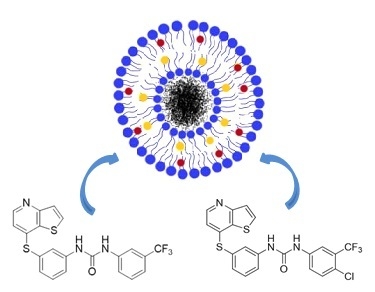
Magnetoliposomes Containing Calcium Ferrite Nanoparticles for Applications in Breast Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Calcium Ferrite Nanoparticles
2.2. Preparation of Magnetoliposomes
2.3. Preparation of Giant Unilamellar Vesicles (GUVs)
2.4. Spectroscopic Measurements
2.4.1. General Methods
2.4.2. FRET Measurements
2.4.3. Fluorescence Anisotropy Measurements
2.4.4. Drug Encapsulation Efficiency
2.5. Structural Characterization
2.5.1. Electron Microscopy (TEM and SEM)
2.5.2. X-Ray Diffraction (XRD)
2.5.3. Dynamic Light Scattering (DLS)
2.6. Magnetic Measurements
2.7. Nanoparticles Encapsulation Efficiency
2.8. Studies in Cell Lines
3. Results and Discussion
3.1. Properties of the Antitumor Compounds
3.1.1. Studies in Cell Lines
3.1.2. Fluorescence Properties
3.2. Nanoparticles Characterization
3.2.1. Absorption Spectra
3.2.2. X-Ray Diffraction (XRD) Measurements
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. Magnetic Properties
3.3. Development of Magnetoliposomes
3.3.1. Magnetoliposomes Characterization
3.3.2. Interaction with Model Membranes
3.4. Drug-Loaded Magnetoliposomes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bremer-Hoffmann, S.; Amenta, V.; Rossi, F. Nanomedicines in the European translational process. Eur. J. Nanomed. 2015, 7, 191–202. [Google Scholar] [CrossRef]
- Chang, E.H.; Harford, J.B.; Eatin, M.A.; Boisseau, P.M.; Dube, A.; Hayeshi, R.; Swai, H.; Lee, D.S. Nanomedicine: Past, present and future—A global perspective. Biochem. Biophys. Res. Commun. 2015, 468, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Xiang, D. Nanoparticles drug delivery systems: An excellent carrier for tumor peptide vaccines. Drug Deliv. 2018, 25, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaatsad, K.; Radomski, M.K.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ahmad, M.Z.; Kazmi, I.; Akhter, S.; Afzal, M.; Gupta, G.; Sinha, V.R. Emergence of Nanomedicine as Cancer Targeted Magic Bullets: Recent Development and Need to Address the Toxicity Apprehension. Curr. Drug Discov. Technol. 2012, 9, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Saldívar-Ramírez, M.M.G.; Sánchez-Torres, C.G.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J.C.; Almanza-Robles, J.M.; Larson, A.; Reséndiz-Hernández, P.J.; Acunã-Gutiérrez, I.O. Study on the efficiency of nanosized magnetite and mixed ferrites in magnetic hyperthermia. J. Mater. Sci. Mater. Electron. 2014, 25, 2229–2236. [Google Scholar] [CrossRef]
- Khanna, L.; Verma, N.K. Synthesis, characterization and in vitro cytotoxicity study of calcium ferrite nanoparticles. Mater. Sci. Semicond. Process. 2013, 16, 1842–1848. [Google Scholar] [CrossRef]
- Hirazawa, H.; Kusamoto, S.; Aono, H.; Naohara, T.; Mori, K.; Hattori, Y.; Maehara, T.; Watanabe, Y. Preparation of fine Mg1−xCaxFe2O4 powder using reverse coprecipitation method for thermal coagulation therapy in an AC magnetic field. J. Alloys Compd. 2008, 4, 467–473. [Google Scholar] [CrossRef]
- Sulaiman, N.H.; Ghazali, M.J.; Majlis, B.Y.; Yunas, J.; Razalia, M. Superparamagnetic calcium ferrite nanoparticles synthesized using a simple sol-gel method. Bio. Med. Mater. Eng. 2015, 26, S103–S110. [Google Scholar] [CrossRef]
- Monnier, C.A.; Burnand, D.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Magnetoliposomes: Opportunities and challenges. Eur. J. Nanomed. 2014, 6, 201–215. [Google Scholar] [CrossRef]
- Yanase, M.; Shinkai, M.; Honda, H.; Wakabayashi, T. Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. Jpn. J. Cancer Res. 1998, 89, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Zhang, Z.R.; Yang, H.; Tan, Q.Y.; Qin, Q.Y.; Qiu, X.L. Lyophilized paclitaxel magnetoliposomes as a potential drug delivery system for breast carcinoma via parenteral administration: In vitro and in vivo studies. Pharm. Res. 2005, 22, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, R.; Barnadas-Rodríguez, R.; Callejas-Fernández, J.; Hidalgo-Alvarez, R.; Estelrich, J. Preparation and characterization of extruded magnetoliposomes. Int. J. Pharm. 2008, 347, 156–162. [Google Scholar] [CrossRef] [PubMed]
- García-Jimeno, S.; Escribano, E.; Queralt, J.; Estelrich, J. Magnetoliposomes prepared by reverse-phase followed by sequential extrusion: Characterization and possibilities in the treatment of inflammation. Int. J. Pharm. 2011, 405, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.V.; Martins, T.M.M.; Goes, A.M.; Fabris, J.D.; Cavalcante, L.C.D.; Outon, L.E.F.; Domingues, R.Z. Thermosensitive gemcitabine-magnetoliposomes for combined hyperthermia and chemotherapy. Nanotechnology 2016, 27, 085105. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Busquets, M.A.; Morán, M.C. Effect of PEGylation on Ligand-Targeted Magnetoliposomes: A Missed Goal. ACS Omega 2017, 2, 6544–6555. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Banerjee, R.; Bellare, J.; Bahadur, D. Preparation and characterization of manganese ferrite-based magnetic liposomes for hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2007, 311, 208–215. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Ramos, J.M.F.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes based on manganese ferrite nanoparticles as nanocarriers for antitumor drugs. RSC Adv. 2016, 6, 17302–17313. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.O.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetoliposomes based on nickel ferrite nanoparticles for biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 18011–18021. [Google Scholar] [CrossRef]
- Cardoso, B.D.; Rio, I.S.R.; Rodrigues, A.R.O.; Fernandes, F.C.T.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetoliposomes containing magnesium ferrite nanoparticles as nanocarriers for the model drug curcumin. R. Soc. Open Sci. 2018, 5, 181017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyman, H.R.; Frey, R.R.; Bousquet, P.F.; Cunha, G.A.; Moskey, M.D.; Ahmed, A.A.; Soni, N.B.; Marcotte, P.A.; Pease, L.J.; Glaser, K.B.; et al. Thienopyridine urea inhibitors of KDR kinase. Bioorg. Med. Chem. Lett. 2007, 17, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.A.; Peixoto, D.R.; Costa, H.J.C.; Froufe, R.C.; Calhelha, R.M.V.; Abreu, I.C.F.R.; Ferreira, R.; Soares, R. Queiroz, Synthesis, antiangiogenesis evaluation and molecular docking studies of 1-aryl-3-[(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas: Discovery of a new substitution pattern for type II VEGFR-2 Tyr kinase inhibitors. Bioorg. Med. Chem. 2015, 23, 6497–6509. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.A.; Peixoto, D.; Queiroz, M.-J.; Soares, R. Antiangiogenic 1-aryl-3-[3-(thieno[3,2-b] pyridin-7-ylthio)phenyl]ureas inhibit MCF-7 and MDA-MB-231 human breast cancer cell lines through PI3K/Akt and MAPK/Erk pathways. J. Cell. Biochem. 2016, 117, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.H.; Van Der Esker, M.W.; Pathmamanoharan, C.; Wiersema, P.H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry 1977, 16, 3932–3935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Niu, H.; Zhang, Y.; Liu, J.; Shia, Y.; Zhang, X.; Cai, Y. Biocompatible phosphatidylcholine bilayer coated on magnetic nanoparticles and their application in the extraction of several polycyclic aromatic hydrocarbons from environmental water and milk samples. J. Chromatogr. A 2012, 1238, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Almeida, B.G.; Rodrigues, J.M.; Queiroz, M.-J.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Magnetoliposomes as carriers for promising antitumor thieno[3,2-b]pyridin-7-arylamines: Photophysical and biological studies. RSC Adv. 2017, 7, 15352–15361. [Google Scholar] [CrossRef]
- Tamba, Y.; Terashima, H.; Yamazaki, M. A membrane filtering method for the purification of giant unilamellar vesicles. Chem. Phys. Lipids 2011, 164, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Tamba, Y.; Masum, S.M.; Yamashita, Y.; Yamazaki, M. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochim. Biophys. Acta 2002, 1564, 173–182. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence—Principles and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 247–261. [Google Scholar]
- Demas, J.N.; Crosby, G.A. The measurement of photoluminescence quantum yields—Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar]
- Fery-Forgues, S.; Lavabre, D. Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J. Chem. Educ. 1999, 76, 1260–1264. [Google Scholar] [CrossRef]
- Johnson, I.; Spence, M.T.Z. Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 11th ed.; Life Technologies: Carlsbad, CA, USA, 2010; pp. 545–587. [Google Scholar]
- Wagner, T.; Eglinger, J. Thorstenwagner/ij-Particlesizer: V1.0.9 Snapshot Release (Version V1.0.9-SNAPSHOT); Zenodo: Genève, Switzerland, 2017. [Google Scholar]
- Tarek, M.; Zaki, M.; EI-Sayed, A.Y. Spectrophotometric determination of iron as phenylfluorone complex sensitized with Triton X-100. Anal. Bioanal. Chem. 1989, 334, 335–339. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.N.C.; Hortelão, A.C.L.; Ramos, J.M.F.; Oliveira, A.D.S.; Calhelha, R.C.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. A new antitumoral heteroarylaminothieno[3,2-b]pyridine derivative: Its incorporation into liposomes and interaction with proteins monitored by fluorescence. Photochem. Photobiol. Sci. 2014, 13, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Mendes, P.M.F.; Silva, P.M.L.; Machado, V.A.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Solid and aqueous magnetoliposomes as nanocarriers for a new potential drug active against breast cancer. Colloids Surf. B Biointerfaces 2017, 158, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawanishi, M.; Ogo, S.; Ikemoto, M.; Totsuka, Y.; Ishino, K.; Wakabayashi, K.; Yagi, T. Genotoxicity and reactive oxygen species production induced by magnetite nanoparticles in mammalian cells. J. Toxicol. Sci. 2013, 38, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.-G.; Miyauchi, M. Efficient Visible Light Active CaFe2O4/WO3 based composite photocatalysts: Effect of interfacial modification. J. Phys. Chem. C 2009, 113, 17132–17137. [Google Scholar] [CrossRef]
- Kim, H.G.; Borse, P.H.; Jang, J.S.; Jeong, E.D.; Jung, O.S.; Suh, Y.J.; Lee, J.S. Fabrication of CaFe2O4/MgFe2O4 bulk heterojunction for enhanced visible light photocatalysis. Chem. Comm. 2009, 39, 5889–5891. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physi. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Lal, G.; Punia, K.; Dolia, S.N.; Alvi, P.A.; Dalela, S.; Kumar, S. Rietveld refinement, Raman, optical, dielectric, Mössbauer and magnetic characterization of superparamagnetic fcc-CaFe2O4 nanoparticles. Ceram. Int. 2019, 45, 5837–5847. [Google Scholar] [CrossRef]
- Samariya, A.; Dolia, S.N.; Prasad, A.S.; Sharma, P.K.; Pareek, S.P.; Dhawan, M.S.; Kumar, S. Size dependent structural and magnetic behaviour of CaFe2O4. Curr. Appl. Phys. 2013, 13, 830–835. [Google Scholar] [CrossRef]
- Mathew, D.S.; Juang, R.-S. An overview of the structure and magnetism of spinel nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Smit, J. Magnetic Properties of Materials; McGraw Hill: New York, NY, USA, 1971; p. 89. [Google Scholar]
- Chauhan, B.S.; Kumar, R.; Jadhav, K.M.; Singh, M. Magnetic study of substituted Mg-Mn ferrites synthesized by citrate precursor method. J. Magn. Magn. Mater. 2004, 283, 71–81. [Google Scholar] [CrossRef]
- Nikam, D.S.; Jadhav, S.V.; Khot, V.M.; Bohara, R.A.; Hong, C.K.; Mali, S.S.; Pawar, S.H. Cation distribution, structural, morphological and magnetic properties of Co1−xZnxFe2O4 (x = 0–1) nanoparticles. RSC Adv. 2015, 5, 2338–2345. [Google Scholar] [CrossRef]
- Widdrat, M.; Kumari, M.; Tompa, E.; Pósfai, M.; Hirt, A.M.; Faivre, D. Keeping nanoparticles fully functional: Long-term storage and alteration of magnetite. ChemPlusChem 2014, 79, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Lentz, B. Membrane ‘fluidity’ as detected by diphenylhexatriene probes. Chem. Phys. Lipids 1989, 50, 171–190. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.R.O.; Matos, J.O.G.; Nova Dias, A.M.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Queiroz, M.J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of Multifunctional Liposomes Containing Magnetic/Plasmonic MnFe2O4/Au Core/Shell Nanoparticles. Pharmaceutics 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Curtis, H.; Barnes, N.S. Biology, 5th ed.; Worth Publishers: New York, NY, USA, 1989. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.J.G.; Castanheira, E.M.S.; Rei, M.C.; Real Oliveira, M.E.C.D. Nile Red and DCM fluorescence anisotropy studies in C12E7/DPPC mixed systems. J. Phys. Chem. B 2002, 106, 12841–12846. [Google Scholar] [CrossRef]
- Feitosa, E.; Alves, F.R.; Niemiec, A.; Oliveira, M.E.C.D.R.; Castanheira, E.M.S.; Baptista, A.L.F. Cationic liposomes in mixed didodecyldimethylammonium bromide and dioctadecyldimethylammonium bromide aqueous dispersions studied by differential scanning calorimetry, Nile Red fluorescence, and turbidity. Langmuir 2006, 22, 3579–3585. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 89th ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Abingdon, UK, 2009. [Google Scholar]












| GI50 (μM) [24] | Compound 1 | Compound 2 |
|---|---|---|
| MCF-7 | 1.2 ± 0.2 | 1.6 ± 0.3 |
| MDA-MB-231 | 5.0 ± 0.7 | 7.0 ± 0.8 |
| GI50 (μM) a | Compound 1 | Compound 2 |
|---|---|---|
| NCI-H460 | 34.6 ± 1.0 | 30.4 ± 1.3 |
| HeLa | 13.7 ± 0.6 | 13.0 ± 0.7 |
| HepG2 | 25.4 ± 2.5 | 24.2 ± 1.5 |
| PLP2 | 34.2 ± 3.3 | 46.8 ± 2.1 |
| Sample | Ox,y,z | i * | Lattice Constant (nm) | Size (nm) | RF | χ2 |
|---|---|---|---|---|---|---|
| CaFe2O4 (original XRD data) | 0.243 | 0.85 | 0.8340 | 4.83 | 2.90 | 1.06 |
| CaFe2O4 (background subtracted XRD data) | 0.241 | 0.85 | 0.8338 | 5.26 | 4.98 | 0.91 |
| System | Lipid | Size ± SD (nm) | PDI ± SD |
|---|---|---|---|
| AMLs | Egg-PC | 129 ± 12 | 0.139 ± 0.04 |
| DPPC | 132 ± 15 | 0.185 ± 0.08 | |
| SMLs | DPPC | 164 ± 23 | 0.203 ± 0.02 |
| Lipid | Anisotropy, r | ||
|---|---|---|---|
| Compound 1 | Compound 2 | ||
| Liposomes | Egg-PC (25 °C) | 0.182 [39] | 0.155 |
| DPPC (25 °C) | 0.251 [39] | 0.181 | |
| DPPC (55 °C) | 0.157 [39] | 0.115 | |
| AMLs | Egg-PC (25 °C) | 0.176 | 0.138 |
| DPPC (25 °C) | 0.232 | 0.189 | |
| DPPC (55 °C) | 0.106 | 0.105 | |
| SMLs | DPPC (25 °C) | 0.212 | 0.175 |
| DPPC (55 °C) | 0.094 | 0.106 | |
| Glycerol | ---- | 0.308 [39] | 0.255 |
| System | EE% ± SD | |
|---|---|---|
| Compound 1 | Compound 2 | |
| AMLs (Egg-PC) | 81.9 ± 8.5 | 86.3 ± 8.2 |
| SMLs (DPPC) | 68.2 ± 9.1 | 75.8 ± 10.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.S.M.; Cardoso, B.D.; Rodrigues, A.R.O.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Queiroz, M.-J.R.P.; Martinho, O.; Baltazar, F.; Calhelha, R.C.; et al. Magnetoliposomes Containing Calcium Ferrite Nanoparticles for Applications in Breast Cancer Therapy. Pharmaceutics 2019, 11, 477. https://doi.org/10.3390/pharmaceutics11090477
Pereira DSM, Cardoso BD, Rodrigues ARO, Amorim CO, Amaral VS, Almeida BG, Queiroz M-JRP, Martinho O, Baltazar F, Calhelha RC, et al. Magnetoliposomes Containing Calcium Ferrite Nanoparticles for Applications in Breast Cancer Therapy. Pharmaceutics. 2019; 11(9):477. https://doi.org/10.3390/pharmaceutics11090477
Chicago/Turabian StylePereira, Daniela S. M., Beatriz D. Cardoso, Ana Rita O. Rodrigues, Carlos O. Amorim, Vítor S. Amaral, Bernardo G. Almeida, Maria-João R. P. Queiroz, Olga Martinho, Fátima Baltazar, Ricardo C. Calhelha, and et al. 2019. "Magnetoliposomes Containing Calcium Ferrite Nanoparticles for Applications in Breast Cancer Therapy" Pharmaceutics 11, no. 9: 477. https://doi.org/10.3390/pharmaceutics11090477
APA StylePereira, D. S. M., Cardoso, B. D., Rodrigues, A. R. O., Amorim, C. O., Amaral, V. S., Almeida, B. G., Queiroz, M.-J. R. P., Martinho, O., Baltazar, F., Calhelha, R. C., Ferreira, I. C. F. R., Coutinho, P. J. G., & Castanheira, E. M. S. (2019). Magnetoliposomes Containing Calcium Ferrite Nanoparticles for Applications in Breast Cancer Therapy. Pharmaceutics, 11(9), 477. https://doi.org/10.3390/pharmaceutics11090477