; McElwee-White, L. Formylation of Amines. Molecules 2014, 19, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.J.; Cundell, A.M. Water Activity Applications in the Pharmaceutical Industry; Parenteral Drug, Association; Davis Healthcare International Publishing: Bethesda, MD, USA; River Grove, IL, USA, 2009. [Google Scholar]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 (Revision, E. 01); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Tomasi, J.; Bonaccorsi, R.; Cammi, R.; del Valle, F.J.O. Theoretical chemistry in solution. Some results and perspectives of the continuum methods and in particular of the polarizable continuum model. J. Mol. Struct. 1991, 234, 401–424. [Google Scholar] [CrossRef]
- Giroto, J.A.; Teixeira, A.C.S.C.; Nascimento, C.A.O.; Guardani, R. Degradation of Poly(ethylene glycol) in Aqueous Solution by Photo-Fenton and H2O2/UV Processes. Ind. Eng. Chem. Res. 2010, 49, 3200–3206. [Google Scholar] [CrossRef]
- Almkvist, G.; Persson, I. Fenton-induced degradation of polyethylene glycol and oak holocellulose. A model experiment in comparison to changes observed in conserved waterlogged wood. Holzforschung 2008, 62, 704. [Google Scholar] [CrossRef]
- Glastrup, J. Degradation of polyethylene glycol. A study of the reaction mechanism in a model molecule: Tetraethylene glycol. Polym. Degrad. Stab. 1996, 52, 217–222. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.; Kwon, D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer 1997, 38, 317–323. [Google Scholar] [CrossRef]
- Fujita, M.; Ueda, T.; Handa, T. Generation of formaldehyde by pharmaceutical excipients and its absorption by meglumine. Chem. Pharm. Bull. 2009, 57, 1096–1099. [Google Scholar] [CrossRef]
- Fukuyama, S.; Kihara, N.; Nakashima, K.; Morokoshi, N.; Koda, S.; Yasuda, T. Mechanism of Optical Isomerization of(S)-N-[1-(2-Fluorophenyl)-3,4,6,7-tetrahydro-4-oxopyrrolo[3,2,1-jk] [l,4]-benzodiazepine-3-y1]-1H-indole-2-carboxamide (FK480) in Soft Capsules Containing Polyethylene Glycol 400 and Glycerol. Pharm. Res. 1994, 11, 1704–1706. [Google Scholar] [CrossRef]
- Jones, G.S.; Savage, S.A.; Ivy, S.; Benitez, P.L.; Ramirez, A. Correction to Kinetic and Mechanistic Insight into the Thermodynamic Degradation of Saxagliptin. J. Org. Chem. 2013, 78, 4627. [Google Scholar] [CrossRef]
- Ramirez, A.; Truc, V.C.; Lawler, M.; Ye, Y.K.; Wang, J.; Wang, C.; Chen, S.; Laporte, T.; Liu, N.; Kolotuchin, S.; et al. The Effect of Additives on the Zinc Carbenoid-Mediated Cyclopropanation of a Dihydropyrrole. J. Org. Chem. 2014, 79, 6233–6243. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.d.; Bernardino, A.M.R.; Souza, M.C.d. Synthetic approaches to amidines. Química Nova 2006, 29, 1301–1306. [Google Scholar] [CrossRef]
- Steinreiber, J.; Faber, K.; Griengl, H. De-racemization of Enantiomers versus De-epimerization of Diastereomers—Classification of Dynamic Kinetic Asymmetric Transformations (DYKAT). Chem. Eur. J. 2008, 14, 8060–8072. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.H.; Cui, C.X.; Qu, Z.B.; Zhu, Y.Y.; Tang, M.S. A computational study on the reaction mechanisms of N-formylation of amines under a Lewis acid catalysis. J. Mol. Struct. THEOCHEM 2010, 951, 89–92. [Google Scholar] [CrossRef]
- Jung, S.H.; Ahn, J.H.; Park, S.K.; Choi, J.K. A practical and convenient procedure for the N-formylation of amines using formic acid. Bull. Korean Chem. Soc. 2002, 23, 149–150. [Google Scholar]
- Linda, P.; Stener, A.; Cipiciani, A.; Savelli, G. Hydrolysis of amides. Kinetics and mechanism of the basic hydrolysis of N-acylpyrroles, N-acylindoles and N-acylcarbazoles. J. Heterocycl. Chem. 1983, 20, 247–248. [Google Scholar] [CrossRef]
- Hoaglund Hyzer, C.; Williamson, M.L.; Jansen, P.E.; Kopach, M.; Brian Scherer, R.; Baertschi, S. Mechanistic Studies of the N-Formylation of Edivoxetine, a Secondary Amine-Containing Drug, in a Solid Oral Dosage Form. J. Pharm. Sci. 2017, 106. [Google Scholar] [CrossRef]
- Hemenway, J.N.; Carvalho, T.C.; Mantri, R.V.; Wu, Y.; Levons, J.K.; Narang, A.S.; Paruchuri, S.R.; Stamato, H.J.; Varia, S.A. Reactive Impurities in PEG: A Case Study. In Excipient Applications in Formulation Design and Drug Delivery; Narang, A.S., Boddu, S.H.S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 67–91. [Google Scholar]
- Waterman, K.C.; Carella, A.J.; Gumkowski, M.J.; Lukulay, P.; MacDonald, B.C.; Roy, M.C.; Shamblin, S.L. Improved protocol and data analysis for accelerated shelf-life estimation of solid dosage forms. Pharm. Res. 2007, 24, 780–790. [Google Scholar] [CrossRef]
- Saringat, H.B.; Alfadol, K.I.; Khan, G.M. The influence of different plasticizers on some physical and mechanical properties of hydroxypropyl methylcellulose free films. Pak. J. Pharm. Sci. 2005, 18, 25–38. [Google Scholar]
- Heinämäki, J.T.; Lehtola, V.M.; Nikupaavo, P.; Yliruusi, J.K. The mechanical and moisture permeability properties of aqueous-based hydroxypropyl methylcellulose coating systems plasticized with polyethylene glycol. Int. J. Pharm. 1994, 112, 191–196. [Google Scholar] [CrossRef]
- Laboulfie, F.; Hémati, M.; Lamure, A.; Diguet, S. Effect of the plasticizer on permeability, mechanical resistance and thermal behaviour of composite coating films. Powder Technol. 2013, 238, 14–19. [Google Scholar] [CrossRef]
- Jarray, A.; Gerbaud, V.; Hemati, M. Polymer-plasticizer compatibility during coating formulation: A multi-scale investigation. Prog. Org. Coat. 2016, 101, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Michoel, A.; Van den Mooter, G. Study of the phase behavior of polyethylene glycol 6000–itraconazole solid dispersions using DSC. Int. J. Pharm. 2004, 272, 181–187. [Google Scholar] [CrossRef] [PubMed]








| Formulation Composition. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Inert tablet core | 200.0 mg | 200.0 mg | 200.0 mg | 200.0 mg |
| SAXA in a form of HCl salt | 2.5 mg 0.00317 mol/gsaxa | 2.5 mg 0.00317 mol/gsaxa | 2.5 mg 0.00317 mol/gsaxa | 2.5 mg 0.00317 mol/gsaxa |
| HPMC 603 | 18.0 mg | 17.5 mg | 17.0 mg | 16.5 mg |
| PEG 6000 | 2.0 mg 0.0182 mol/gsaxa | 2.5 mg 0.0227 mol/gsaxa | 3.0 mg 0.0272 mol/gsaxa | 3.5 mg 0.0318 mol/gsaxa |
| Film-coated tablet (total) | 222.5 mg | 222.5 mg | 222.5 mg | 222.5 mg |
| PEG to SAXA ratio (w/w) | 0.8 | 1.0 | 1.2 | 1.4 |
| PEG to HPMC ratio (w/w) | 0.111 | 0.143 | 0.176 | 0.212 |
| T (°C) No. of Days | 30 °C | 40 °C | 50 °C | 60 °C |
|---|---|---|---|---|
| 7 | - | - | - | X |
| 14 | - | - | X | X |
| 30 | X | X | X | - |
| 45 | - | - | X | - |
| 60 | X | X | X | - |
| 90 | X | X | - | - |
| 120 | X | X | - | - |
| 150 | X | X | - | - |
| 180 | X | X | - | - |
| 30 °C | 40 °C | 50 °C | PEG to SAXA Weight Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10% RH | 30% RH | 50% RH | 10% RH | 30% RH | 50% RH | 10% RH | 30% RH | 50% RH | |
| 5.68 | 5.70 | 5.65 | 5.63 | 5.63 | 5.58 | 5.63 | 5.62 | 5.57 | 0.8 |
| 5.66 | 5.63 | 5.61 | 5.63 | 5.60 | 5.56 | 5.62 | 5.59 | 5.55 | 1.0 |
| 5.60 | 5.49 | 5.61 | 5.53 | 5.51 | 5.56 | 5.53 | 5.51 | 5.55 | 1.2 |
| 5.48 | 5.40 | 5.27 | 5.43 | 5.40 | 5.25 | 5.45 | 5.37 | 5.23 | 1.4 |
| ri | ki’(40 °C) | Ai | Ea,i (kJ mol−1) |
|---|---|---|---|
| r1 | 0.803 ± 0.003 d−1 | 0.803 ± 0.009 d−1 | ~0 |
| r2 | 0.0246 ± 0.0002 d−1 | 0.0246 ± 0.0003 d−1 | ~0 |
| r3 | 4.66 ± 0.01 d−1 | (8.2 ± 0.2) × 103 d−1 | 19.46 ± 0.05 |
| r4 | 0.06672 ± 0.00004 d−1 | (7.3 ±0.3) × 1013 d−1 | 90.2 ± 0.1 |
| r5 | 0.00025735 ± 0.00000008 L mol−1 d−1 * | (1.51 ±0.03) × 1011 L mol−1 d−1 * | 88.54 ± 0.04 |
| r6 | 0.09322 ± 0.00006 L mol−1 d−1 * | 0.09322 ± 0.00006 L mol−1 d−1 * | ~0 |
| r7 | 0.1524 ± 0.0001 L gsaxa mol−2 d−1 * | (3.0 ± 0.3) × 1024 L gsaxa mol−2 d−1 * | 151.6 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robnik, B.; Likozar, B.; Wang, B.; Stanić Ljubin, T.; Časar, Z. Understanding and Kinetic Modeling of Complex Degradation Pathways in the Solid Dosage Form: The Case of Saxagliptin. Pharmaceutics 2019, 11, 452. https://doi.org/10.3390/pharmaceutics11090452
Robnik B, Likozar B, Wang B, Stanić Ljubin T, Časar Z. Understanding and Kinetic Modeling of Complex Degradation Pathways in the Solid Dosage Form: The Case of Saxagliptin. Pharmaceutics. 2019; 11(9):452. https://doi.org/10.3390/pharmaceutics11090452
Chicago/Turabian StyleRobnik, Blaž, Blaž Likozar, Baifan Wang, Tijana Stanić Ljubin, and Zdenko Časar. 2019. "Understanding and Kinetic Modeling of Complex Degradation Pathways in the Solid Dosage Form: The Case of Saxagliptin" Pharmaceutics 11, no. 9: 452. https://doi.org/10.3390/pharmaceutics11090452
APA StyleRobnik, B., Likozar, B., Wang, B., Stanić Ljubin, T., & Časar, Z. (2019). Understanding and Kinetic Modeling of Complex Degradation Pathways in the Solid Dosage Form: The Case of Saxagliptin. Pharmaceutics, 11(9), 452. https://doi.org/10.3390/pharmaceutics11090452